Abstract
Objectives: This study aimed to evaluate whether the addition of pressurized carbon dioxide (PCD) influences the antimicrobial efficacy of 2.5% sodium hypochlorite (NaOCl) against Enterococcus faecalis biofilm in root canals and dentinal tubules. Methods: Forty extracted human mandibular premolars with single canals were contaminated with E. faecalis for 10 days and randomly assigned to four groups (n = 10): 2.5% NaOCl, 2.5% NaOCl + CO2, sterile saline, and sterile saline + CO2. The pH and temperature of the NaOCl solution were measured before and after CO2 incorporation. Microbial load was assessed by CFU counts before and after irrigation, and in dentin samples from the cervical, middle, and apical thirds. Oxidative stress was evaluated via lipid peroxidation (TBARS), protein carbonyl content, and total protein quantification. Biofilm metabolic activity was analyzed using the XTT reduction assay. Data were analyzed using one-way ANOVA on ranks and two-way repeated measures ANOVA (α = 0.05), a very large effect size (Cohen’s d) ≈ 1.756 was assumed. Results: All irrigation protocols significantly reduced bacterial load (p < 0.05). Both NaOCl groups outperformed the saline controls (p = 0.009). The addition of CO2 to NaOCl slightly enhanced disinfection in the main canal but did not improve antimicrobial action in dentinal tubules. CO2 incorporation reduced the pH of NaOCl from ~13.4 to 7.4 and slightly increased the temperature, making the solution more chemically reactive. However, both oxidative stress markers and the XTT assay showed that the combination with CO2 impaired the antimicrobial effectiveness of NaOCl. Conclusions: Despite the improvement in bacterial reduction in the root canal lumen, the combination of PCD with NaOCl failed to enhance intratubular disinfection and reduced the oxidative damage and metabolic inactivation of the biofilm. CO2 pressurization appears to limit the antimicrobial action of NaOCl.
1. Introduction
Effective root canal decontamination remains one of the main challenges in endodontics, particularly when dealing with resistant microorganisms such as Enterococcus faecalis [1]. The persistence of microbial communities and their virulence factors within the root canal system is a leading cause of endodontic treatment failure [2]. Although these secondary endodontic infections are polymicrobial in nature, they are predominantly composed of Gram-positive facultative anaerobes, notably E. faecalis [3,4,5]. This species is frequently implicated in endodontic failure due to its ability to survive in hostile environments—such as its tolerance to highly alkaline conditions—and to form resilient biofilms in anatomically complex areas, including dentinal tubules [6,7,8,9,10]. In addition to its biofilm-forming capacity and coaggregation with other species, E. faecalis expresses multiple virulence factors that contribute to its resistance to conventional endodontic disinfection protocols [11,12]. Therefore, the eradication of mature biofilms remains a significant challenge in endodontic therapy.
Sodium hypochlorite (NaOCl) is the most widely used endodontic irrigant due to its broad-spectrum antimicrobial activity and ability to dissolve organic tissues [13,14]. Various concentrations of NaOCl, ranging from 0.5% to 6%, have been tested for root canal disinfection [15,16]. Although the antimicrobial effect of NaOCl increases with concentration, higher concentrations also lead to increased cytotoxicity in periapical tissues [17,18,19]. Therefore, achieving effective cleaning, lubrication, and disinfection within the root canal system is critical to the success of endodontic therapy [20]. Additionally, it has been reported that lowering the pH of NaOCl to a range between 6.0 and 7.5 may enhance its antimicrobial efficacy. This is because when dissolved in water, chlorine undergoes hydrolysis and predominantly forms hypochlorous acid (HOCl)—the most active disinfecting species—which is favored at lower pH levels. As a result, NaOCl becomes chemically more reactive and effective under mildly acidic conditions [21].
To improve the efficacy of NaOCl, several enhancement strategies have been investigated, including temperature elevation [22], ultrasonic activation [23,24], and negative pressure irrigation systems [25]. Moreover, clinical scenarios such as endodontic treatment in teeth with immature apices [26], or procedures based on revascularization protocols—which rely on the regenerative potential of the periapical tissues—still require further clarification regarding irrigation protocols and potential adjuvants. These cases are particularly sensitive due to the increased risk of irrigant extrusion beyond the apex [27].
Among potential adjuncts, pressurized carbon dioxide (PCD) has emerged as a promising candidate due to its recognized antimicrobial properties. PCD has shown substantial efficacy in inactivating a variety of pathogenic organisms in both aqueous and non-aqueous environments [28,29,30]. Its favorable physicochemical properties—such as low cost, non-toxicity, low viscosity, and zero surface tension—facilitate rapid penetration into porous and complex structures [28]. Studies have indicated that combining PCD with NaOCl enhances bacterial inactivation in certain contexts, such as seawater disinfection [28], possibly due to pH reduction that increases the proportion of HOCl [31].
In addition to microbial inactivation, it is essential to evaluate whether CO2 enhances the oxidative stress exerted by NaOCl on bacterial biofilms. The innate immune system commonly employs oxidative stress as a defense mechanism, involving the production of reactive oxygen species (ROS) such as superoxide (O2−) and hydroxyl radicals (OH−), as well as non-radical oxidants like hydrogen peroxide (H2O2) and reactive nitrogen/chlorine species including nitric oxide (NO) and HOCl [32,33]. These oxidants contribute not only to microbial destruction but also to tissue damage and inflammation. Thus, understanding how oxidative mechanisms are modulated during irrigation is essential to balance microbial elimination with biocompatibility [32,33].
Although various strategies have been proposed to enhance the antimicrobial action of NaOCl [22,23,24,25], no studies to date have evaluated the effect of combining NaOCl with PCD for root canal disinfection. In particular, the impact of this combination on oxidative damage to microbial structures—such as lipid peroxidation and protein oxidation—remains entirely unexplored in the endodontic literature. Given the growing interest in improving irrigant performance through physical–chemical modifications, this gap represents a relevant area for investigation. Therefore, the present in vitro study aimed to evaluate the efficacy of combining PCD with NaOCl against E. faecalis biofilm in root canals and dentinal tubules, using microbiological culture methods and biochemical assays to assess lipid peroxidation and protein oxidation. The null hypothesis was that the addition of PCD to NaOCl would not result in a significant difference in CFU reduction or oxidative damage when compared to NaOCl alone.
2. Materials and Methods
2.1. Sample Selection and Preparation
This study was approved by the Research Ethics Committee of the School of Dentistry, Araçatuba—UNESP (CAAE: 34692620.8.0000.5420, approved on 23 September 2020). Forty human mandibular premolars recently extracted for periodontal or orthodontic reasons were selected. Teeth with anatomical alterations, previous treatments, or structural damage—such as fractures, open apices, root curvature > 10°, or restorations—were excluded. All the extracted teeth were stored in 0.1% thymol solution and refrigerated at 2–4 °C from the time of extraction until use (approximately 6 months).
Residual periodontal tissues were removed with periodontal curettes. The crowns were sectioned transversely with a water-cooled diamond disk (American Burrs, Palhoça, Santa Catarina, Brazil), and the root segments were standardized to 16 mm in length. Working length was determined by inserting a size 10 K-file (Dentsply Sirona, Ballaigues, Switzerland) into the canal until its tip was visible at the apical foramen under magnification [34]. Root canals were instrumented using rotary files up to size 40.04 (MK Life, Porto Alegre, RS, Brazil) according to the manufacturer’s speed and torque specifications. During instrumentation, 1 mL of 2.5% NaOCl (Rioquímica, São José do Rio Preto, SP, Brazil) was used for irrigation after each file.
The irrigation protocol for cleaning dentinal tubule entrances was adapted from Ferraz et al. (2001) [35]. All the samples were subjected to an ultrasonic bath for 10 min in 17% EDTA (Biodinâmica, Ibiporã, PR, Brazil), followed by 10 min in 5.25% NaOCl (Apothimed, Araçatuba, SP, Brazil) to remove the smear layer. Irrigants were neutralized with 5 mL of 5% sodium thiosulfate (Merck KGaA, Darmstadt, Germany). Each tooth was immersed in approximately 700 mL of Brain Heart Infusion (BHI) broth (Kasvi, Pinhais, Paraná, Brazil) and ultrasonically agitated for 1 min to promote penetration of the culture medium into canal irregularities. The samples were then autoclaved for 30 min at 121 °C and incubated at 37 °C for 48 h to confirm sterilization efficacy.
2.2. Cultivation of E. faecalis and Specimen Contamination
The contamination protocol was adapted from Carvalho et al. (2019) [36]. A pure culture of E. faecalis (ATCC 29212, ATCC, Manassas, VA, USA) was grown in BHI broth at 37 °C for 24 h. The culture was transferred to fresh BHI and incubated overnight at 37 °C to reach exponential growth. The cellular suspension was adjusted in a spectrophotometer (BioTek Instruments, Winooski, VT, USA) at 600 OD to achieve a turbidity of 1.5 × 10−8 colony-forming units (CFU/mL), equivalent to 0.5 McFarland standard [37], to be used for biofilm formation.
