Breaking Barriers: Evaluating Challenges in Advancing Periodontal Ligament Cell-Derived Organoids
Abstract
1. Introduction
2. Methods
3. Results
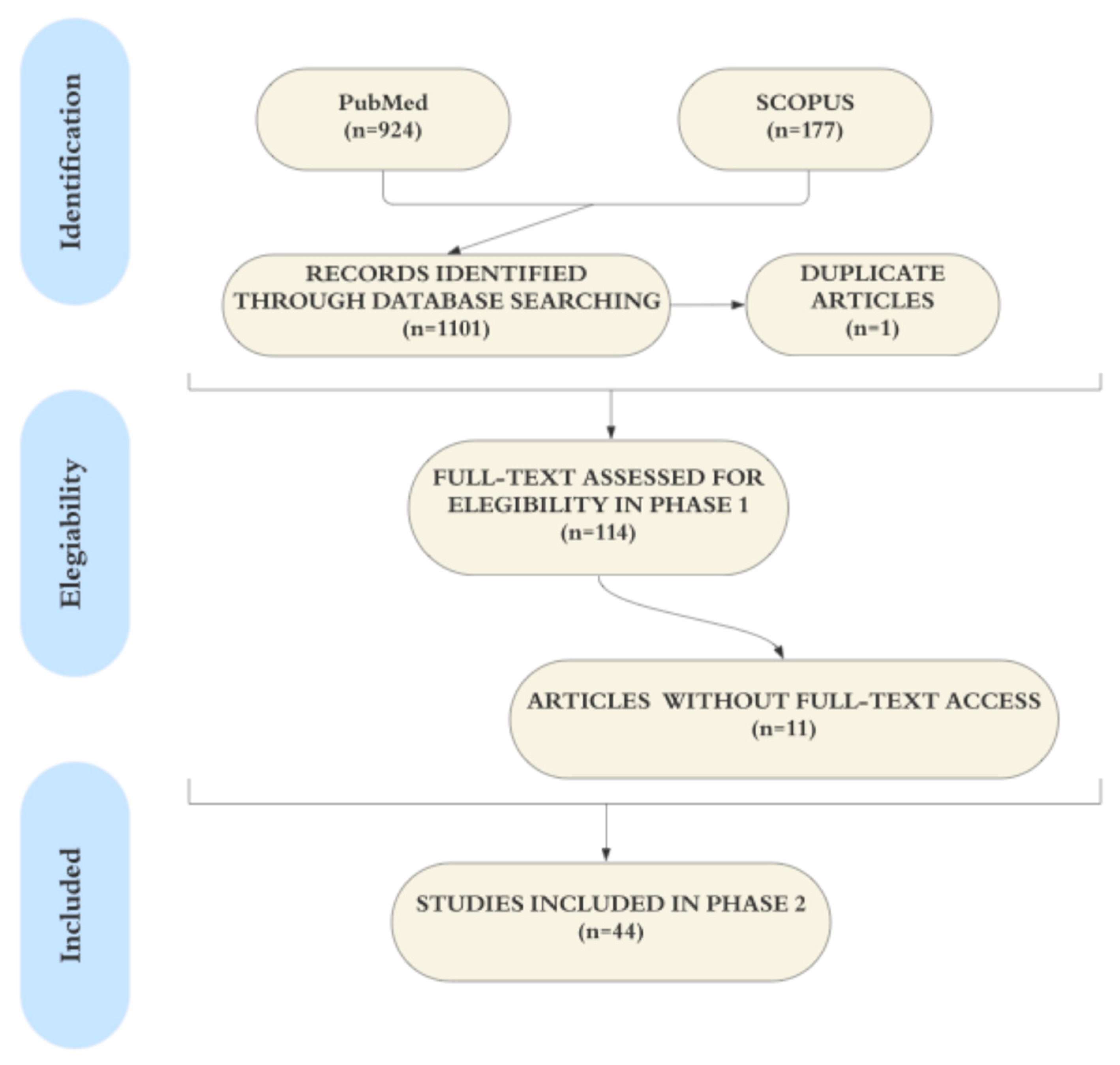
3.1. Study Selection
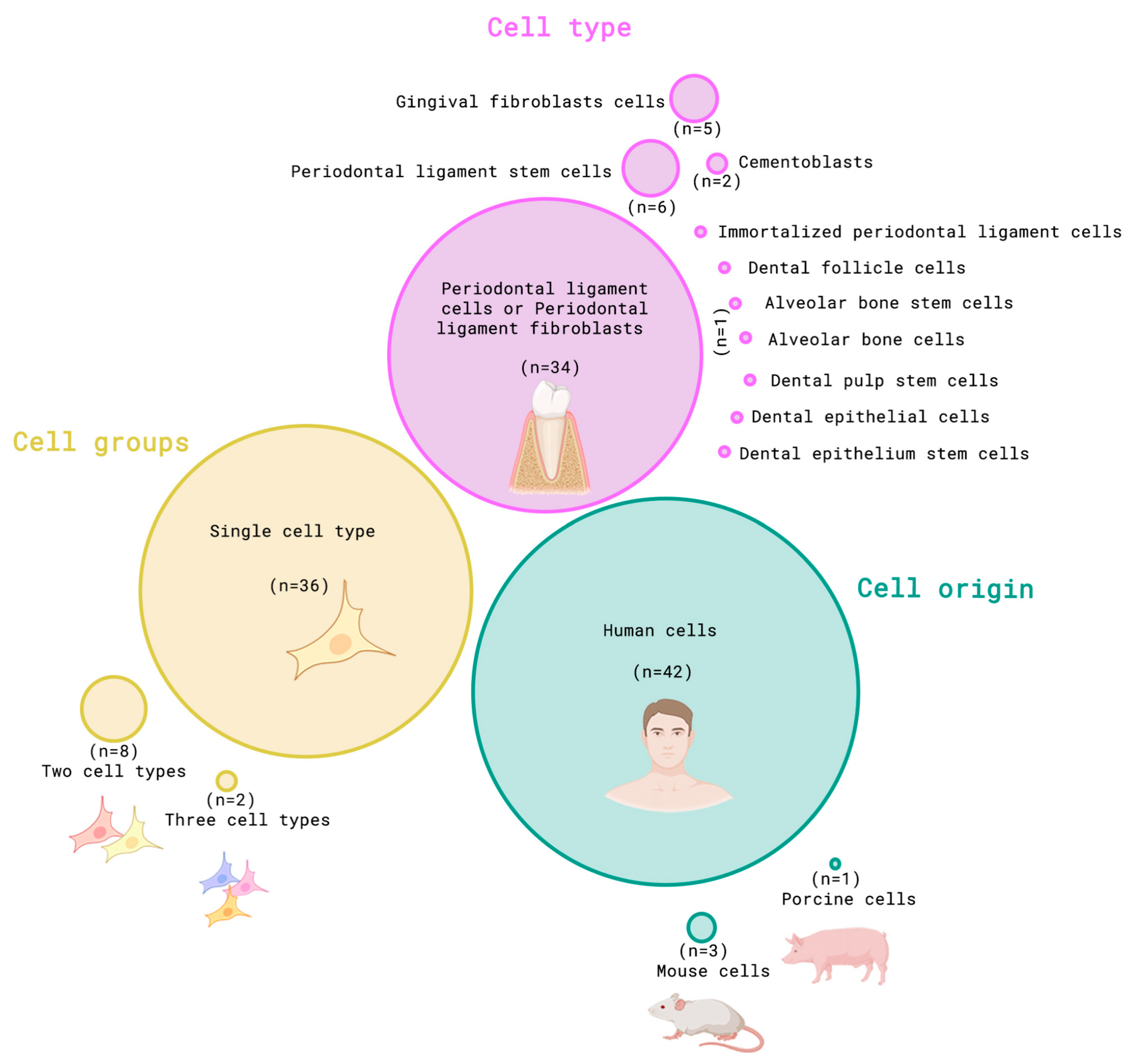
3.2. Cells
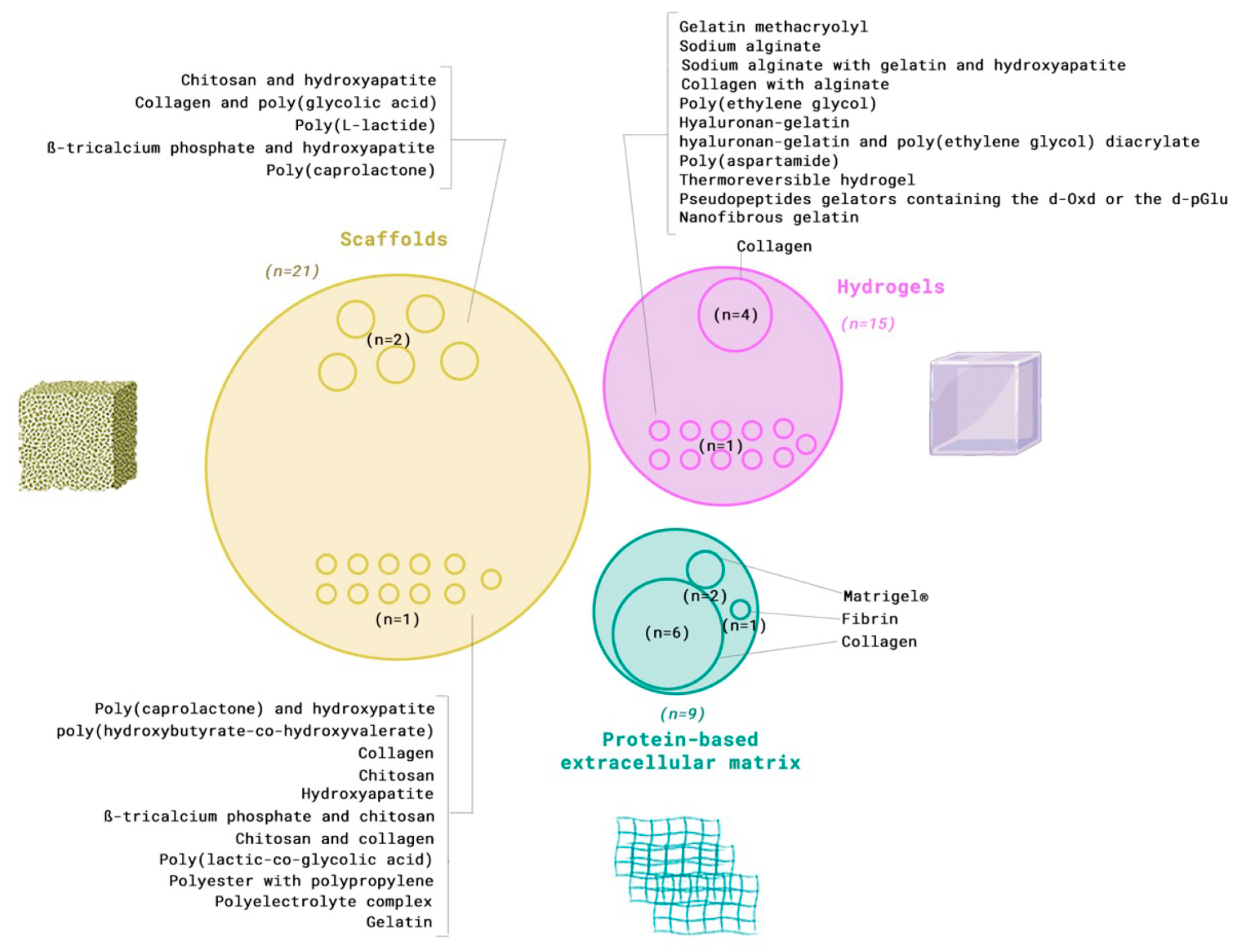
3.3. Extracellular Matrix Component
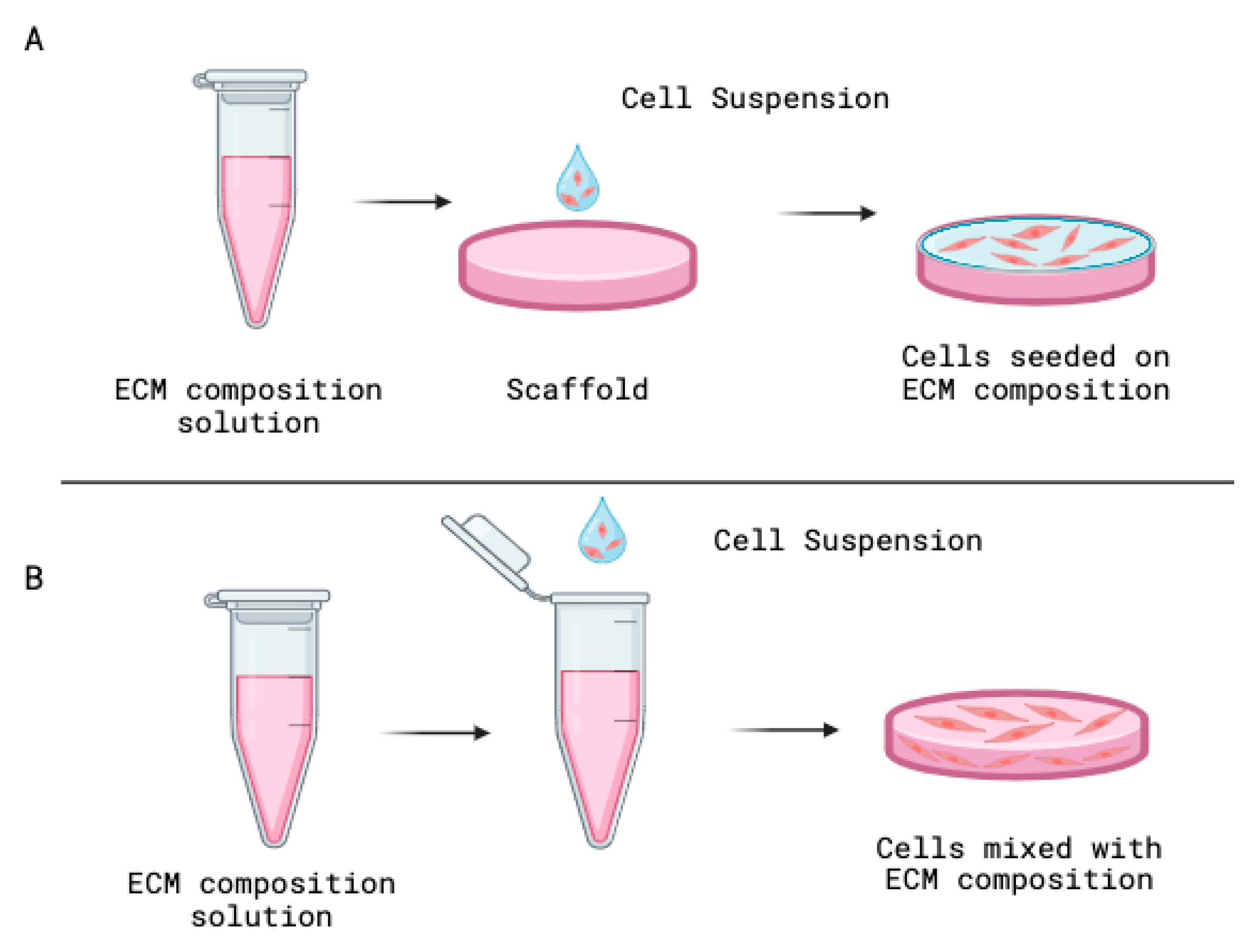
3.4. Organoids Preparation
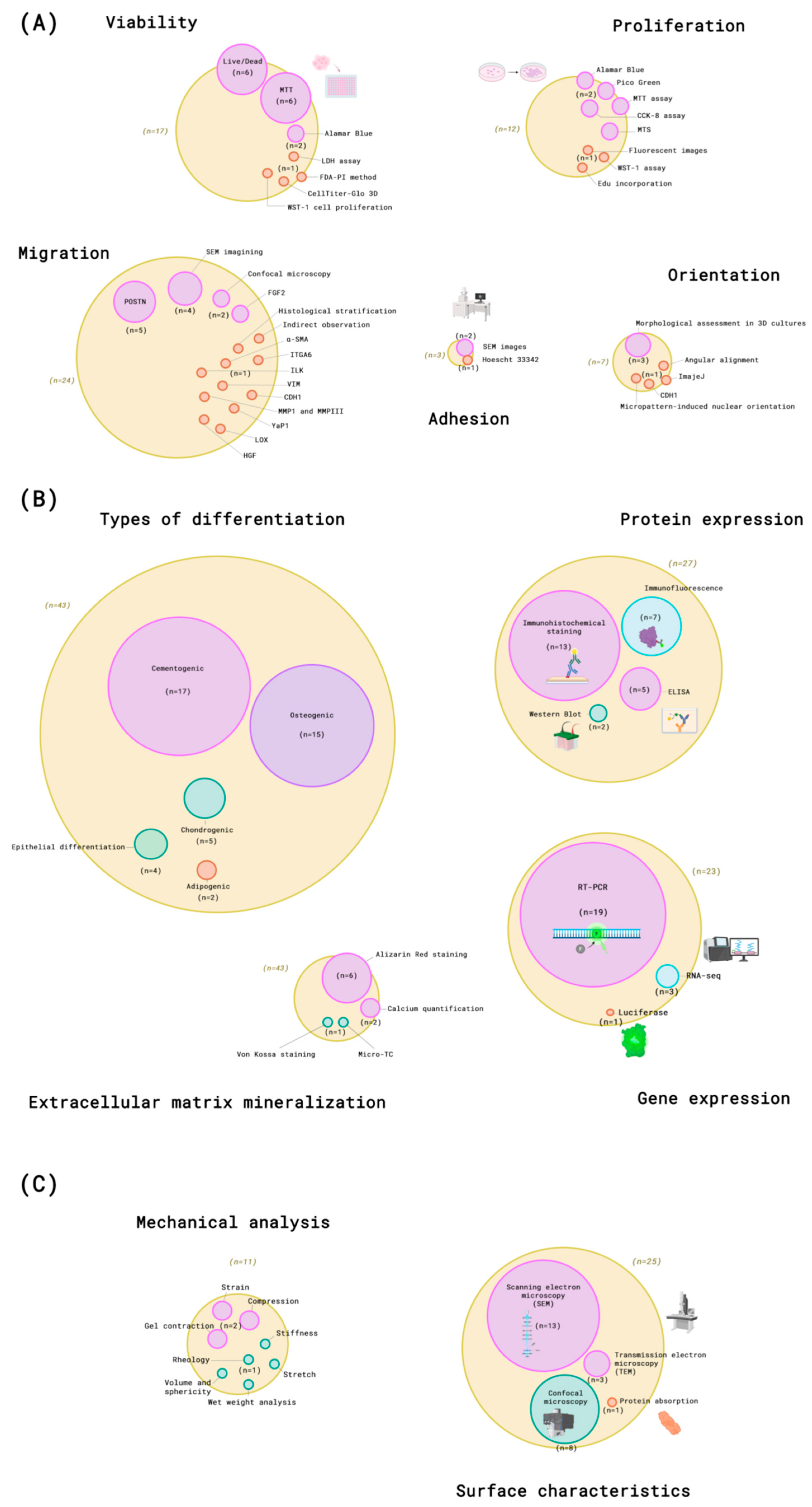
3.5. Characterization Methods
3.5.1. Viability
3.5.2. Proliferation
3.5.3. Adhesion
3.5.4. Migration
3.5.5. Orientation
3.5.6. Differentiation
3.5.7. Surface Characteristics
3.5.8. Mechanical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2016, 14, 910–919. [Google Scholar] [CrossRef]
- Lampe, K.J.; Mooney, R.G.; Bjugstad, K.B.; Mahoney, M.J. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J. Biomed. Mater. Res. A 2010, 94, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Roman, V.; Mihaila, M.; Radu, N.; Marineata, S.; Diaconu, C.C.; Bostan, M. Cell Culture Model Evolution and Its Impact on Improving Therapy Efficiency in Lung Cancer. Cancers 2023, 15, 4996. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Nguyen, T.; Benoit, D.S.W. Matrix Control of Periodontal Ligament Cell Activity via Synthetic Hydrogel Scaffolds. Tissue Eng. Part A 2021, 27, 733–747. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Eng. Part C Methods 2016, 22, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, T.; Horvath, J.M.; Blitterswijk, C.A.; LaPointe, V.L.S. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J. Tissue Eng. Regen. Med. 2019, 13, 1815–1829. [Google Scholar] [CrossRef]
- Poornima, K.; Francis, A.P.; Hoda, M.; Eladl, M.A.; Subramanian, S.; Veeraraghavan, V.P.; El-Sherbiny, M.; Asseri, S.M.; Hussamuldin, A.B.A.; Surapaneni, K.M.; et al. Implications of Three-Dimensional Cell Culture in Cancer Therapeutic Research. Front. Oncol. 2022, 12, 891673. [Google Scholar] [CrossRef]
- Hermans, F.; Hemeryck, L.; Bueds, C.; Torres Pereiro, M.; Hasevoets, S.; Kobayashi, H.; Lambrechts, D.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Organoids from mouse molar and incisor as new tools to study tooth-specific biology and development. Stem Cell Rep. 2023, 18, 1166–1181. [Google Scholar] [CrossRef]
- Cardoso, B.D.; Castanheira, E.M.S.; Lanceros-Méndez, S.; Cardoso, V.F. Recent Advances on Cell Culture Platforms for In Vitro Drug Screening and Cell Therapies: From Conventional to Microfluidic Strategies. Adv. Healthc. Mater. 2023, 12, 2202936. [Google Scholar] [CrossRef]
- Biju, T.S.; Priya, V.V.; Francis, A.P. Role of three-dimensional cell culture in therapeutics and diagnostics: An updated review. Drug Deliv. Transl. Res. 2023, 13, 2239–2253. [Google Scholar] [CrossRef]
- Zheng, L.; Zhan, Y.; Wang, C.; Fan, Q.; Sun, D.; Li, Y.; Xiong, Y. Technological advances and challenges in constructing complex gut organoid systems. Front. Cell Dev. Biol. 2024, 12, 1432744. [Google Scholar] [CrossRef]
- Özkan, A.; LoGrande, N.T.; Feitor, J.F.; Goyal, G.; Ingber, D.E. Intestinal organ chips for disease modelling and personalized medicine. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 751–773. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, C.; Zhu, L.; Zhao, T.; Wang, Z.; Yi, X.; Yan, F.; Wang, X.; Li, C.; Cui, T.; et al. Liver organoids: Updates on generation strategies and biomedical applications. Stem Cell Res. Ther. 2024, 15, 244. [Google Scholar] [CrossRef]
- Mikawa, R.; Gotoh, S. Past and future of alveolar organoids for lung regenerative medicine. Stem Cells 2024, 42, 491–498. [Google Scholar] [CrossRef]
- Zhang, X.; Contessi Negrini, N.; Correia, R.; Sharpe, P.T.; Celiz, A.D.; Angelova Volponi, A. Generating Tooth Organoids Using Defined Bioorthogonally Cross-Linked Hydrogels. ACS Macro Lett. 2024, 13, 1620–1626. [Google Scholar] [CrossRef]
- Hemeryck, L.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Establishing Organoids from Human Tooth as a Powerful Tool Toward Mechanistic Research and Regenerative Therapy. J. Vis. Exp. 2022, 182, e63671. [Google Scholar]
- Sano, T.; Renn, T.Y.; Kanematsu, T.; Hsieh, M.S.; Hsu, C.C.; Chang, W.J. Organoid in dentistry: Models for oral biology and disease. J. Dent. Sci. 2025, 20, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Schweinitzer, S.; Kadousaraei, M.J.; Aydin, M.S.; Mustafa, K.; Rashad, A. Measuring cell proliferation in bioprinting research. Biomed. Mater. 2024, 19, 031001. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Lai, C.C.; Lin, D.J. Collagen Scaffolds Laden with Human Periodontal Ligament Fibroblasts Promote Periodontal Regeneration in SD Rat Model. Polymers 2023, 15, 2649. [Google Scholar] [CrossRef] [PubMed]
