The Effect of Simvastatin on Odontoblastic Differentiation of Human Dental Pulp Stem Cells: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Dental Pulp Stem Cell (hDPSC) Culture
2.2. Viability Assay
2.3. Alkaline Phosphatase Activity
2.4. Alizarin Red Staining Assay
2.5. Odontogenic-Related Gene Expression Analysis
2.6. Statistical Analysis
3. Results
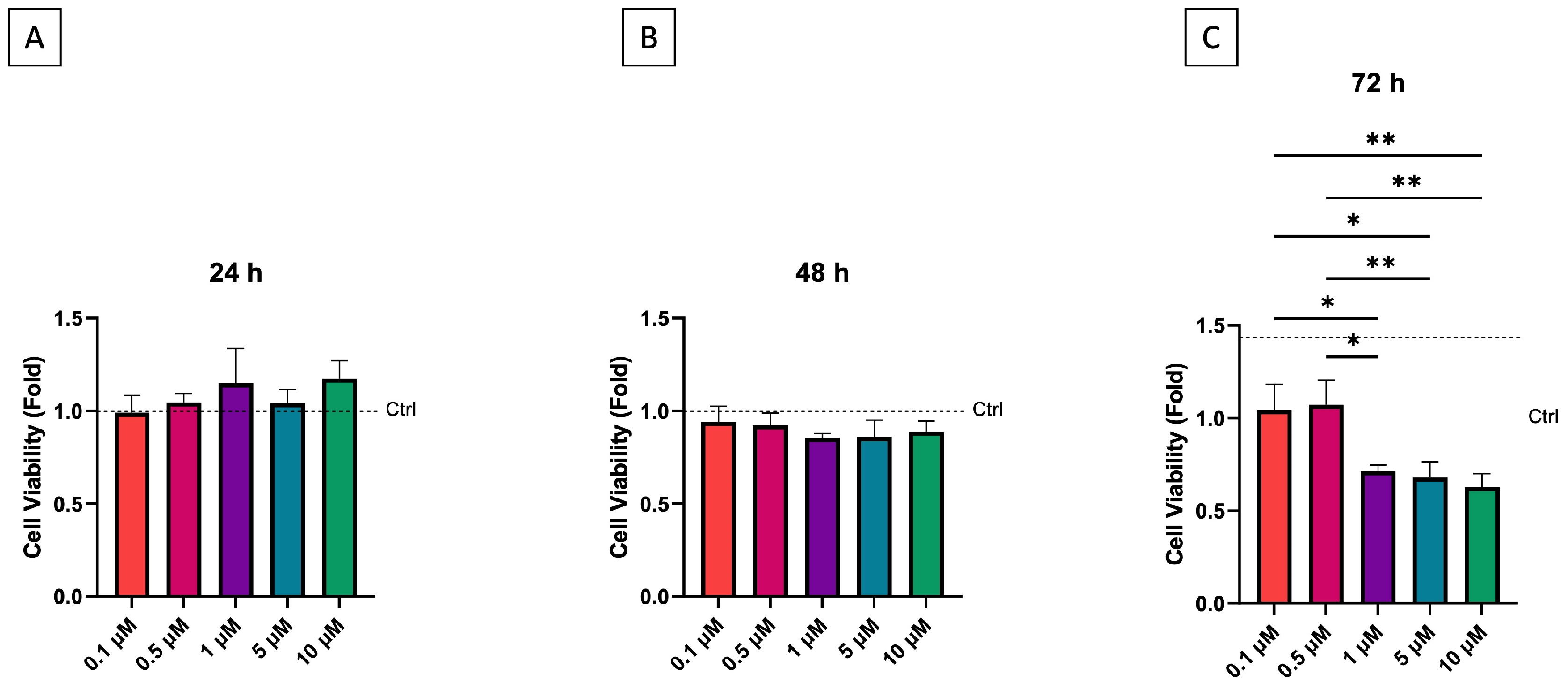
3.1. Cell Viability
3.2. Osteogenic Differentiation of hDPSCs
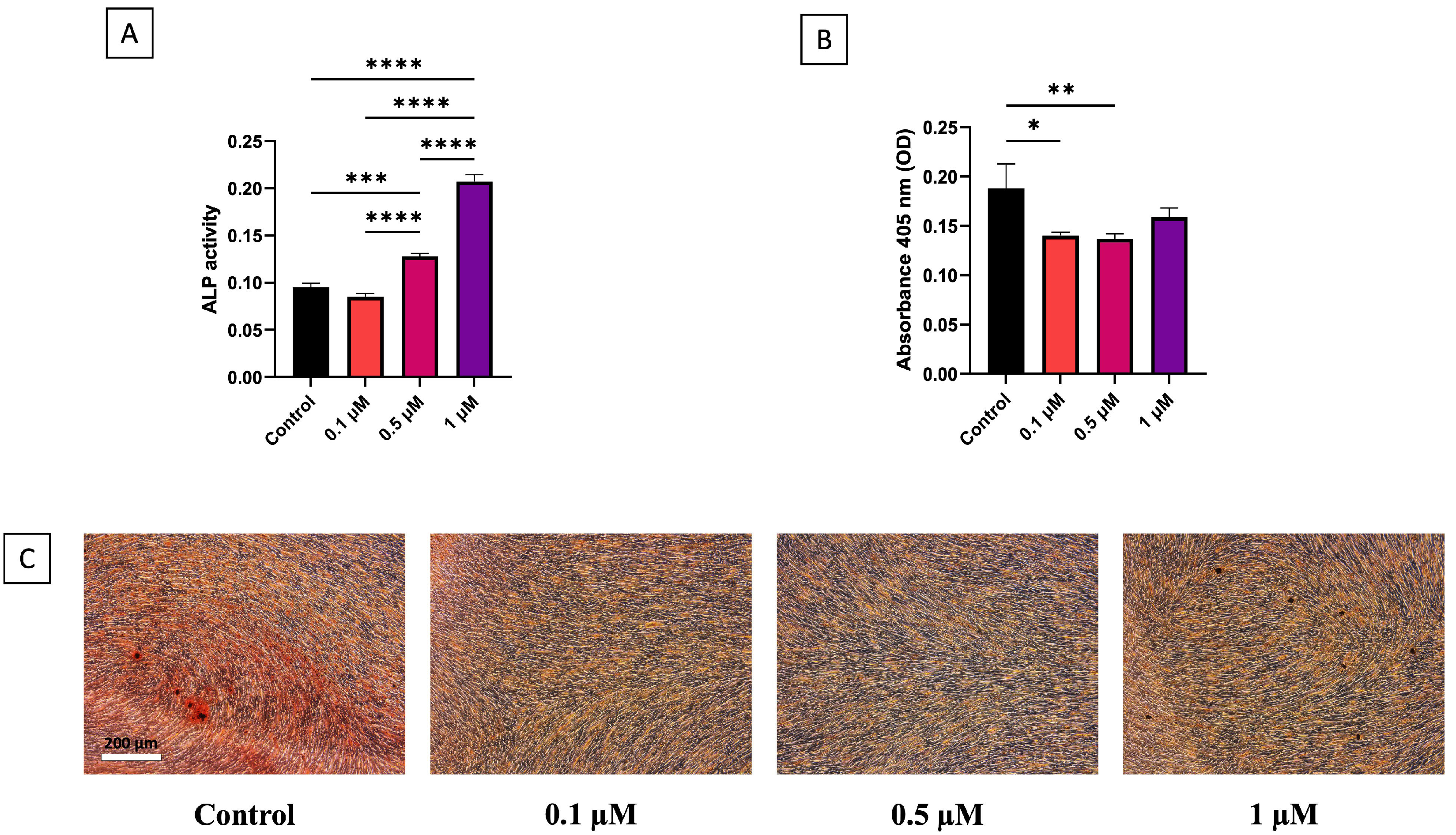
3.3. Odontogenic Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| hDPSCs | human Dental Pulp Stem Cells |
| ALP | Alkaline Phosphatase |
| ARS | Alizarin Red Staining |
| VPT | Vital Pulp Therapy |
| BMP | Bone Morphogenetic Protein |
| PCR | Polymerase Chain Reaction |
| OCN | Osteocalcin |
| MEPE | Matrix Extracellular Phosphoglycoprotein |
| DSPP | Dentin Sialophosphoprotein |
| DMP-1 | Dentin Matrix Protein 1 |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| OSX | Osterix |
References
- AAE Position statement on vital pulp therapy. J. Endod. 2021, 47, 1340–1344. [CrossRef]
- European Society of Endodontology (ESE); Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef]
- Silva, E.J.N.L.; Pinto, K.P.; Riche, F.N.S.J.; Carestiato, M.G.H.ì.; Martins, J.N.R.; Duncan, H.F.; Versiani, M.A.; De-Deus, G. A meta-analysis of calcium silicate-based cements and calcium hydroxide as promoters of hard tissue bridge formation. Int. Endod. J. 2025, 58, 685–714. [Google Scholar] [CrossRef]
- Kabra, S.; Thosar, N.R.; Malviya, N.S. Exploring the Synergistic Effect of Simvastatin in Oral Health Applications: A Literature Review. Cureus 2023, 15, e44411. [Google Scholar] [CrossRef]
- Glynis, A.; Foschi, F.; Kefalou, I.; Koletsi, D.; Tzanetakis, G.N. Regenerative Endodontic Procedures for the Treatment of Necrotic Mature Teeth with Apical Periodontitis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2021, 47, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.A.; Dhar, V.; Chen, C.Y.; Crystal, Y.O.; Guelmann, M.; Marghalani, A.A.; AlShamali, S.; Xu, Z.; Glickman, G.N.; Wedeward, R. Use of Vital Pulp Therapies in Primary Teeth 2024. Pediatr. Dent. 2024, 46, 13–26. [Google Scholar]