Aliquots of 800 μL were introduced into 2 mL Eppendorf tubes containing the specimens, followed by sequential centrifugation at 1400, 2000, 3600, and 5600× g in double 5 min cycles. The inoculum was renewed after each cycle. After eight cycles, the samples were transferred to new 2 mL tubes with sterile BHI broth, vortexed (Phoenix Luferco, Araraquara, Brazil), and incubated at 37 °C for 24 h. For 10 days, the specimens were maintained in sterile BHI broth, with centrifugation (3600× g for 5 min at 25 °C) on alternate days to enhance bacterial penetration into the root canal system and for a mature biofilm. The culture medium was renewed every 48 h on the centrifugation days. All the steps were performed aseptically in a laminar flow cabinet (Veco Bioseg 12 Ltd.a, Campinas, Brazil). On the tenth day, the samples were removed, excess medium discarded, and external root surfaces cleaned with sterile gauze.
2.3. Experimental Groups
Sample size calculation was based on previous studies [37,38], indicating 10 teeth per group. A very large effect size (Cohen’s d) ≈ 1.756 was assumed, with a significance level (α) of 0.05 and a statistical power (1–β) of 0.80 [39]. After completing the sample size determination (n), the teeth were placed in 2 mL Eppendorf tubes, randomly numbered, and blindly allocated by a single operator into four experimental groups according to the irrigation protocol employed:
- The 2.5% NaOCl group: Root canals were irrigated and manually agitated with a #15 K-file using 15 mL of 2.5% NaOCl, 5 mL of 17% EDTA, and a final 5 mL of 2.5% NaOCl for 20 s each. Subsequently, 1 mL of 5% sodium thiosulfate was used for 1 min to inactivate NaOCl. A total of 20 mL of 2.5% NaOCl was used for irrigation [36,40].
- The 2.5% NaOCl + CO2 group: Root canals were irrigated and manually agitated with a #15 K-file using 15 mL of 2.5% NaOCl + CO2 (NaOCl was carbonated using a jet aeration device -Sodastream Industries LTD, Kfar Saba, Israel), 5 mL of 17% EDTA, and a final 5 mL of 2.5% NaOCl + CO2 for 20 s each. Subsequently, 1 mL of 5% sodium thiosulfate was used for 1 min to inactivate NaOCl. A total of 20 mL of 2.5% NaOCl + CO2 was used for irrigation [36,40].
- Sterile saline group: Root canals were irrigated and manually agitated with a #15 K-file using 15 mL of sterile saline solution, 5 mL of 17% EDTA, and a final 5 mL of sterile saline solution for 20 s each. Subsequently, 1 mL of 5% sodium thiosulfate was used for 1 min. A total of 20 mL of sterile saline solution was used for irrigation [36,40].
- Sterile saline + CO2 group: Root canals were irrigated and manually agitated with a #15 K-file using 15 mL of sterile saline solution + CO2 using the jet aeration system, 5 mL of 17% EDTA, and a final 5 mL of sterile saline solution + CO2 solution for 20 s each. Subsequently, 1 mL of 5% sodium thiosulfate was used for 1 min. A total of 20 mL of sterile saline solution + CO2 was used for irrigation [36,40].
After the contamination period, the specimens were mounted on a sterile aluminum platform. The apical foramen of each sample was sealed with a fast-setting epoxy resin to prevent apical leakage and to simulate a closed canal system. All the irrigation procedures were carried out using 30-G NaviTip needles (Ultradent Products Inc., Indaiatuba, Brazil), positioned 1 mm short of the working length.
CO2 was incorporated into the 2.5% NaOCl + CO2 and Sterile Saline + CO2 groups using a jet aeration system (Sodastream Industries LTD, Kfar Saba, Israel). A CO2 gas cylinder was connected to the device, and the gas was infused into 800 mL of 2.5% NaOCl by pressing the release button 10 times to ensure consistent CO2 saturation. The same procedure was applied to 800 mL of sterile saline to maintain consistency across experimental groups.
2.4. Microbiological Collection
The sampling method used in this study was based on the protocol described by Yamamoto et al. (2021) [37]. Briefly, microbiological samples were collected from all the root canals before (S1) and after (S2) irrigation using three sterile paper points (Dentsply Maillefer). Each point remained in the canal for one minute before being transferred to 2 mL Eppendorf tubes containing 1 mL of Ringer’s solution (Sigma-Aldrich, St. Louis, MI, USA). The tubes were vortexed for 30 s. The samples were homogenized and serially diluted to 10−4 (S1) and 10−1 (S2). Each dilution was plated on BHI agar and incubated at 37 °C for 48 h. Colony-forming units (CFU/mL) were then counted.
2.5. Dentine Samples
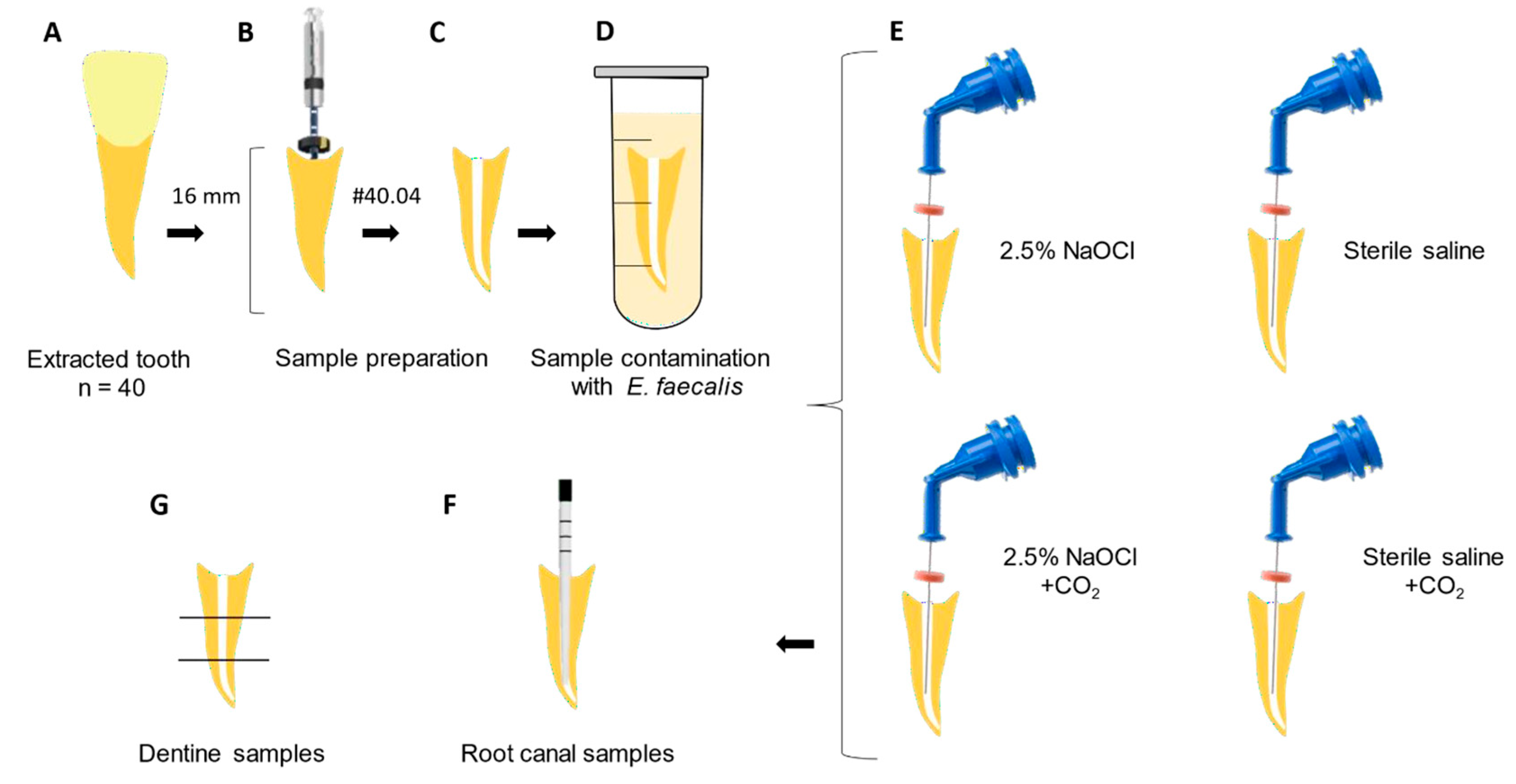
Each root was sectioned into three segments: cervical, middle, and apical thirds (Figure 1). Dentin debris was collected from the canal walls using sterile, incrementally sized diamond-tipped conical burs [4137 (ISO 025), KG Sorensen, Serra, ES, Brazil] mounted on a low-speed electric handpiece (Dentsply Sirona). The collected dentin shavings were individually stored in 2 mL Eppendorf tubes containing Ringer’s solution [41], plated on BHI agar, and incubated at 37 °C for 48 h for CFU quantification.