- Mahdi Souzani, A.; Rajeshwari, H.R.S.; Selvaganapathy, P.R.; Kishen, A. Impact of 3D collagen-based model and hydrostatic pressure on periodontal ligament fibroblast: A morpho-biochemical analysis. J. Mech. Behav. Biomed. Mater. 2023, 147, 106092. [Google Scholar] [CrossRef]
- Gauthier, R.; Attik, N.; Chevalier, C.; Salles, V.; Grosgogeat, B.; Gritsch, K.; Trunfio-Sfarghiu, A.M. 3D Electrospun Polycaprolactone Scaffolds to Assess Human Periodontal Ligament Cells Mechanobiological Behaviour. Biomimetics 2023, 8, 108. [Google Scholar] [CrossRef]
- Makkar, H.; Atkuru, S.; Tang, Y.L.; Sethi, T.; Lim, C.T.; Tan, K.S.; Sriram, G. Differential immune responses of 3D gingival and periodontal connective tissue equivalents to microbial colonization. J. Tissue Eng. 2022, 13, 204173142211116. [Google Scholar] [CrossRef]
- Ono, T.; Tomokiyo, A.; Ipposhi, K.; Yamashita, K.; Alhasan, M.A.; Miyazaki, Y.; Kunitomi, Y.; Tsuchiya, A.; Ishikawa, K.; Maeda, H. Generation of biohybrid implants using a multipotent human periodontal ligament cell line and bioactive core materials. J. Cell. Physiol. 2021, 236, 6742–6753. [Google Scholar] [CrossRef]
- Zou, R.; Wu, S.; Wang, Y.; Kang, X.; Zhao, S.; Shi, H.; Zheng, D.; Gao, B.; Ma, S.; Niu, L.; et al. Role of integrinlinked kinase in static compressive stressinduced autophagy via phosphatidylinositol 3 kinase in human periodontal ligament cells. Int. J. Mol. Med. 2021, 48, 167. [Google Scholar] [CrossRef]
- Kim, M.G.; Park, C.H. The Topographical Optimization of 3D Microgroove Pattern Intervals for Ligamentous Cell Orientations: In Vitro. Int. J. Mol. Sci. 2020, 21, 9358. [Google Scholar] [CrossRef] [PubMed]
- Firth, F.A.; Milne, T.J.; Seo, B.; Farella, M. An in-vitro mechanical strain three-dimensional culture model: Periodontal ligament cell viability, apoptosis, and endoplasmic reticulum stress response. Eur. J. Oral Sci. 2020, 128, 120–127. [Google Scholar] [CrossRef]
- Saminathan, A.; Vinoth, K.J.; Low, H.H.; Cao, T.; Meikle, M.C. Engineering three-dimensional constructs of the periodontal ligament in hyaluronan–gelatin hydrogel films and a mechanically active environment. J. Periodontal Res. 2013, 48, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Hegedűs, O.; Juriga, D.; Sipos, E.; Voniatis, C.; Juhász, Á.; Idrissi, A.; Zrínyi, M.; Varga, G.; Jedlovszky-Hajdú, A.; Nagy, K.S. Free thiol groups on poly(aspartamide) based hydrogels facilitate tooth-derived progenitor cell proliferation and differentiation. PLoS ONE 2019, 14, e0226363. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; van den Beucken, J.; Yuan, C.; Jansen, J.; Yang, F. Spheroid formation and stemness preservation of human periodontal ligament cells on chitosan films. Oral Dis. 2018, 24, 1083–1092. [Google Scholar] [CrossRef]
- Mino, C.; Iwata, T.; Kawata, T. Adhesion of human periodontal ligament cells by three-dimensional culture to the sterilized root surface of extracted human teeth. J. Oral Sci. 2017, 59, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Okada, M.; Inami, K.; Hashimoto, Y.; Matsumoto, N. Cell survival and gene expression under compressive stress in a three-dimensional in vitro human periodontal ligament-like tissue model. Cytotechnology 2016, 68, 249–260. [Google Scholar] [CrossRef]
- An, S.; Gao, Y.; Ling, J. Characterization of human periodontal ligament cells cultured on three-dimensional biphasic calcium phosphate scaffolds in the presence and absence of L-ascorbic acid, dexamethasone and β-glycerophosphate in vitro. Exp. Ther. Med. 2015, 10, 1387–1393. [Google Scholar] [CrossRef]
- Alves, L.B.; Mariguela, V.C.; Grisi, M.F.d.M.; Souza, S.L.S.d.; Novaes Junior, A.B.; Taba Junior, M.; de Oliveira, P.T.; Palioto, D.B. Expression of osteoblastic phenotype in periodontal ligament fibroblasts cultured in three-dimensional collagen gel. J. Appl. Oral Sci. 2015, 23, 206–214. [Google Scholar] [CrossRef][Green Version]
- Yamada, R.; Kitajima, K.; Arai, K.; Igarashi, M. Cytokeratin expression of engrafted three-dimensional culture tissues using epithelial cells derived from porcine periodontal ligaments. J. Oral Pathol. Med. 2014, 43, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Heckler, A.F.; Mirzaei, Z.; Pereira, I.; Simmons, C.A.; Gong, S.G. Development of a three-dimensional in vitro model system to study orthodontic tooth movement. Arch. Oral Biol. 2013, 58, 1498–1510. [Google Scholar] [CrossRef]
- Liao, W.; Okada, M.; Sakamoto, F.; Okita, N.; Inami, K.; Nishiura, A.; Hashimoto, Y.; Matsumoto, N. In vitro human periodontal ligament-like tissue formation with porous poly-l-lactide matrix. Mater. Sci. Eng. C 2013, 33, 3273–3280. [Google Scholar] [CrossRef]