- Silva, E.J.N.L.; Pinto, K.P.; Belladonna, F.G.; Ferreira, C.M.A.; Versiani, M.A.; De-Deus, G. Success rate of permanent teeth pulpotomy using bioactive materials: A systematic review and meta-analysis of randomized clinical trials. Int. Endod. J. 2023, 56, 1024–1041. [Google Scholar] [CrossRef]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Lin, J.; Zeng, Q.; Wei, X.; Zhao, W.; Cui, M.; Gu, J.; Lu, J.; Yang, M.; Ling, J. Regenerative Endodontics Versus Apexification in Immature Permanent Teeth with Apical Periodontitis: A Prospective Randomized Controlled Study. J. Endod. 2017, 43, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; De Deus, G.; Kristoffersen, I.M.; Wiig, E.; Reseland, J.E.; Johnsen, G.F.; Silva, E.J.N.L.; Haugen, H.J. Regenerative Endodontics by Cell Homing: A Review of Recent Clinical trials. J. Endod. 2023, 49, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Clinical Perspectives of Pulp Regeneration. J. Endod. 2020, 46, S161–S174. [Google Scholar] [CrossRef]
- Ahmed, H.M.A.; El-Karim, I.; Duncan, H.F.; Krastl, G.; Galler, K. Implications of root, pulp chamber, and canal anatomy on pulpotomy and revitalization procedures. Clin. Oral. Investig. 2023, 27, 6357–6369. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Sonoyama, W.; Ono, M.; Akiyama, K.; Fujisawa, T.; Oshima, M.; Tsuchimoto, Y.; Matsuka, Y.; Yasuda, T.; Shi, S.; et al. Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. J. Endod. 2009, 35, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Al-Natour, B.; Rankin, R.; McKenna, R.; McMillan, H.; Zhang, S.D.; About, I.; Khan, A.A.; Galicia, J.C.; Lundy, F.T.; El-Karim, I.A. Identification and validation of novel biomarkers and therapeutics for pulpitis using connectivity mapping. Int. Endod. J. 2021, 54, 1571–1580. [Google Scholar] [CrossRef]
- Chen, P.Y.; Sun, J.S.; Tsuang, Y.H.; Chen, M.H.; Weng, P.W.; Lin, F.H. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr. Res. 2010, 30, 191–199. [Google Scholar] [CrossRef]
- Jia, W.; Zhao, Y.; Yang, J.; Wang, W.; Wang, X.; Ling, L.; Ge, L. Simvastatin promotes dental pulp stem cell-induced coronal pulp regeneration in pulpotomized teeth. J. Endod. 2016, 42, 1049–1054. [Google Scholar] [CrossRef]
- Del Giudice, C.; Rengo, C.; Maglitto, M.; Armogida, N.G.; Iaculli, F.; Rengo, S.; Menale, C.; Spagnuolo, G. Cytotoxicity effects and differentiation potential of ormocer-based and nanohybrid composite resins on human dental pulp stem cells. Dent. Mater. 2024, 40, 984–992. [Google Scholar] [CrossRef]
- Soares, D.G.; Zhang, Z.; Mohamed, F.; Eyster, T.W.; de Souza Costa, C.A.; Ma, P.X. Simvastatin and nanofibrous poly(l-lactic acid) scaffolds to promote the odontogenic potential of dental pulp cells in an inflammatory environment. Acta Biomater. 2018, 68, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Karanxha, L.; Park, S.J.; Son, W.J.; Nör, J.E.; Min, K.S. Combined effects of simvastatin and enamel matrix derivative on odontoblastic differentiation of human dental pulp cells. J. Endod. 2013, 39, 76–82. [Google Scholar] [CrossRef]