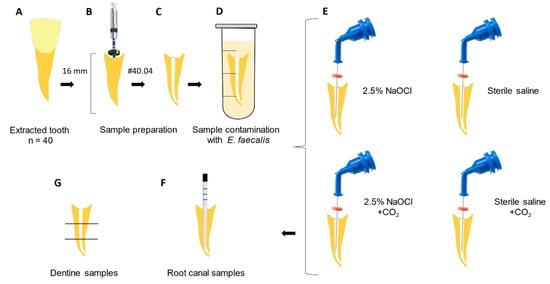
Figure 1.
Study design: (A) Extracted tooth used in experimental groups. (B) Sample preparation: standardization of the root canal length (16 mm) and endodontic treatment with Rotatory Files #40/04. (C) Root canal filled with BHI solution. (D) Sample contamination with E. faecalis for 10 days. (E) Experimental groups. (F) Root canal sample collection after irrigation protocols. (G) Dentine sample collection.
2.6. pH Measurement
The pH of the 2.5% NaOCl solution was measured in its pure form and after the addition of CO2 at different pressure levels (1 to 10 gas injections) using a digital pH meter (model PHS3BW, Bel Engineering, Monza, Italy) calibrated at 25 °C. The measurements were taken at 30 and 60 s after each CO2 injection.
2.7. Assessment of Oxidative Damage Products
The oxidative damage assay was based on Rodrigues et al. (2025) [42]. The E. faecalis strain was reactivated on BHI agar and transferred to BHI broth, then incubated at 37 °C for 7 h. After incubation, the bacterial suspension was adjusted using a spectrophotometer to reach a turbidity equivalent to the McFarland 0.5 standard (1.5 × 108 CFU/mL). A total of 45 mL of the inoculum was transferred to 50 mL Falcon tubes and centrifuged at 10,000× g rpm at 4 °C for 10 min to form a pellet, and then the supernatant was discarded. The centrifugation process was repeated 12 times to increase the concentration of the bacterial pellet. After the final centrifugation, sterile saline was added to resuspend the pellet, and the suspension was aliquoted into 60 microcentrifuge tubes (1.5 mL).
The tubes were centrifuged again to concentrate the pellet. The supernatant was discarded, and each group (n = 10) received the treatment protocol corresponding to its experimental group. The pellets were washed with 2 mL of 0.9% NaCl to remove residual treatment agents. Homogenates were prepared in 50 mmol/L sodium phosphate buffer (pH 7.4) containing 0.2% (v/v) Triton X-100 and 2 mmol/L phenylmethylsulfonyl fluoride (PMSF). The samples were sonicated for 10 s at 100% amplitude and centrifuged at 10,000× g rpm for 10 min at 4 °C. The supernatants were collected for analysis of thiobarbituric acid-reactive substances (TBARS) and protein quantification.
2.8. Metabolic Activity Assessment
The metabolic activity of the biofilms was evaluated using the XTT reduction assay (2,3-bis [2-methoxy-4-nitro-5-sulfophenyl]-5-[(phenylamino)carbonyl]-2H-tetrazolium; Sigma-Aldrich, St. Louis, MO, USA), following a protocol adapted from Jacob et al. (2020) [43]. The E. faecalis strain (ATCC 29212) was initially cultured on BHI agar (Kasvi, Paraná, Brazil) for 24 h at 37 °C. Subsequently, it was transferred to liquid BHI broth and incubated at 37 °C until reaching the exponential growth phase, with the optical density adjusted to OD600 ≈ 0.08 (~1.5 × 108 CFU/mL). The standardized inoculum (200 µL) was distributed into 96-well plates and statically incubated at 37 °C for 72 h to allow biofilm formation. After this period, the biofilms were subjected to the treatments described for each experimental group. CO2 activation was performed as previously described, using a solution pre-saturated with the gas under standardized conditions. Preliminary tests defined the final protocol: direct application of 25 µL of the solution for 30 s. After treatment, wells were washed twice with PBS buffer (pH 7.0).
The XTT assay was conducted using a fresh solution composed of XTT (150 mg/L) and phenazine methosulfate (PMS, 10 mg/L) mixed in a 1:1 (v/v) ratio. Each well received 200 µL of the solution, followed by incubation for 3 h at 37 °C in the dark under mild orbital shaking (120 rpm). After incubation, the 200 µL was transferred to a new plate, and absorbance was measured at 490 nm. The absorbance values were corrected by the negative controls (without biofilm) and used as a relative measure of metabolic activity, considering uniform adhesion to the well bottoms. The experiments were performed in duplicate (n = 10 per group) and repeated in two independent assays to ensure reproducibility.
2.9. Statistical Analysis
The CFU data were statistically analyzed using Sigma Plot 12.0 for Windows (Systat Software Inc., San Jose, CA, USA). Two-way repeated measures ANOVA followed by the Student–Newman–Keuls post hoc test was employed for intergroup comparisons and analysis across different sampling time points. A one-way analysis of variance on ranks was conducted to evaluate the percentage reduction in CFU counts. Intragroup comparisons among the cervical, middle, and apical thirds were also conducted using two-way repeated measures ANOVA followed by the Student–Newman–Keuls post hoc test. One-way ANOVA followed by Tukey’s post hoc test was applied for the analysis of the other assays. A significance level of 5% (p < 0.05) was adopted for all the statistical tests.
3. Results
Table 1 presents the intergroup comparison of E. faecalis counts before and after the irrigation protocols, along with the percentage of bacterial reduction. Cultivable bacteria were detected in all the baseline samples (S1, 40/40). All the irrigation protocols significantly reduced the bacterial load (p < 0.05). Irrigation with 2.5% NaOCl demonstrated superior antibacterial efficacy compared to sterile saline (p = 0.001), regardless of CO2 supplementation. Notably, the addition of CO2 enhanced the decontamination potential of 2.5% NaOCl. However, incorporating CO2 into the sterile saline solution did not lead to a significant improvement in bacterial reduction (p > 0.05).

Table 1.
Mean (±Standard Deviation) of CFU/mL counts (log10) before and after irrigation protocols (IP).
Table 2 presents the results of the inter- and intra-group analyses. The 2.5% NaOCl + CO2 group demonstrated statistically significant differences among all root thirds, with the highest reduction in CFU/mL observed in the cervical third. In contrast, no significant differences were detected among the cervical, middle, and apical thirds in either of the sterile saline groups.

Table 2.
Mean (±Standard Deviation) of CFU/mL counts (log10) after irrigation protocols (IP) in the different thirds.