- Hoz, L.; Romo, E.; Zeichner-David, M.; Sanz, M.; Nuñez, J.; Gaitán, L.; Mercado, G.; Arzate, H. Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol. Int. 2012, 36, 129–136. [Google Scholar] [CrossRef]
- Oortgiesen, D.A.W.; Yu, N.; Bronckers, A.L.J.J.; Yang, F.; Walboomers, X.F.; Jansen, J.A. A Three-Dimensional Cell Culture Model to Study the Mechano-Biological Behavior in Periodontal Ligament Regeneration. Tissue Eng. Part C Methods 2012, 18, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Jung, I.H.; Park, J.C.; Jung, U.W.; Kim, C.S.; Lee, Y.K.; Kim, C.-K.; Choi, S.-H. Effect of seeding using an avidin-biotin binding system on the attachment of periodontal ligament fibroblasts to nanohydroxyapatite scaffolds: Three-dimensional culture. J. Periodontal Implant Sci. 2011, 41, 73–78. [Google Scholar] [CrossRef]
- Berahim, Z.; Moharamzadeh, K.; Rawlinson, A.; Jowett, A.K. Biologic Interaction of Three-Dimensional Periodontal Fibroblast Spheroids With Collagen-Based and Synthetic Membranes. J. Periodontol. 2011, 82, 790–797. [Google Scholar] [CrossRef]
- Akman, A.C.; Tığlı, R.S.; Gümüşderelioğlu, M.; Nohutcu, R.M. bFGF-loaded HA-chitosan: A promising scaffold for periodontal tissue engineering. J. Biomed. Mater. Res. A 2010, 92, 953–962. [Google Scholar] [CrossRef]
- Liao, F.; Chen, Y.; Li, Z.; Wang, Y.; Shi, B.; Gong, Z.; Cheng, X. A novel bioactive three-dimensional β-tricalcium phosphate/chitosan scaffold for periodontal tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 489–496. [Google Scholar] [CrossRef]
- Peng, L.; Cheng, X.; Zhuo, R.; Lan, J.; Wang, Y.; Shi, B.; Li, S. Novel gene-activated matrix with embedded chitosan/plasmid DNA nanoparticles encoding PDGF for periodontal tissue engineering. J. Biomed. Mater. Res. A 2009, 90, 564–576. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Cheng, X.R.; Chen, Y.; Shi, B.; Chen, X.H.; Xu, D.X.; Ke, J. Three-dimensional Nanohydroxyapatite/Chitosan Scaffolds as Potential Tissue Engineered Periodontal Tissue. J. Biomater. Appl. 2007, 21, 333–349. [Google Scholar] [CrossRef]
- Lee, Y.; Nahm, D.; Jung, Y.; Choi, J.; Kim, S.G.; Cho, M.; Kim, M.-H.; Chae, C.-H.; Kim, S.-G. Differential Gene Expression of Periodontal Ligament Cells After Loading of Static Compressive Force. J. Periodontol. 2007, 78, 446–452. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, H.; Zhao, Y.; Jiang, T. Three-Dimensional Culture of Human Periodontal Ligament Cells on Highly Porous Polyglycolic Acid Scaffolds in vitro. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 1–4 September 2005; IEEE: Piscataway, NJ, USA, 2005; pp. 4908–4911. [Google Scholar]
- Phuegyod, S.; Pramual, S.; Wattanavichean, N.; Assawajaruwan, S.; Amornsakchai, T.; Sukho, P.; Svasti, J.; Surarit, R.; Niamsiri, N. Microbial Poly(hydroxybutyrate-co-hydroxyvalerate) Scaffold for Periodontal Tissue Engineering. Polymers 2023, 15, 855. [Google Scholar] [CrossRef]
- Liang, Y.; Shakya, A.; Liu, X. Biomimetic Tubular Matrix Induces Periodontal Ligament Principal Fiber Formation and Inhibits Osteogenic Differentiation of Periodontal Ligament Stem Cells. ACS Appl. Mater. Interfaces 2022, 14, 36451–36461. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M.; Ishikawa, H.; Tamaoki, S.; Maeda, H.; Ohno, J. Embedded Human Periodontal Ligament Stem Cells Spheroids Enhance Cementogenic Differentiation via Plasminogen Activator Inhibitor 1. Int. J. Mol. Sci. 2022, 23, 2340. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, M.; Liu, Y.; Shi, C.; Wang, Y.; Liu, T.; Huang, Y.; Zhong, P.; Dai, J.; Liu, X. The performance of 3D bioscaffolding based on a human periodontal ligament stem cell printing technique. J. Biomed. Mater. Res. A 2021, 109, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zong, W.; Xu, X.; Chen, J. Improved biphasic calcium phosphate combined with periodontal ligament stem cells may serve as a promising method for periodontal regeneration. Am. J. Transl. Res. 2018, 10, 4030–4041. [Google Scholar]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-Dimensional Printed Multiphase Scaffolds for Regeneration of Periodontium Complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Dabija-Wolter, G.; Bakken, V.; Cimpan, M.R.; Johannessen, A.C.; Costea, D.E. In vitro reconstruction of human junctional and sulcular epithelium. J. Oral Pathol. Med. 2013, 42, 396–404. [Google Scholar] [CrossRef]
- Zanna, N.; Focaroli, S.; Merlettini, A.; Gentilucci, L.; Teti, G.; Falconi, M.; Tomasini, C. Thixotropic Peptide-Based Physical Hydrogels Applied to Three-Dimensional Cell Culture. ACS Omega 2017, 2, 2374–2381. [Google Scholar] [CrossRef]