- Wang, J.H.; He, D.E. Simvastatin treatment promotes proliferation of human dental pulp stem cells via modulating PI3K/AKT/miR-9/KLF5 signalling pathway. J. Cell. Mol. Med. 2021, 25, 10892–10901. [Google Scholar] [CrossRef]
- Mortada, I.; Mortada, R. Dental pulp stem cells and osteogenesis: An update. Cytotechnology 2018, 70, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Aiello, E.; Cooper, P.R.; Marrelli, B.; Makeeva, I.; Islam, M.; Spagnuolo, G.; Maged, D.; De Vito, D.; Tatullo, M. A Dedifferentiation Strategy to Enhance the Osteogenic Potential of Dental Derived Stem Cells. Front. Cell Dev. Biol. 2021, 9, 668558. [Google Scholar] [CrossRef]
- Spagnuolo, G. Bioactive Dental Materials: The Current Status. Materials 2022, 15, 2016. [Google Scholar] [CrossRef]
- Bossù, M.; Iaculli, F.; Di Giorgio, G.; Salucci, A.; Polimeni, A.; Di Carlo, S. Different Pulp Dressing Materials for the Pulpotomy of Primary Teeth: A Systematic Review of the Literature. J. Clin. Med. 2020, 9, 838. [Google Scholar] [CrossRef]
- Spagnuolo, G.; De Luca, I.; Iaculli, F.; Barbato, E.; Valletta, A.; Calarco, A.; Valentino, A.; Riccitiello, F. Regeneration of dentin-pulp complex: Effect of calcium-based materials on hDPSCs differentiation and gene expression. Dent. Mater. 2023, 39, 485–491. [Google Scholar] [CrossRef]
- Duncan, H.F. Present status and future directions-Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55 (Suppl. S3), 497–511. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Lee, Y.M.; Hong, S.O.; Kim, E.C. Simvastatin promotes odontoblastic differentiation and expression of angiogenic factors via heme oxygenase-1 in primary cultured human dental pulp cells. J. Endod. 2010, 36, 447–452. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, Y.J.; Yeh, C.L.; Lin, T.A.; Lin, C.P. Development of calcium phosphate/calcium sulfate biphasic biomedical material with hyaluronic acid containing collagenase and simvastatin for vital pulp therapy. Dent. Mater. 2020, 36, 755–764. [Google Scholar] [CrossRef]
- Lau, C.P.Y.; Fung, C.S.H.; Wong, K.C.; Wang, Y.H.; Huang, L.; Tsui, S.K.W.; Lee, O.K.; Kumta, S.M. Simvastatin Possesses Antitumor and Differentiation-Promoting Properties That Affect Stromal Cells in Giant Cell Tumor of Bone. J. Orthop. Res. 2020, 38, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, C.; Changb, J.; Sun, J. Odontogenic differentiation of human dental pulp cells induced by silicate-based bioceramics via activation of P38/MEPE pathway. RSC Adv. 2015, 5, 72536. [Google Scholar] [CrossRef]