3.1. Results of pH Measurement
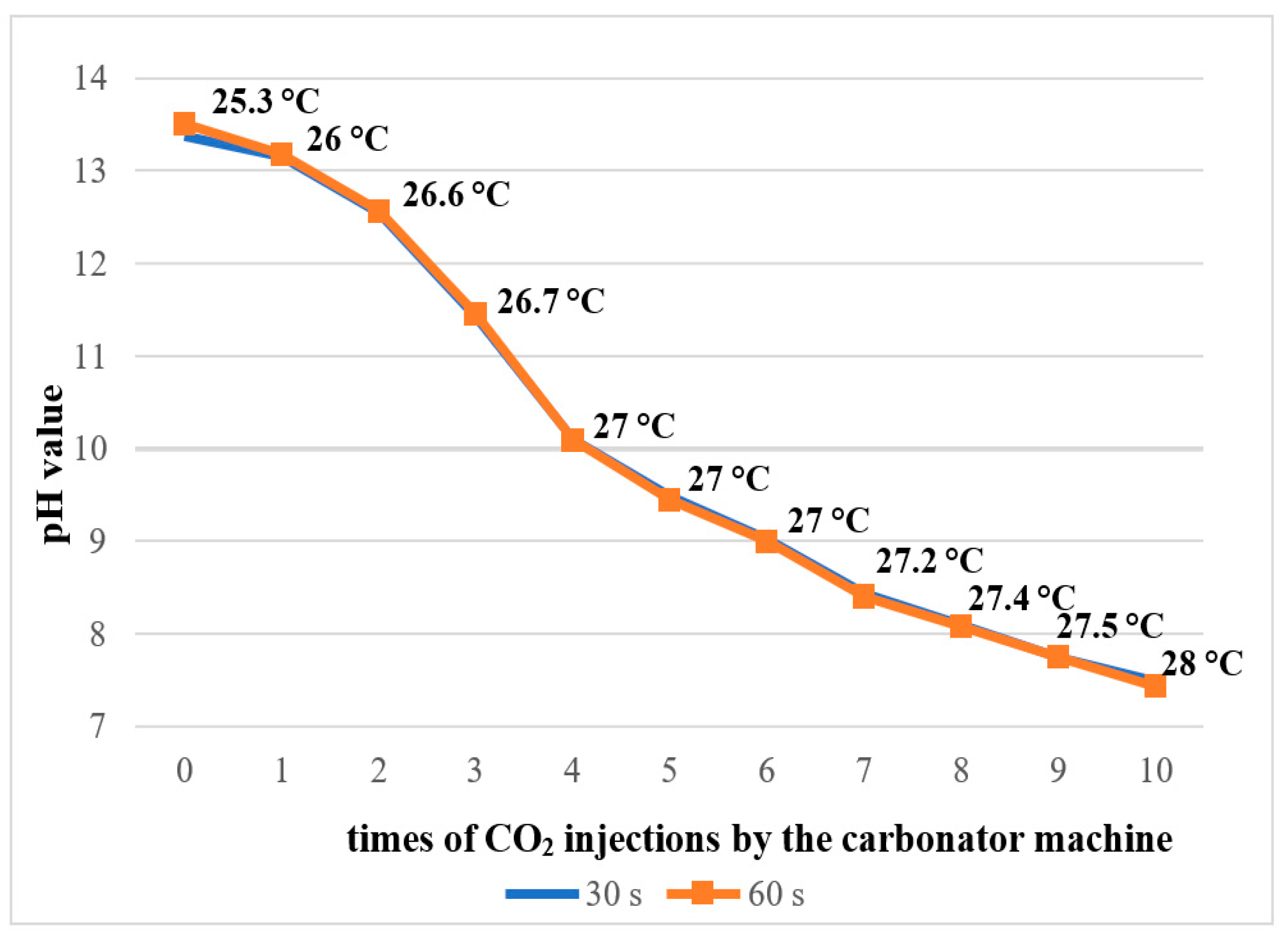
The pure 2.5% NaOCl solution exhibited an alkaline pH ranging from 13.39 to 13.51 at 25 °C. As the number of CO2 gas injections increased, the pH progressively decreased, reaching a final value of 7.43 after 10 injections (Figure 2). A corresponding increase in solution temperature was also observed, from 25.3 °C in the untreated solution to 28 °C following the 10 CO2 injections.
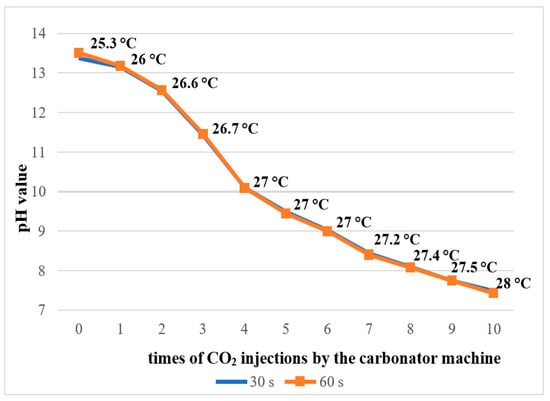
Figure 2.
pH measurement and temperature of 2.5% NaOCl solution during the addition of CO2 by the carbonator machine.
3.2. Oxidative Damage
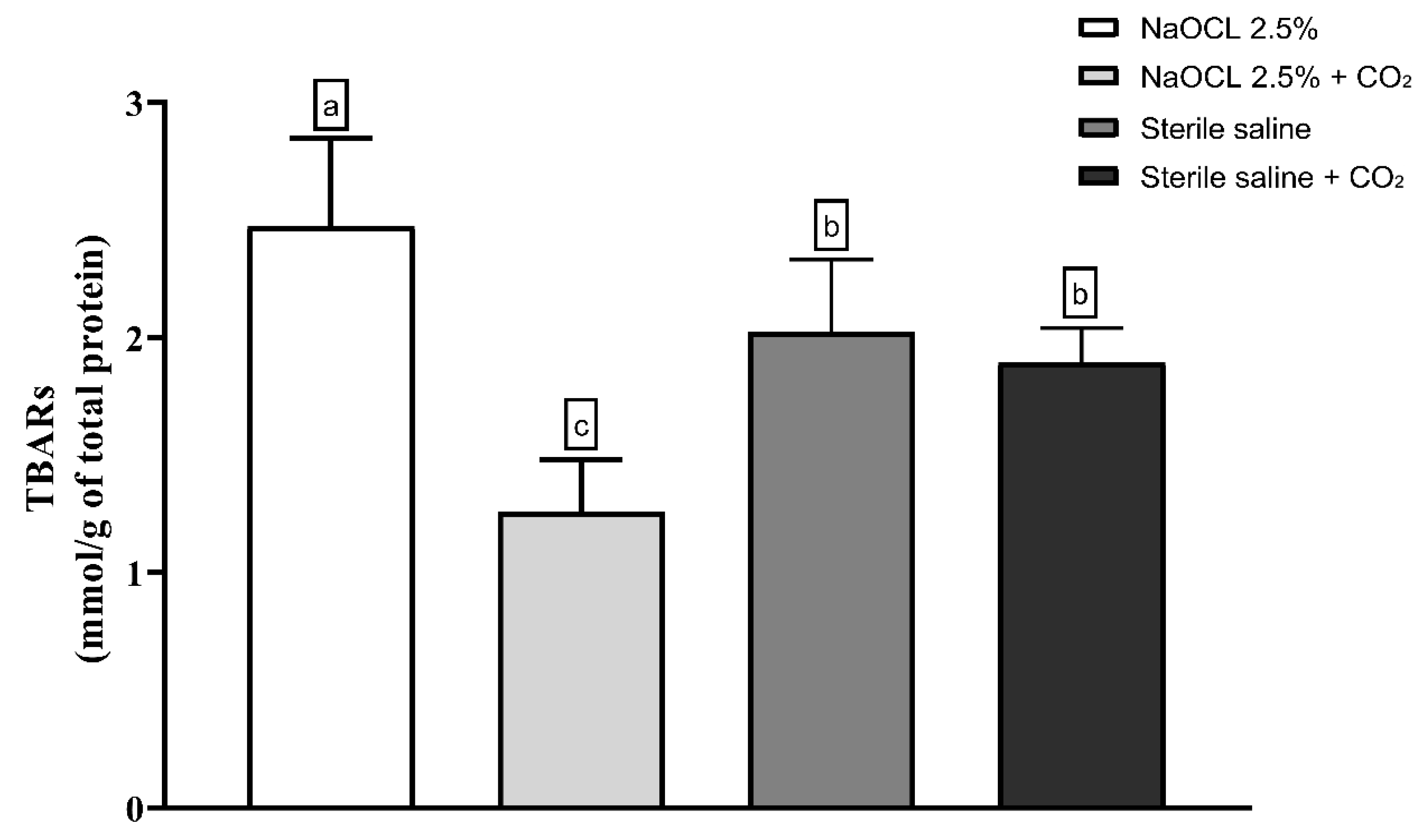
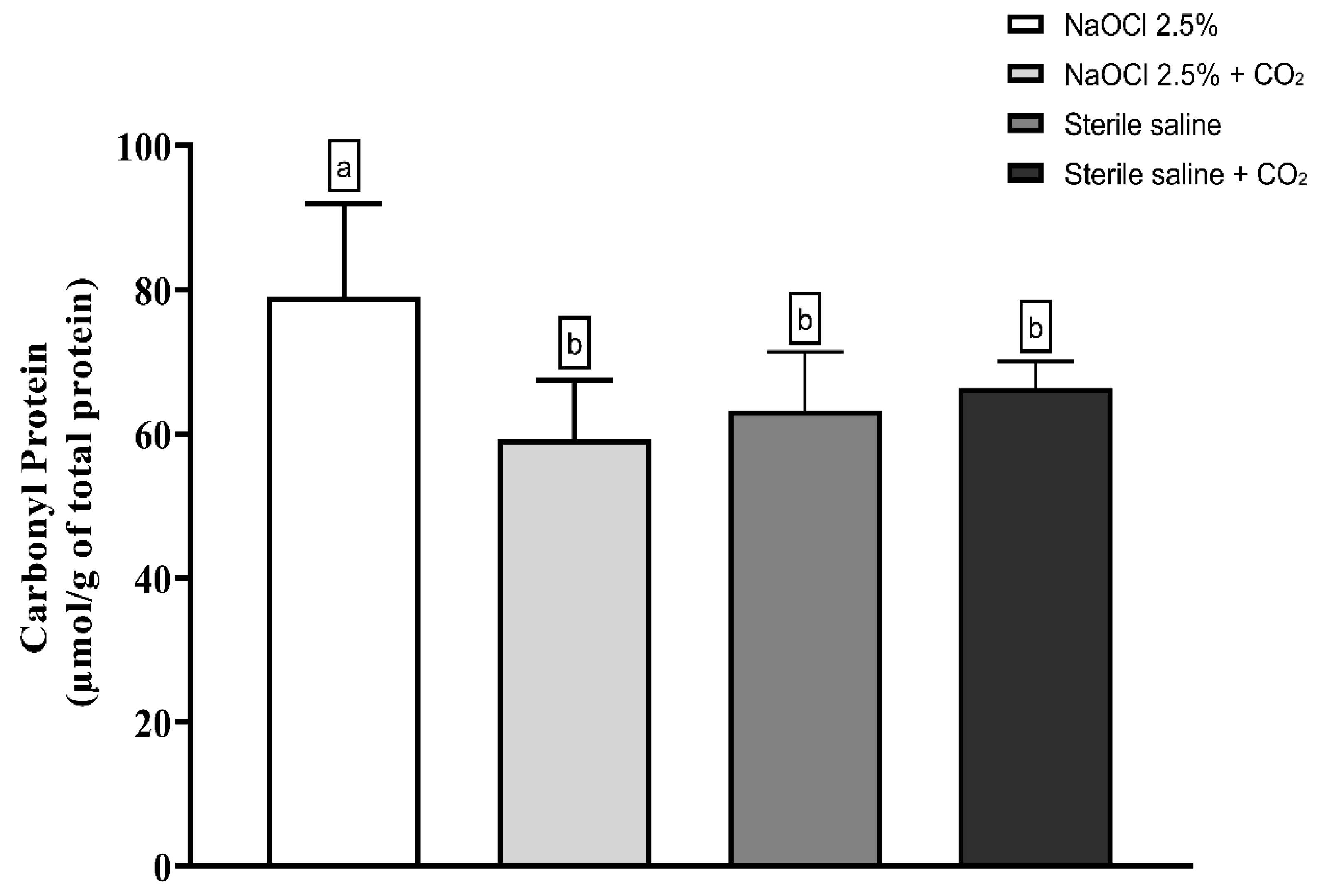
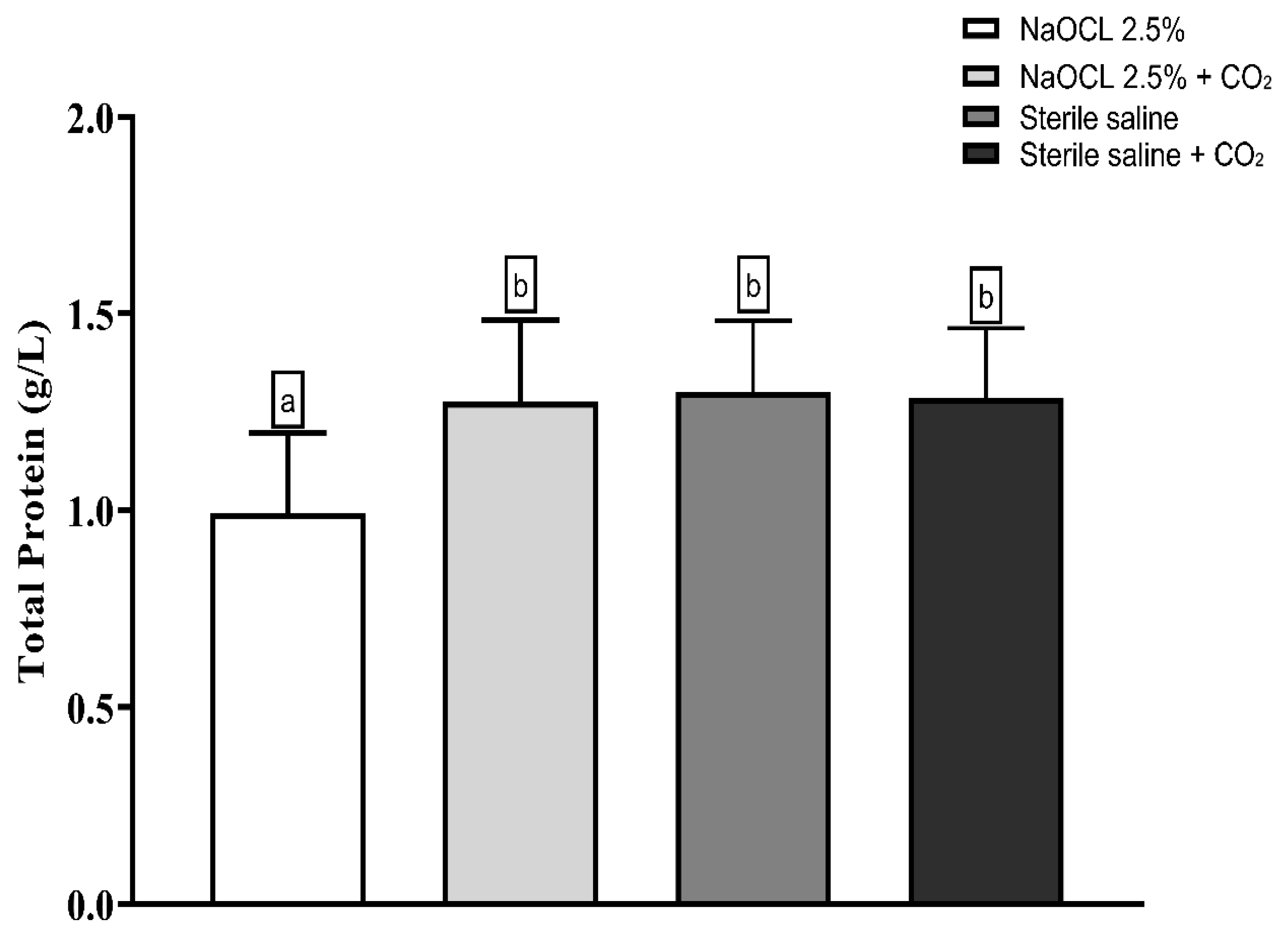
Lipid peroxidation was evaluated using the thiobarbituric acid-reactive substances (TBARS) assay. The results showed that the addition of pressurized CO2 reduced the ability of 2.5% NaOCl to induce lipid damage (Figure 3). Similarly, protein oxidation—measured by the