- Hillmann, G.; Gebert, A.; Geurtsen, W. Matrix expression and proliferation of primary gingival fibroblasts in a three-dimensional cell culture model. J. Cell Sci. 1999, 112, 2823–2832. [Google Scholar] [CrossRef]
- Lin, H.H.; Chao, P.H.G.; Tai, W.C.; Chang, P.C. 3D-Printed Collagen-Based Waveform Microfibrous Scaffold for Periodontal Ligament Reconstruction. Int. J. Mol. Sci. 2021, 22, 7725. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Pieles, O.; Pfeifer, C.; Alt, V.; Morsczeck, C.; Docheva, D. Dental follicle cell differentiation towards periodontal ligament-like tissue in a self-assembly three-dimensional organoid model. Eur. Cells Mater. 2021, 42, 20–33. [Google Scholar] [CrossRef]
- Jin, Q.-M.; Zhao, M.; Webb, S.A.; Berry, J.E.; Somerman, M.J.; Giannobile, W.V. Cementum engineering with three-dimensional polymer scaffolds. J. Biomed. Mater. Res. A 2003, 67, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Reuther, T.; Kohl, A.; Komposch, G.; Tomakidi, P. Morphogenesis and proliferation in mono- and organotypic co-cultures of primary human periodontal ligament fibroblasts and alveolar bone cells. Cell Tissue Res. 2003, 312, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hamano, T.; Teramoto, A.; Iizuka, E.; Abe, K. Effects of polyelectrolyte complex (PEC) on human periodontal ligament fibroblast (HPLF) function. I. Three-dimensional structure of HPLF cultured on PEC. J. Biomed. Mater. Res. 1998, 41, 257–269. [Google Scholar] [CrossRef]
- Golda, A.; Gasiorek, A.; Dobosz, E.; Oruba, Z.; Lamont, R.J.; Potempa, J.; Koziel, J. Organotypic model of the gingiva for studying bacterial and viral pathogens implicated in periodontitis. J. Oral Microbiol. 2024, 16, 2292382. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Kim, M.S.; Lee, K.E.; Nam, O.H.; Jang, J.H.; Choi, S.C.; Lee, H.S. Comparison of 2- and 3-Dimensional Cultured Periodontal Ligament Stem Cells; a Pilot Study. Appl. Sci. 2021, 11, 1083. [Google Scholar] [CrossRef]
- Marsee, A.; Roos, F.J.M.; Verstegen, M.M.A.; Gehart, H.; de Koning, E.; Lemaigre, F.; Forbes, S.J.; Peng, W.C.; Huch, M.; Takebe, T. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 2021, 28, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Liu, H.; Yang, X.; Li, R.; Zhao, H.; Shang, Z. Organoids in the oral and maxillofacial region: Present and future. Int. J. Oral Sci. 2024, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, H.; Wang, C.; Cao, Z. The Applications and Potentials of Extracellular Vesicles from Different Cell Sources in Periodontal Regeneration. Int. J. Mol. Sci. 2023, 24, 5790. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Nuñez, J.; Vignoletti, F.; Caffesse, R.G.; Sanz, M. Cellular therapy in periodontal regeneration. Periodontology 2000, 79, 107–116. [Google Scholar] [CrossRef]
- Schroeder, H.E.; Listgarten, M.A. The gingival tissues: The architecture of periodontal protection. Periodontology 2000, 13, 91–120. [Google Scholar] [CrossRef]
| Author (Year) | Cell Lineage (Origin) | Extracellular Matrix Composition | Organoid Preparation | Characterization Methods | Results | Main Findings |
|---|---|---|---|---|---|---|
| Gauthier, et al. (2023) [22] | Periodontal ligament cells (human) | Fibrous polycaprolactone scaffold | Cells were seeded into the scaffolds after their synthetize through electrospinning. |
|
| Polycaprolactone was a suitable material to mimic the periodontal ligament collagen bundles. |
| Phuegyod, et al. (2023) [48] | Gingival fibroblasts + Periodontal ligament stem cells (human) | Poly(hydroxybutyrate-co-hydroxyvalerate) scaffold | The scaffolds were prepared as a cylindrical shape, and cells were seeded later. |
|
| Microbial-derived P(HB-50HV) scaffold can be used as a biomaterial for periodontal tissue engineering and stem cell applications. |
| Ono, et al. (2021) [24] | Periodontal ligament cells (human) | Tubular scaffold with titanium and hydroxyapatite core | Single-cell spheroids were picked and inserted into the 3D positions of the needle array to form a pre-designed 3D tubular model. |
|
| Exhibited high cell viability, included abundant collagen fibers, and expressed factors strongly associated with PDL tissue. |
| Lin, et al. (2021) [57] | Immortalized periodontal ligament cells (human) | 3D-printed collagen-based scaffold | Cells were seeded onto the collagen microfibrous scaffolds. |
|
| Preserved cell viability and promoted healing and regeneration under shear stress. |
| Kim, et al. (2020) [26] | Periodontal ligament cells (human) | 3D-printed poly-ε-caprolactone scaffold | Poly-ε-caprolactone solution was cast into the support material mold. Cells were seeded into all scaffolds. |
|