- Rewthamrongsris, P.; Phothichailert, S.; Chokechanachaisakul, U.; Janjarussakul, P.; Kornsuthisopon, C.; Samaranayake, L.; Osathanon, T. Simvastatin modulates osteogenic differentiation in Stem Cells isolated from Apical Papilla. BMC Oral. Health 2025, 25, 398. [Google Scholar] [CrossRef]
- Xue, D.; Gong, Z.; Zhu, F.; Qiu, Y.; Li, X. Simvastatin increases cell viability and suppresses the expression of cytokines and vascular endothelial growth factor in inflamed human dental pulp stem cells in vitro. Adv. Clin. Exp. Med. 2018, 27, 1615–1623. [Google Scholar] [CrossRef]
- Mahendran, K.; Ponnusamy, C.; Maloor, S.A. Histological evaluation of pulpal response to direct pulp capping using statins with α-tricalcium phosphate and mineral trioxide aggregate in human teeth. J. Conserv. Dent. 2019, 22, 441–448. [Google Scholar] [CrossRef]
- Parisay, I.; Moodi, M.; Boskabady, M.; Bagheri, H.; Salari, R.; Hoseinzadeh, M. Physical and drug- releasing properties of a cement containing simvastatin (SimCeram). BMC Oral. Health 2025, 25, 684. [Google Scholar] [CrossRef]
- Chak, R.K.; Singh, R.K.; Mutyala, J.; Killi, N.K. Clinical Radiographic Evaluation of 3Mixtatin and MTA in Primary Teeth Pulpotomies: A Randomized Controlled. Int. J. Clin. Pediatr. Dent. 2022, 15, S80–S86. [Google Scholar] [CrossRef] [PubMed]
- Bossù, M.; Mancini, P.; Bruni, E.; Uccelletti, D.; Preziosi, A.; Rulli, M.; Relucenti, M.; Donfrancesco, O.; Iaculli, F.; Di Giorgio, G.; et al. Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases. Biology 2021, 10, 470. [Google Scholar] [CrossRef]
- Iaculli, F.; Rodríguez-Lozano, F.J.; Briseño-Marroquín, B.; Wolf, T.G.; Spagnuolo, G.; Rengo, S. Vital Pulp Therapy of Permanent Teeth with Reversible or Irreversible Pulpitis: An Overview of the Literature. J. Clin. Med. 2022, 11, 4016. [Google Scholar] [CrossRef]
- Back, L.S.; Manso, I.S.; Sordi, M.B.; Magrin, G.L.; Aragonês, Á.; Magini, R.S.; Gruber, R.; Cruz, A.C.C. Evaluating Bioassays for the Determination of Simvastatin’s Osteogenic Activity: A Systematic Review. J. Funct. Biomater. 2025, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Diniz, J.A.; Dourado, A.C.A.G.; Barbirato, D.D.S.; da Silveira, K.G.; Vasconcellos, R.J.H.; Laureano Filho, J.R. Effect of simvastatin topical use on alveolar bone after tooth extraction: A scoping review. Clin. Oral Investig. 2024, 28, 86. [Google Scholar] [CrossRef]