concentration of carbonylated proteins—was also attenuated when NaOCl was combined with CO2 (Figure 4). Furthermore, analysis of total protein levels revealed that only NaOCl without CO2 was effective in significantly reducing protein content (Figure 5), suggesting a greater impact on bacterial cell viability.
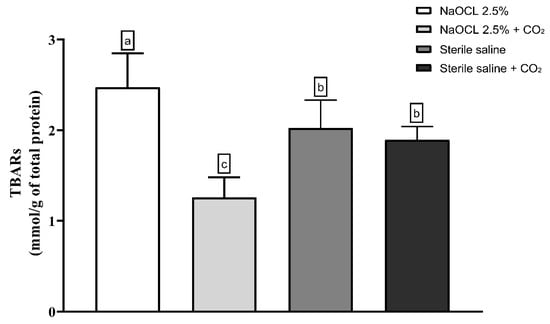
Figure 3.
Mean and Standard Deviation of TBARS concentrations in E. faecalis cells after treatment with different irrigating solutions: NaOCl 2.5%, NaOCl 2.5% + CO2, saline solution, and saline solution + CO2. Different lowercase letters indicate statistically significant differences among the groups (p < 0.05).
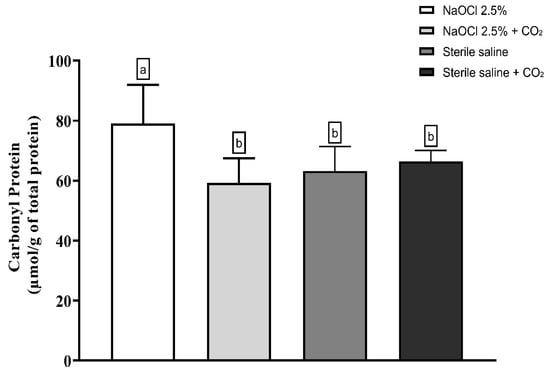
Figure 4.
Mean and Standard Deviation of carbonyl protein concentrations in E. faecalis cells after treatment with different irrigating solutions: NaOCl 2.5%, NaOCl 2.5% + CO2, saline solution, and saline solution + CO2. Different lowercase letters indicate statistically significant differences among the groups (p < 0.05).