| Controllable microgroove intervals can specifically organize human PDL cells by 3D printing. Surface topography can angularly guide human PDL cells. |
| Shi, et al. (2018) [52] | Periodontal ligament stem cells (human) | Porous β-tricalcium phosphate and hydroxyapatite scaffold | Cell suspension was dropped onto the BCP by means of the pipetting technique. |
|
| The incorporation of PDLSC and the improved BCP significantly restored the lost periodontium. BCP is one of promising bioactive materials for periodontal tissue regeneration. |
| Yan, et al. (2018) [30] | Periodontal ligament cells (human) | Chitosan film scaffold | Chitosan scaffold was prepared, and the cells were seeded. |
|
| Compared to cells cultured in monolayer, periodontal ligament cells did not proliferate, but exhibited higher self-renewal gene expression, colony-forming unit, and osteogenic capacity. |
| Liao, et al.(2016) [32] | Periodontal ligament fibroblasts (human) | Porous poly(L-lactide) scaffold | Cells were seeded and centrifuged into a round-shaped PLLA matrix. |
|
| The in vitro hPdLLT model system effectively mimicked the reaction and gene expression of the human periodontal ligament in response to orthodontic force. |
| An, et al. (2015) [33] | Periodontal ligament cells (human) | β-tricalcium phosphate and hydroxyapatite scaffold | Cells were seeded on the scaffolds. |
|
| Three-dimensional porous BCP scaffolds can stimulate the osteoblastic differentiation of hPDLC in the presence and absence of osteogenic inducer. |
| Lee, et al. (2014) [53] | Dental pulp stem cells + Periodontal ligament stem cells + Alveolar bone stem cells (human) | 3D-printed poly(caprolactone)-hydroxyapatite scaffold | PCL/HA scaffolds were fabricated, and the cells were seeded. |
|
| Method for the regeneration of multiphase periodontal tissues by spatiotemporal delivery of multiple proteins. |
| Liao, et al. (2013) [37] | Periodontal ligament fibroblasts (human) | Porous poly(L-lactide) scaffold | PLLA matrix was prepared, and the cells were seeded. |
|
| The results indicated in vitro human periodontal ligament-like tissue, and it could be used in future periodontal ligament tissue engineering. |
| Jang, et al. (2011) [40] | Periodontal ligament fibroblasts (human) | Nanohydroxyapatite scaffold with avidin biotin binding system | Cells were loaded onto the scaffolds. |
|
| The high-affinity ABBS enhances the ability of periodontal ligament fibroblasts to attach to three-dimensionally constructed n-HA scaffolds. |
| Berahim, et al. (2011) [41] | Periodontal ligament fibroblasts (human) | Collagen and poly(glycolic acid)-based membrane | Membrane squares were placed, and periodontal spheroids were pipetted into each ring. |
|
|
Were able to grow three-dimensionally on the biologic membranes and may have the potential to be used together with guided tissue regeneration approaches. |
| Akman, et al. (2010) [42] | Periodontal ligament cells + Cementoblasts (human) | Chitosan and hydroxyapatite scaffold loaded with basic fibroblast growth factor | Porous chitosan scaffolds were prepared, and the cells were added drop-by-drop with a micropipette. |
|
| bFGF-loaded HA-chitosan scaffolds supported the cellular structure, proliferation, and mineralization. |
| Liao, et al. (2010) [43] | Periodontal ligament cells (human) | β-tricalcium phosphate and chitosan scaffold | Cell suspensions were seeded into each scaffold. |
|
| The scaffold could promote the differentiation of hPDLC towards osteoblast and cementoblast phenotypes. |
| Peng, et al. (2009) [44] | Periodontal ligament cells (human) | Chitosan and collagen scaffold | Chitosan/collagen composite scaffold was prepared, and the cell suspension was added. |
|
|
-PDLC achieved high proliferation and maintained a fibroblast figure, and the periodontal connective tissue-like structure formed in the scaffolds.
The novel gene-activated matrix (GAM) had potential in the application of periodontal tissue engineering. |
| Zhang, et al. (2007) [45] | Periodontal ligament cells (human) | Porous nanohydroxyapatite and chitosan scaffold | HA/chitosan composites were mixed, and cells were suspended and poured onto each scaffold. |
|
|
1% HA/chitosan scaffold had potential applications as biomaterials in periodontal tissue
engineering. |
| Wang, et al. (2005) [47] | Periodontal ligament cells (human) | Poly(glycolic acid) scaffold | PGA scaffolds were prepared and PDL cells were seeded. |
|
| May serve as a viable approach for promoting periodontal tissue regeneration and provides a possibility of PDL regeneration on dental implants. |
| Jin, et al. (2003) [59] | Cementoblasts + Periodontal ligament fibroblasts + Dental follicle cells (mice) | Poly(lactic-co-glycolic acid) scaffold | Scaffolds were prepared, and cells were added. |