- Zhang, M.; Bian, Y.Q.; Tao, H.M.; Yang, X.F.; Mu, W.D. Simvastatin induces osteogenic differentiation of MSCs via Wnt/β-catenin pathway to promote fracture healing. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2896–2905. [Google Scholar] [PubMed]
- Bronze-Uhle, E.S.; Melo, C.C.D.S.B.; da Silva, I.S.P.; Stuani, V.T.; Bueno, V.H.; Rinaldo, D.; de Souza Costa, C.A.; Lisboa Filho, P.N.; Soares, D.G. Simvastatin-Loaded Chitosan Microspheres as a Biomaterial for Dentin Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2025, 113, e35536. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, S.; Shirban, F.; Bagherniya, M.; Johnston, T.P.; Sahebkar, A. The effects of statins on dental and oral health: A review of preclinical and clinical studies. J. Transl. Med. 2020, 18, 155. [Google Scholar] [CrossRef] [PubMed]

| Primer | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
|---|---|---|
| OCN | CCACCGAGACACCATGAGAG | CCATAGGGCTGGGAGGTCAG |
| MEPE | GGTTATACAGATCTTCAAGAGAGAG | GTTGGTACTTTCAGCTGCATCACT |
| DSPP | AGAAGGACCTGGCCAAAAAT | TCTCCTCGGCTACTGCTGTT |
| DMP-1 | TGGGGATTATCCTGTGCTCT | TACTTCTGGGGTCACTGTCG |
| GAPDH | TCAGCAATGCCTCCTGCAC | TCTGGGTGGCAGTGATGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giudice, C.; Iaculli, F.; Rengo, C.; Salucci, A.; Spagnuolo, G.; Riccitiello, F.; Bossù, M.; Polimeni, A.; Di Giorgio, G. The Effect of Simvastatin on Odontoblastic Differentiation of Human Dental Pulp Stem Cells: An In Vitro Study. Dent. J. 2025, 13, 428. https://doi.org/10.3390/dj13090428
Del Giudice C, Iaculli F, Rengo C, Salucci A, Spagnuolo G, Riccitiello F, Bossù M, Polimeni A, Di Giorgio G. The Effect of Simvastatin on Odontoblastic Differentiation of Human Dental Pulp Stem Cells: An In Vitro Study. Dentistry Journal. 2025; 13(9):428. https://doi.org/10.3390/dj13090428
Chicago/Turabian StyleDel Giudice, Carmela, Flavia Iaculli, Carlo Rengo, Alessandro Salucci, Gianrico Spagnuolo, Francesco Riccitiello, Maurizio Bossù, Antonella Polimeni, and Gianni Di Giorgio. 2025. "The Effect of Simvastatin on Odontoblastic Differentiation of Human Dental Pulp Stem Cells: An In Vitro Study" Dentistry Journal 13, no. 9: 428. https://doi.org/10.3390/dj13090428
APA StyleDel Giudice, C., Iaculli, F., Rengo, C., Salucci, A., Spagnuolo, G., Riccitiello, F., Bossù, M., Polimeni, A., & Di Giorgio, G. (2025). The Effect of Simvastatin on Odontoblastic Differentiation of Human Dental Pulp Stem Cells: An In Vitro Study. Dentistry Journal, 13(9), 428. https://doi.org/10.3390/dj13090428