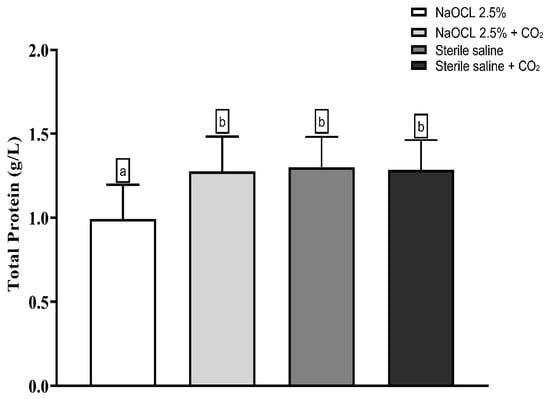
Figure 5.
Mean and Standard Deviation of total protein concentrations in E. faecalis cells after treatment with different irrigating solutions: NaOCl 2.5%, NaOCl 2.5% + CO2, saline solution, and saline solution + CO2. Different lowercase letters indicate statistically significant differences among the groups (p < 0.05).
3.3. Evaluation of Metabolic Activity
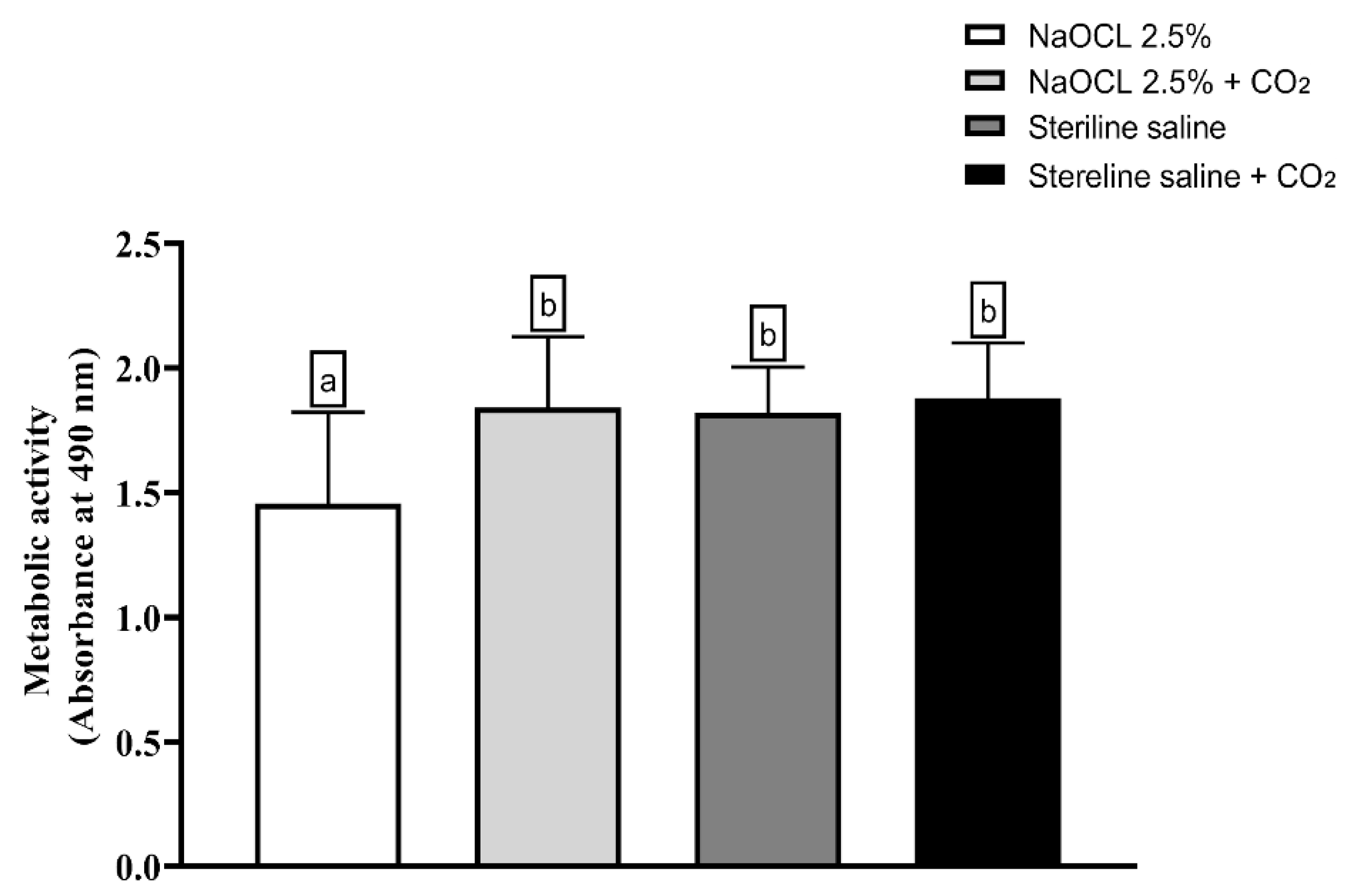
The analysis of the metabolic activity of E. faecalis biofilms is shown in Figure 6 demonstrated that only the group treated with NaOCl showed a statistically significant reduction in cell viability compared to the control (p < 0.05). The other groups—saline solution, saline solution with CO2, NaOCl with CO2, and control—did not show significant differences among themselves, indicating that the addition of CO2 compromised the antimicrobial activity of NaOCl.
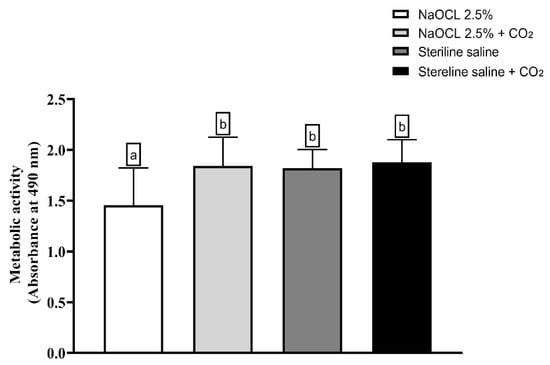
Figure 6.
Analysis of the metabolic activity of Enterococcus faecalis ATCC 29212 monospecies biofilms exposed to the different experimental groups described in the graph. Different lowercase letters indicate statistically significant differences between groups (one-way ANOVA, Tukey’s post hoc test; p < 0.05).
4. Discussion
This study investigated the potential of combining PCD with NaOCl for disinfecting root canals and dentinal tubules contaminated with E. faecalis. Although NaOCl remains the gold standard in endodontic irrigation due to its broad-spectrum antimicrobial activity and tissue-dissolving properties, strategies to enhance its penetration and efficacy—particularly within dentinal tubules—continue to pose a significant challenge.
Our results showed that 2.5% NaOCl significantly reduced the bacterial load in the main canal space compared to saline controls, which aligns with previous studies emphasizing its superior efficacy [44,45]. The addition of CO2 led to a modest improvement in disinfection within the main canal, potentially due to reduced surface tension and pH modification that may enhance hypochlorous acid availability. However, this synergistic effect was not observed in the dentinal tubules, where the anatomical complexity and limited irrigant penetration remain critical obstacles [46].
One plausible explanation for the lack of enhanced intratubular efficacy is the absence of irrigant activation. In industrial settings, PCD typically requires mechanical agitation to promote gas solubilization and enhance penetration into target substrates [47]. In endodontics, passive ultrasonic irrigation (PUI) and other activation techniques have been shown to significantly enhance irrigant distribution and disrupt bacterial biofilms [48,49]. To better reflect clinical conditions and explore the full potential of this strategy, future studies could include irrigant activation protocols combined with CO2 and assess their efficacy against a wider range of oral pathogens.
The biochemical analysis revealed that the addition of CO2 to NaOCl resulted in significantly reduced oxidative damage to lipids and proteins, suggesting interference with the formation of ROS such as HOCl. This finding deserves further attention, as the acidification of NaOCl can influence the chemical equilibrium between chlorine species. While a slight pH reduction (~pH 6–7.5) may favor the predominance of HOCl over hypochlorite ions (OCl−), excessive acidification, as observed with CO2 saturation to pH 7.4, may destabilize the oxidative activity of NaOCl and compromise its antimicrobial action [21,46]. Thus, future investigations should assess controlled acidification strategies that balance antimicrobial potency with chemical stability.
Another important aspect concerns the design of the oxidative stress assays. In this study, biochemical analyses were conducted using bacterial pellets in suspension rather than dentin-adhered biofilms, which may limit the translational relevance of the findings. Although this method enables standardized quantification of oxidative damage, it does not accurately reproduce the protective architecture and microenvironment of biofilms residing within dentinal tubules. However, this approach offers important advantages, including experimental reproducibility, precise control of treatment conditions, and the ability to quantify oxidative damage without interference from dentin matrix components. It also enables the isolation of the direct effects of irrigants on bacterial components, helping to elucidate fundamental mechanisms of action. As a preliminary screening tool, this model provides valuable insights that can guide the design of more complex in situ experiments. Notably, the metabolic activity and cellular fragility of the E. faecalis biofilm observed in the XTT assay were consistent with the oxidative damage findings. Future studies should employ dentin-adhered biofilm models alongside advanced imaging or spectroscopic techniques to more accurately assess oxidative stress in anatomically complex systems.