|
| Delivery of selected cells via PLGA scaffolds may serve as a viable approach for promoting periodontal tissue regeneration. |
| Hillmann, et al. (1999) [56] | Gingival fibroblasts (human) | Polyester with polypropylene scaffold coated with fibronectin | Polyester with polypropylene scaffold was pretreated and coated with fibronectin. Cells were seeded onto the polyester mesh. |
|
| A dynamic model for performing studies on the interactions of cultured cells with extracellular matrix molecules, the pathogenesis of inflammatory processes, and the interactions with biomaterials. |
| Hamano, et al. (1998) [61] | Periodontal ligament fibroblast (human) | Polyelectrolyte complex | Both the polyanions and the synthetic polycations were dissolved and mixed. Cells were seeded later. |
|
| PECs affected the cell cycle and promoted proliferation and differentiation of hPLF. |
| Author (Year) | Cell Lineage (Origin) | Extracellular Matrix Composition | Organoid Preparation | Characterization Methods | Results | Main Findings |
|---|---|---|---|---|---|---|
| Schweinitzer, et al. (2024) [19] | Periodontal ligament fibroblasts (human) | Gelatin methacryloyl (GelMA ® ) or sodium alginate hydrogels | Cells were mixed into the hydrogels. |
|
| Discrepancies between these assays, attributable to their mechanisms of action and their different protocols. |
| Chang. (2023) [20] | Periodontal ligament fibroblasts (human) | Collagen hydrogels | Cells were mixed into the hydrogels. |
|
| Periodontal tissue regeneration was achieved. Hydrogels incorporating cells were superior compared to hydrogels. |
| Hermans, et al. (2023) [9] | Dental epithelium stem cells (mice) | Hydrogels (Matrigel®) | Cells seeded into the hydrogel droplets |
|
| A valuable tool to study mouse tooth DE/DESC, dental epithelial–mesenchymal interactions, and AB/JE differentiation while allowing further elucidation of tooth-type-specific features. |
| Liang, et al. (2022) [49] | Periodontal ligament stem cells (human) | Nanofibrous gelatin | Scaffold synthetized through electrospinning, and cells were seeded onto gelatin scaffolds. |
|
| Tubular matrix mimicked the physical architecture and chemical compositions of the ECM of PDL, controlled and aligned PDLSC migration inside the tubules, and inhibited the osteogenesis. |
| Yasunaga, et al. (2022) [50] | Periodontal ligament stem cells (human) | Collagen hydrogel | The prepared collagen gel mixtures were applied, and the spheroids transferred to the mixtures in the plates. |
|
| RhPAI-1 treatment of embedded hPLSC spheroids enhanced the cementogenic. |
| Tian, et al. (2021) [51] | Periodontal ligament stem cells (human) | Sodium alginate, gelatin, and nano-hydroxyapatite mixed into a hydrogel | The hydrogels were prepared, and cell was added. |
|
| Hydrogels showed effective stimulation for cell survival, proliferation, and osteoblast differentiation. |
| Fraser, et al. (2021) [5] | Periodontal ligament cells (human) | Poly(ethylene glycol) hydrogel | One-milliliter syringe was used as a hydrogel mold. Cells were pipetted into cylindrical syringe molds and formed under UV light for 3 min. |
|
| Hydrogels expressed key PDL matrix genes but did not the mineralization. |
| Zou, et al. (2021) [25] | Periodontal ligament cells (human) | Collagen–alginate hydrogel | Type I collagen solution, DMEM, sodium alginate solution, CaSO4, and NaOH solution were mixed. The hPDLC suspension was added. The mixed solution was added to the pre-designed mold. |
|
| The static compressive stress can induce autophagy in hPDLC regulated by ILK and PI3K and upregulate ILK expression in a PI3K-dependent manner. |
| Firth, et al. (2020) [27] | Periodontal ligament cells (human) | Thiol-modified hyaluronan–gelatin, poly(ethylene glycol) diacrylate cross-linked hydrogel | The cells were seeded on thiol-modified hyaluronan–gelatin, polyethylene glycol diacrylate (PEGDA) cross-linked hydrogel. |
|
| Three-dimensional mechanical strain PDL cell culture models offer a method to study the role of endoplasmic reticulum stress and unfolded protein response (UPR). |
| Saminathan, et al. (2013) [28] | Periodontal ligament cells (human) | Hyaluronan–gelatin hydrogel film | Glycosil and Gelin-S were mixed and PDL cells added. |
|
|
Cells organized into a bilayer and stimulated cell proliferation. Suitable for investigating the pathogenesis of periodontal diseases. |
| Hegedűs, et al. (2019) [29] | Periodontal ligament cells (human) | Poly(aspartamide)-based hydrogel | The poly(aspartamide) gel discs were prepared, and the cells were seeded. |
|
|
PDLC attaches and grows on hydrogels.