In summary, while the addition of CO2 to NaOCl presents a conceptually promising approach, the findings of this study suggest that under non-activated conditions, this combination does not improve disinfection in dentinal tubules and may even reduce the chemical aggressiveness and antimicrobial effectiveness of NaOCl. This insight reinforces the notion that the uncritical adoption of new irrigation adjuncts can lead to unintended antagonistic interactions, ultimately diminishing treatment outcomes. Therefore, the use of adjuncts such as PCD must be guided by a comprehensive understanding of their physicochemical interactions with conventional irrigants and the specific conditions required for their optimal performance. A more rigorous control of pH, application of irrigant activation, and clinically relevant oxidative stress assessment models are essential next steps.
5. Conclusions
Within the limitations of this in vitro study, the combination of PCD with 2.5% NaOCl resulted in a modest improvement in bacterial reduction within the root canal lumen but did not enhance antimicrobial activity in dentinal tubules. Furthermore, the addition of CO2 significantly attenuated oxidative damage to lipids and proteins and reduced biofilm metabolic inactivation—indicating a marked decrease in the overall bactericidal efficacy of NaOCl. Although conceptually promising, this approach requires further refinement, particularly regarding pH modulation, chemical stability, and delivery dynamics. A clinically relevant implication of these findings is that the association of substances with NaOCl—especially when applied without proper activation—may limit their therapeutic potential.
Author Contributions
Conceptualization, R.d.C.J., F.C.M., J.G.d.A. and C.L.; methodology, J.G.d.A., A.F.F.N., C.L., G.W.L.R., A.P.F.R., R.N.d.F., R.J.B., L.C.O., Y.G.C.d.M., N.A.G., A.H.C.-N., F.C.M. and R.d.C.J.; validation, J.G.d.A., A.F.F.N., C.L., G.W.L.R., A.P.F.R., R.N.d.F., R.J.B., L.C.O., Y.G.C.d.M., N.A.G., A.H.C.-N., F.C.M. and R.d.C.J.; formal analysis, J.G.d.A., A.F.F.N., C.L., G.W.L.R., A.P.F.R., R.N.d.F., R.J.B., L.C.O., Y.G.C.d.M., N.A.G., A.H.C.-N., F.C.M. and R.d.C.J.; investigation, J.G.d.A., A.F.F.N., C.L., G.W.L.R., A.P.F.R., R.N.d.F., R.J.B., L.C.O., Y.G.C.d.M., N.A.G., A.H.C.-N., F.C.M. and R.d.C.J.; resources, R.d.C.J., J.G.d.A. and A.F.F.N.; data curation, J.G.d.A., C.L., G.W.L.R., L.C.O. and Y.G.C.d.M.; writing—original draft preparation, J.G.d.A., R.d.C.J., C.L., L.C.O. and G.W.L.R.; writing—review and editing, J.G.d.A., R.d.C.J. A.H.C.-N., F.C.M. and Y.G.C.d.M.; visualization, J.G.d.A., A.F.F.N., C.L., G.W.L.R., A.P.F.R., R.N.d.F., R.J.B., L.C.O., Y.G.C.d.M., N.A.G., A.H.C.-N., F.C.M. and R.d.C.J.; supervision, R.d.C.J., A.H.C.-N. and F.C.M.; project administration, J.G.d.A. and R.d.C.J.; funding acquisition, J.G.d.A., R.d.C.J. and A.F.F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel, Brazil (CAPES Code 001), and the São Paulo Research Foundation (FAPESP 2021/02260-6). This study was financed in part by PROPG/UNESP through call nº 23/2025.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Araçatuba School of Dentistry, São Paulo State University (protocol code CAAE: 34692620.8.0000.5420, Approved on 23 September 2020).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahmed, S.; Hassan, S.J.; Gajdhar, S.; Alhazmi, L.S.; Khalifah, R.Y.; Alrifai, J.A.; Aljhdali, S.S.; Maqbul, M.S. Prevalence of Enterococcus faecalis and Candida albicans in endodontic retreatment Cases: A comprehensive study. Saudi Dent. J. 2024, 36, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef]
- Barbosa-Ribeiro, M.; Arruda-Vasconcelos, R.; Louzada, L.M.; dos Santos, D.G.; Andreote, F.D.; Gomes, B.P.F.A. Microbiological analysis of endodontically treated teeth with apical periodontitis before and after endodontic retreatment. Clin. Oral Investig. 2020, 25, 2017–2027. [Google Scholar] [CrossRef]
- Pinheiro, E.T.; Gomes, B.P.F.A.; Ferraz, C.C.R.; Teixeira, F.B.; Zaia, A.A.; Filho, F.J.S. Evaluation of root canal microorganisms isolated from teeth with endodontic failure and their antimicrobial susceptibility. Oral Microbiol. Immunol. 2003, 18, 100–103. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Polymerase chain reaction–based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 85–94. [Google Scholar] [CrossRef]
- Distel, J.; Hatton, J.; Gillespie, M. Biofilm formation in medicated root canals. J. Endod. 2002, 28, 689–693. [Google Scholar] [CrossRef]
- Guerreiro-Tanomaru, J.M.; de Faria-Júnior, N.B.; Duarte, M.A.H.; Ordinola-Zapata, R.; Graeff, M.S.Z.; Tanomaru-Filho, M. Comparative Analysis of Enterococcus faecalis Biofilm Formation on Different Substrates. J. Endod. 2013, 39, 346–350. [Google Scholar] [CrossRef]
- Sedgley, C.M.; Molander, A.; Flannagan, S.E.; Nagel, A.C.; Appelbe, O.K.; Clewell, D.B.; Dahlén, G. Virulence, phenotype and genotype characteristics of endodontic Enterococcus spp. Oral Microbiol. Immunol. 2004, 20, 10–19. [Google Scholar] [CrossRef]
- Gomes, B.P.F.A.; Souza, S.F.C.; Ferraz, C.C.R.; Teixeira, F.B.; Zaia, A.A.; Valdrighi, L.; Souza-Filho, F.J. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int. Endod. J. 2003, 36, 267–275. [Google Scholar] [CrossRef]
- Weckwerth, P.H.; Zapata, R.O.; Vivan, R.R.; Filho, M.T.; Maliza, A.G.A.; Duarte, M.A.H. In Vitro Alkaline pH Resistance of Enterococcus faecalis. Braz. Dent. J. 2013, 24, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Ribeiro, M.; De-Jesus-Soares, A.; Zaia, A.A.; Ferraz, C.C.; Almeida, J.F.; Gomes, B.P. Antimicrobial Susceptibility and Characterization of Virulence Genes of Enterococcus faecalis Isolates from Teeth with Failure of the Endodontic Treatment. J. Endod. 2016, 42, 1022–1028. [Google Scholar] [CrossRef]
- Figdor, D.; Davies, J.K.; Sundqvist, G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol. Immunol. 2003, 18, 234–239. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Camargo, C.H.R.; Valera, M.C.; Camargo, S.E.A.; Mancini, M.N.G. Smear Layer removal by auxiliary chemical substances in biomechanical preparation: A scanning electron microscope study. J. Endod. 2008, 34, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z. Sodium hypochlorite in endodontics: An update review. Int. Dent. J. 2008, 58, 329–341. [Google Scholar] [CrossRef]
- Aveiro, E.; Chiarelli-Neto, V.M.; De-Jesus-Soares, A.; Zaia, A.A.; Ferraz, C.C.R.; Almeida, J.F.A.; Marciano, M.A.; Feres, M.; Gomes, B.P.F.A. Efficacy of reciprocating and ultrasonic activation of 6% sodium hypochlorite in the reduction of microbial content and virulence factors in teeth with primary endodontic infection. Int. Endod. J. 2019, 53, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Cheung, G.S. Extension of bactericidal effect of sodium hypochlorite into dentinal tubules. J. Endod. 2014, 40, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Gazzaneo, I.; Vieira, G.C.; Pérez, A.R.; Alves, F.R.; Gonçalves, L.S.; Mdala, I.; Siqueira, J.F.; Rôças, I.N. Root Canal Disinfection by Single- and Multiple-instrument Systems: Effects of Sodium Hypochlorite Volume, Concentration, and Retention Time. J. Endod. 2019, 45, 736–741. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Shen, Y.; Haapasalo, M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J. Endod. 2011, 37, 1380–1385. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Effectiveness of endodontic disinfecting solutions against young and old enterococcus faecalis biofilms in dentin canals. J. Endod. 2012, 38, 1376–1379. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]
- Rossi-Fedele, G.; Guastalli, A.R.; Doğramacı, E.J.; Steier, L.; De Figueiredo, J.A.P. Influence of pH changes on chlorine-containing endodontic irrigating solutions. Int. Endod. J. 2011, 44, 792–799. [Google Scholar] [CrossRef]
- Yared, G.; Ramli, G.A.A. Antibacterial Ability of Sodium Hypochlorite Heated in the Canals of Infected Teeth: An Ex Vivo Study. Cureus 2020, 12, e6975. [Google Scholar] [CrossRef]
- Hage, W.; De Moor, R.J.G.; Hajj, D.; Sfeir, G.; Sarkis, D.K.; Zogheib, C. Impact of Different Irrigant Agitation Methods on Bacterial Elimination from Infected Root Canals. Dent. J. 2019, 7, 64. [Google Scholar] [CrossRef]
- Penukonda, R.; Teja, K.V.; Kacharaju, K.R.; Xuan, S.Y.; Mohan, D.A.; Sheun, L.Y.; Cernera, M.; Iaculli, F. Comparative evaluation of smear layer removal with Ultra-X device and XP-Endo Finisher file system: An ex-vivo study: Smear removal on using various activation devices. G. Ital. Endod. 2023, 37. [Google Scholar] [CrossRef]
- Zeng, C.; Meghil, M.M.; Miller, M.; Gou, Y.; Cutler, C.W.; Bergeron, B.E.; Niu, L.; Ma, J.; Tay, F.R. Antimicrobial efficacy of an apical negative pressure root canal irrigation system against intracanal microorganisms. J. Dent. 2018, 72, 71–75. [Google Scholar] [CrossRef]
- Abdellatif, D.; Pisano, M.; Cecere, L.; Natoli, V.; Mancino, D.; Euvrard, E.; Iandolo, A. Activation of Irrigants in Root Canals with Open Apices: A Narrative Review. J. Clin. Med. 2024, 13, 6611. [Google Scholar] [CrossRef] [PubMed]
- Teja, K.V.; Mustafa, M.; Esposito, L.; Iaculli, F.; Cernera, M. Novel non-obturation based concept of regeneration: Apical debris extrusion. G. Ital. Endod. 2024, 38. [Google Scholar] [CrossRef]
- Dang, T.-L.T.; Imai, T.; Van Le, T.; Nguyen, D.-M.K.; Higuchi, T.; Kanno, A.; Yamamoto, K.; Sekine, M. Synergistic effect of pressurized carbon dioxide and sodium hypochlorite on the inactivation of Enterococcus sp. in seawater. Water Res. 2016, 106, 204–213. [Google Scholar] [CrossRef]
- Dehghani, F.; Annabi, N.; Titus, M.; Valtchev, P.; Tumilar, A. Sterilization of ginseng using a high pressure CO2 at moderate temperatures. Biotechnol. Bioeng. 2008, 102, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Park, H.J.; Yim, D.S.; Kim, H.T.; Choi, I.-G.; Kim, K.H. Analysis of survival rates and cellular fatty acid profiles of Listeria monocytogenes treated with supercritical carbon dioxide under the influence of cosolvents. J. Microbiol. Methods 2008, 75, 47–54. [Google Scholar] [CrossRef]
- Vo, H.T.; Imai, T.; Teeka, J.; Sekine, M.; Kanno, A.; Van Le, T.; Higuchi, T.; Phummala, K.; Yamamoto, K. Comparison of disinfection effect of pressurized gases of CO2, N2O, and N2 on Escherichia coli. Water Res. 2013, 47, 4286–4293. [Google Scholar] [CrossRef]
- Hernández-Ríos, P.; Pussinen, P.J.; Vernal, R.; Hernández, M. Oxidative Stress in the Local and Systemic Events of Apical Periodontitis. Front. Physiol. 2017, 8, 869. [Google Scholar] [CrossRef]
- Georgiou, A.C.; Cornejo Ulloa, P.; Van Kessel, G.M.H.; Crielaard, W.; Van der Waal, S.V. Reactive oxygen species can be traced locally and systemically in apical periodontitis: A systematic review. Arch. Oral. Biol. 2021, 129, 105167. [Google Scholar] [CrossRef]
- Castelo-Baz, P.; Martín-Biedma, B.; Cantatore, G.; Ruíz-Piñón, M.; Bahillo, J.; Rivas-Mundiña, B.; Varela-Patiño, P. In vitro comparison of passive and continuous ultrasonic irrigation in simulated lateral canals of extracted teeth. J. Endod. 2012, 38, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.C.R.; Gomes, B.P.F.A.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J. Endod. 2001, 27, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.C.; Zuolo, M.L.; Arruda-Vasconcelos, R.; Marinho, A.C.S.; Louzada, L.M.; Francisco, P.A.; Pecorari, V.G.A.; Gomes, B.P.F.d.A. Effectiveness of XP-Endo Finisher in the reduction of bacterial load in oval-shaped root canals. Braz. Oral Res. 2019, 33, e021. [Google Scholar] [CrossRef]
- Yamamoto, L.Y.; Loureiro, C.; Cintra, L.T.A.; Leonardo, R.d.T.; Banci, H.A.; Ribeiro, A.P.F.; Sivieri-Araujo, G.; Jacinto, R.d.C. Antibiofilm activity of laser ablation with indocyanine green activated by different power laser parameters compared with photodynamic therapy on root canals infected with Enterococcus faecalis. Photodiagn. Photodyn. Ther. 2021, 35, 102377. [Google Scholar] [CrossRef]
- Asnaashari, M.; Veshveshadi, O.; Aslani, F.; Hakimiha, N. Evaluation the antibacterial efficacy of sodium hypochlorite in combination with two different photodynamic therapy protocols against Enterococcus Faecalis in Infected root canals: An in-vitro experiment. Photodiagn. Photodyn. Ther. 2023, 43, 103722. [Google Scholar] [CrossRef] [PubMed]
- Lile, I.E.; Hajaj, T.; Veja, I.; Hosszu, T.; Vaida, L.L.; Todor, L.; Stana, O.; Popovici, R.-A.; Marian, D. Comparative Evaluation of Natural Mouthrinses and Chlorhexidine in Dental Plaque Management: A Pilot Randomized Clinical Trial. Healthcare 2025, 13, 1181. [Google Scholar] [CrossRef]
- Marinho, A.C.S.; Martinho, F.C.; Gonçalves, L.M.; Rabang, H.R.C.; Gomes, B.P.F.A. Does the Reciproc file remove root canal bacteria and endotoxins as effectively as multifile rotary systems? Int. Endod. J. 2014, 48, 542–548. [Google Scholar] [CrossRef]
- Berber, V.B.; Gomes, B.P.F.A.; Sena, N.T.; Vianna, M.E.; Ferraz, C.C.R.; Zaia, A.A.; Souza-Filho, F.J. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. Int. Endod. J. 2006, 39, 10–17. [Google Scholar] [CrossRef]
- Rodrigues, G.W.L.; Gouveia, S.D.B.V.; Oliveira, L.C.; de Freitas, R.N.; Dourado, N.G.; Sacoman, C.A.; Ribeiro, A.P.F.; Chaves-Neto, A.H.; Sivieri-Araújo, G.; Leonardo, R.d.T.; et al. Comparative analysis of antimicrobial activity and oxidative damage induced by laser ablation with indocyanine green versus aPDT with methylene blue and curcumin on E. coli biofilm in root canals. Odontology 2025, 1–9. [Google Scholar] [CrossRef]
- Jacob, V.P.; Paião, L.I.; da Silva, A.C.G.; Magario, M.K.W.; Kaneko, T.Y.; Martins, C.M.; Monteiro, D.R.; Mori, G.G. Antimicrobial action of NeoMTA Plus on mono- and dual-species biofilms of Enterococcus faecalis and Candida albicans: An in vitro study. Arch. Oral Biol. 2020, 120, 104925. [Google Scholar] [CrossRef]
- Zehnder, M. Root Canal Irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Shalavi, S.; Giardino, L.; Palazzi, F.; Mashouf, R.Y.; Soltanian, A. Antimicrobial effect of three new and two established root canal irrigation solutions. Gen. Dent. 2012, 60, 534. [Google Scholar]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ballal, N.V.; Gandhi, P.; Shenoy, P.A.; Dummer, P.M.H. Evaluation of various irrigation activation systems to eliminate bacteria from the root canal system: A randomized controlled single blinded trial. J. Dent. 2020, 99, 103412. [Google Scholar] [CrossRef]
- Orozco, E.I.F.; Toia, C.C.; Cavalli, D.; Khoury, R.D.; Cardoso, F.G.d.R.; Bresciani, E.; Valera, M.C. Effect of passive ultrasonic activation on microorganisms in primary root canal infection: A randomized clinical trial. J. Appl. Oral Sci. 2020, 28, e20190100. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).