-The presence of thiol groups enhances viability and facilitates the osteogenic direction of the differentiating cells. |
| Zanna, et al. (2017) [55] | Gingival fibroblasts (human) | Pseudopeptides gelators containing the d-Oxd or the d-pGlu [Oxd = (4R,5S)-4-methyl-5-carboxyl-oxazolidin-2-one, pGlu = pyroglutamic acid] moiety and selected amino acids hydrogel | The mixture was stirred until gel formation, and the cells were seeded. |
|
| Hydrogels allow the growth of encapsulated cells with very good viability. These hydrogels may be used for 3D cell culture in regenerative medicine. |
| Mino, et al. (2017) [31] | Periodontal ligament cells (human) | Mebiol Gel (hydrogel) | hPDLC cells were mixed with Mebiol Gel. |
|
| PDL cells can adhere to sterile root surfaces and can serve as scaffolds for PDL regeneration |
| Heckler, et al. (2013) [36] | Periodontal ligament fibroblasts (human) | Type I bovine collagen hydrogel | hPDLF cells were mixed with sterile PureCol (type I bovine collagen ) and polymerized. |
|
| 3D model of orthodontic tooth movement offers promise for use as a model system in future studies. |
| Oortgiesen, et al. (2012) [39] | Periodontal ligament fibroblasts (rats) | Type I rat collagen hydrogel | Cells were mixed with collagen hydrogel. |
|
| 3D model to mimic an authentic PDL space, and it also provided a useful and well-controlled approach to study cell response to mechanical loading and other stimuli. |
| Author (Year) | Cell Lineage (Origin) | Extracellular Matrix Composition | Organoid Preparation | Characterization Methods | Results | Main Findings |
|---|---|---|---|---|---|---|
| Golda, et al. (2024) [62] | Telomerase-immortalized gingival keratinocytes (human) + Immortalized gingival fibroblasts-hTERT (human) | Hydrogel | Gingival fibroblasts were mixed with hydrogel. Then, keratinocytes were added to the top. |
|
| The OTG model resembled the morphology of the human gingiva; the pathogens penetrated deep into the tissue, forming a biofilm on the cell surface. |
| Mahdi Souzani, et al. (2023) [21] |
P
eriodontal ligament
fibroblasts
(human) | Bovine type I collagen | Cells were mixed into bovine type I collagen |
|
| This is the first to investigate the effect of hydrostatic pressure on a 3D model of hPDLF, showing that low pressure promotes even distribution of cells and greater expression of F-actin and periostin, while high or no pressure reduces these effects. |
| Makkar, et al. (2022) [23] | Gingival fibroblasts + Periodontal ligament fibroblasts (human) | Human fibrin-based matrix | PEG–fibrinogen solution was mixed with fibroblast cell suspension. |
|
| The gingival and periodontal CTEs exhibited differential responses to various bacterial challenges. |
| Alves, et al. (2015) [34] | Periodontal ligament fibroblast (human) | Collagen solution | Scaffolds were prepared by mixing the collagen solution and polymerizing. The cells were seeded. |
|
| 3D collagen scaffolds demonstrated strong potential for promoting mineralized matrix formation in periodontal regeneration. |
| Yamada, et al. (2014) [35] | Dental epithelial cells + Periodontal fibroblasts (porcine) | Hydrated collagen gel | Collagen was mixed with DMEM and FBS. Cells were added. |
|
| 3D cultures formed a stratified epithelial structure, suggesting that differentiation of three-dimensional culture tissues differs in vivo and in vitro. |
| Dabija-Wolter, et al. (2013) [54] | Gingival fibroblasts + Periodontal ligament fibroblasts (human) | Collagen matrix | Cell was seeded on top of a reconstituted collagen I biomatrix supplemented or not with fibroblasts. |
|
| These models as reliable tools for studying periodontal bacteria–host interactions. |
| Lee, et al. (2007) [46] | Periodontal ligament cells (human) | Calf skin collagen gel | Cells were cultured in a three-dimensional collagen gel hydrogel. |
|
| The changed expression of IL-6 and ALP in response to the static compressive force in PDL cells suggests promotion of bone resorption and reduction in mineralization, supporting the role of PDL cells in orthodontic tooth movement. |
| Reuther, et al. (2003) [60] | Periodontal ligament fibroblasts + Alveolar bone cells (human) | Type I collagen matrix | Osteoblasts were incorporated into a collagen type I solution and polymerized. Pdl cells were seeded onto the osteoblast-containing collagen. |
|
| A novel experimental tool to further elucidate the underlying mechanisms of the growth and differentiation of hPDLF and bone tissue. |
| Genes and Proteins | Number of Studies (n) | Differentiation | References |
|---|---|---|---|
| Activating Transcription Factor 4 (ATF4) | 1 | Cementogenic | [27] |
| Activating Transcription Factor 6 (ATF6) | 1 | Cementogenic | [27] |
| Activating Transcription Factor 6 beta (ATF6β) | 1 | Cementogenic | [27] |
| Alkaline Phosphatase | 16 | Osteogenic | [5,20,22,29,30,31,33,34,38,43,44,46,47,54,55,59] |
| Alpha-Smooth Muscle Actin (α-SMA) | 1 | Osteogenic | [20] |
| Ameloblastin (AMBN) | 1 | Chondrogenic/Epithelial | [9] |
| Amelotin (AMTN) | 1 | Chondrogenic/Epithelial | [9] |
| Amphiregulin (AREG) | 1 | Chondrogenic/Epithelial | [9] |
| Asporin (ASPN) | 1 | Cementogenic/Osteogenic | [5] |
| Autophagy-Related Gene 5 (ATG5) | 1 | Osteogenic/Adipogenic | [25] |
| Bcl-2-Associated X Protein (BAX) | 1 | Cementogenic | [27] |
| Beclin 1 (BECN1) | 1 | Osteogenic | [25] |
| Betacellulin (BTC) | 1 | Chondrogenic/Epithelial | [9] |
| Bone Gamma-Carboxyglutamate Protein (BGLAP) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Bone Morphogenetic Protein 2 (BMP2) | 2 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28,32] |
| Bone Sialoprotein (BSP) | 4 | Cementogenic/Osteogenic | [40,44,49,57] |
| Calnexin (CANX) | 1 | Cementogenic/Adipogenic | [27] |
| Calreticulin (CALR) | 1 | Cementogenic/Adipogenic | [27] |
| Caspase 3 Apoptotic Cysteine Protease (CASP3) | 1 | Cementogenic/Adipogenic | [27] |
| Caspase 7 Apoptotic Cysteine Protease (CASP7) | 1 | Cementogenic/Adipogenic | [27] |
| Catabolite Activator Protein (CAP) | 2 | Cementogenic/Osteogenic | [44,54] |
| Cementum Protein 1 (CEMP1) | 3 | Cementogenic/Osteogenic | [24,54,57] |
| Collagen Type I Alpha 1 Chain (COL1A1) | 13 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [5,24,28,32,34,38,40,42,46,49,53,57,59] |
| Collagen Type II Alpha 1 Chain (COL2A1) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Collagen Type III Alpha 1 Chain (COL3A1) | 5 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic | [24,27,28,53,59] |
| Collagen Type XII Alpha 1 Chain (COL12A1) | 2 | Cementogenic/Osteogenic | [24,49] |
| Cyclic AMP-Responsive Element-Binding Protein 3 (CREB3) | 1 | Cementogenic/Adipogenic | [27] |
| Cyclin D (CCND) | 1 | Epithelial | [60] |
| Cyclooxygenase-2 (COX2) | 1 | Cementogenic/Osteogenic | [40] |
| Cytokeratin (KRT) | 1 | Cementogenic/Osteogenic | [55] |
| Dentin Sialophosphoprotein (DSPP) | 1 | Cementogenic/Osteogenic | [57] |
| E-cadherin (CDH1) | 1 | Epithelial | [60] |
| Epidermal Growth Factor (EGF) | 1 | Chondrogenic/Epithelial | [9] |
| Epigen (EPGN) | 1 | Chondrogenic/Epithelial | [9] |
| Epiregulin (EREG) | 1 | Chondrogenic/Epithelial | [9] |
| Fibroblast Growth Factor 2 (FGF2) | 2 | Cementogenic/Osteogenic/Chondrogenic | [32,38] |
| GLI Family Zinc Finger 1 (GLI1) | 1 | Chondrogenic/Epithelial | [9] |
| Heparin-Binding EGF-Like Growth Factor (HBEGF) | 1 | Chondrogenic/Epithelial | [9] |
| Hepatocyte Growth Factor (HGF) | 1 | Cementogenic/Osteogenic | [24] |
| Integrin Subunit Alpha 6 (ITGA6) | 1 | Chondrogenic/Epithelial | [9] |
| Integrin-Linked Kinase (ILK) | 1 | Osteogenic | [25] |
| Leucine-Rich Repeats and Immunoglobulin-Like Domains 1 (LRIG1) | 1 | Chondrogenic/Ephitelial | [9] |
| Lysyl Oxidase (LOX) | 1 | Cementogenic/Adipogenic | [27] |
| Matrix Metalloproteinase 1 and 3 (MMP1 and MMPIII) | 2 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Meis Homeobox 1 (MEIS1) | 1 | Chondrogenic/Epithelial | [9] |
| Myogenic Differentiation 1 (MYOD1) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Nanog Homeobox (NANOG) | 1 | Chondrogenic/Epithelial | [30] |
| Neuregulin 1 (NRG1) | 1 | Chondrogenic/Epithelial | [9] |
| Neuregulin 2 (NRG2) | 1 | Chondrogenic/Epithelial | [9] |
| Neuregulin 3 (NRG3) | 1 | Chondrogenic/Epithelial | [9] |
| Neuregulin 4 (NRG4) | 1 | Chondrogenic/Epithelial | [9] |
| Octamer-binding Transcription 4 (Oct4) | 1 | Chondrogenic/Epithelial | [30] |
| Odontogenic Ameloblast-Associated Protein (ODAM) | 2 | Chondrogenic/Epithelial | [9,36] |
| Osteocalcin (OCN) | 5 | Cementogenic/Osteogenic | [24,33,34,49,53] |
| Osteopontin (OPN) | 2 | Cementogenic/Osteogenic | [33,34] |
| Osteoprotegerin (OPG) | 1 | Cementogenic/Osteogenic/Chondrogenic | [32] |
| Paired-Like Homeodomain 2 (PITX2) | 1 | Chondrogenic/Epithelial | [9] |
| Periodontal Ligament-Associated Protein-1 (PLAP1) | 3 | Cementogenic/Osteogenic/Chondrogenic | [24,32,38] |
| Periostin (POSTN) | 5 | Cementogenic/Osteogenic/Epithelial | [5,21,42,53,60] |
| Peroxisome Proliferator-Activated Receptor Gamma (PPARG) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Phosphoinositide 3-Kinase (PI3K) | 1 | Osteogenic | [25] |
| Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL) | 1 | Cementogenic/Osteogenic/Chondrogenic | [32] |
| Runt-Related Transcription Factor 2 (Runx2) | 6 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28,33,34,40,42,53] |
| SRY-Box Transcription Factor 2 (Sox2) | 2 | Chondrogenic/Epithelial | [9,30] |
| SRY-Box Transcription Factor 21 (SOX21) | 1 | Chondrogenic/Epithelial | [9] |
| SRY-Box Transcription Factor 9 (SOX9) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Stem Cell Marker (STRO-1) | 1 | Cementogenic/Osteogenic | [56] |
| T-Box Transcription Factor 1 (TBX1) | 1 | Chondrogenic/Epithelial | [9] |
| Tissue Inhibitor of Metalloproteinases 1 (TIMP1) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Transcription Factor Osterix (Sp7) | 2 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28,53] |
| Transforming Growth Factor Beta 1 (TGFB1) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Tumor Necrosis Factor Receptor Superfamily Member 11B (TNFRSF11B) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Tumor Necrosis Factor Superfamily Member 11 (TNFSF11) | 1 | Cementogenic/Osteogenic/Chondrogenic/Adipogenic/Epithelial | [28] |
| Twist Family BHLH Transcription Factor 1 (TWIST1) | 1 | Cementogenic/Osteogenic | [53] |
| Vascular Endothelial Growth Factor A (VEGFA) | 1 | Cementogenic/Osteogenic | [24] |
| Vimentin (VIM) | 1 | Cementogenic/Osteogenic | [56] |
| Yes-Associated Protein 1 (YaP1) | 1 | Cementogenic/Osteogenic | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, L.d.O.; Sordi, M.B.; Birjandi, A.A.; Sharpe, P.T.; Cruz, A.C.C. Breaking Barriers: Evaluating Challenges in Advancing Periodontal Ligament Cell-Derived Organoids. Dent. J. 2025, 13, 422. https://doi.org/10.3390/dj13090422
Matos LdO, Sordi MB, Birjandi AA, Sharpe PT, Cruz ACC. Breaking Barriers: Evaluating Challenges in Advancing Periodontal Ligament Cell-Derived Organoids. Dentistry Journal. 2025; 13(9):422. https://doi.org/10.3390/dj13090422
Chicago/Turabian StyleMatos, Luiza de Oliveira, Mariane Beatriz Sordi, Anahid Ahmadi Birjandi, Paul Thomas Sharpe, and Ariadne Cristiane Cabral Cruz. 2025. "Breaking Barriers: Evaluating Challenges in Advancing Periodontal Ligament Cell-Derived Organoids" Dentistry Journal 13, no. 9: 422. https://doi.org/10.3390/dj13090422
APA StyleMatos, L. d. O., Sordi, M. B., Birjandi, A. A., Sharpe, P. T., & Cruz, A. C. C. (2025). Breaking Barriers: Evaluating Challenges in Advancing Periodontal Ligament Cell-Derived Organoids. Dentistry Journal, 13(9), 422. https://doi.org/10.3390/dj13090422







