Determinants of Severe Oral Mucositis Development Despite Photobiomodulation Therapy in Stem Cell Transplant Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Adopted Protocols for Prevention and Control of Oral Mucositis
2.3. General Heath Monitoring Protocols Applied for the HSCT Patients
2.4. Statistical Analysis
3. Results
3.1. Study Population
| Gender | n | Percentage | OM Mean * | Age Range (Years) |
|---|---|---|---|---|
| Male | 71 | 50.35 | 2.23 | 17–75 |
| Female | 70 | 49.65 | 2.51 | 21–72 |
| Conditioning Regimens ** | Allogeneic transplants | Autologous transplants | ||
| n | Percentage | n | Percentage | |
| BEAC | 1 | 0.71 | 7 | 4.96 |
| BEAM | 0 | 0 | 5 | 3.55 |
| Bu-Cy | 12 | 8.51 | 1 | 0.71 |
| Bu-Flu | 26 | 18.44 | 0 | 0 |
| BuMel | 7 | 4.96 | 2 | 1.42 |
| Cy-TBI | 14 | 9.93 | 0 | 0 |
| Flu-Bu-Cy | 18 | 12.77 | 0 | 0 |
| Flu-Cy | 3 | 2.13 | 0 | 0 |
| Flu-Cy-TBI | 11 | 7.8 | 0 | 0 |
| Flu-Melphalan | 9 | 6.38 | 0 | 0 |
| Melphalan | 0 | 0 | 25 | 17.73 |
| Total | 101 | 71.63 | 40 | 28.37 |
3.2. Correlation Between the Independent Variables and OM Grade
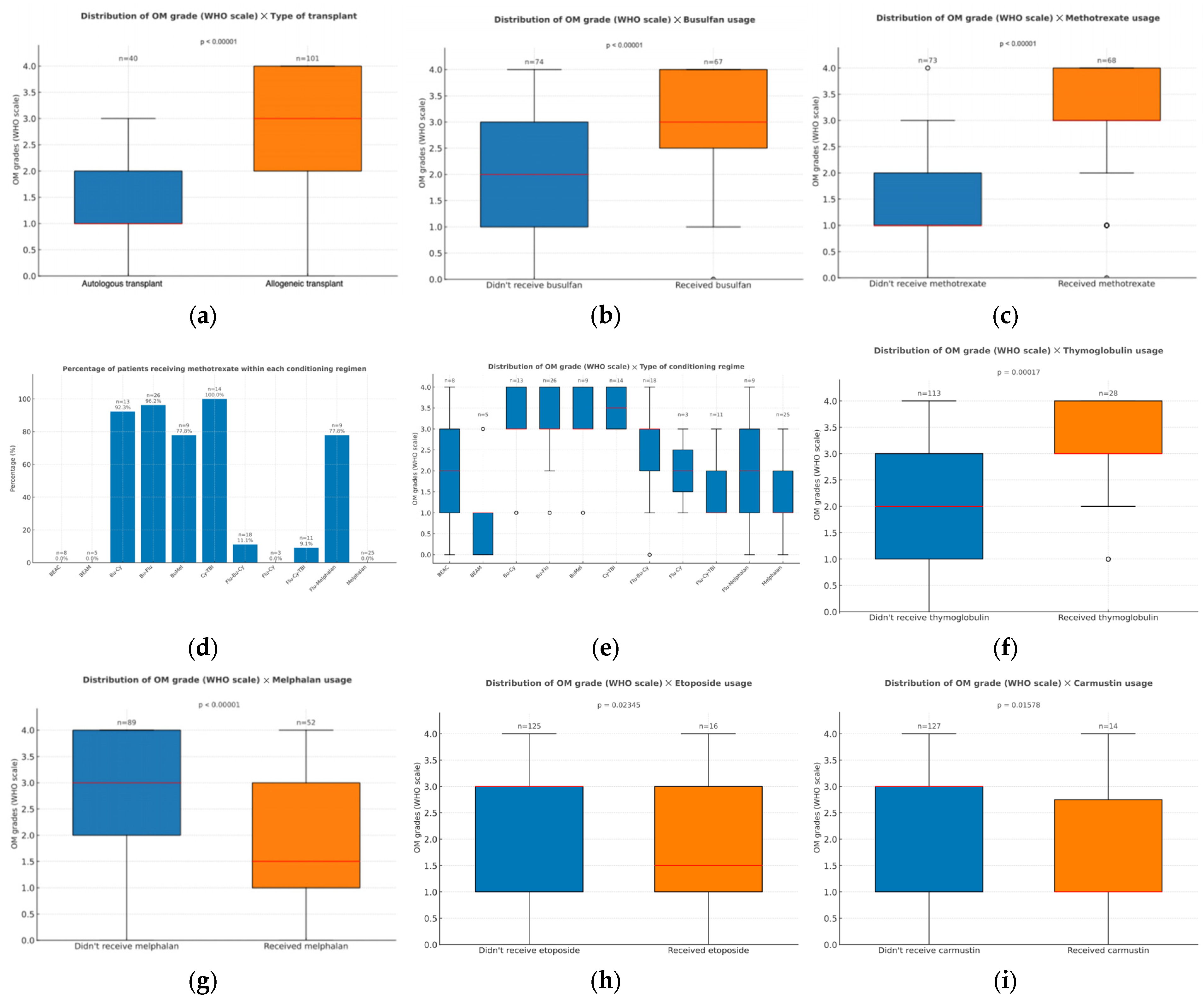
3.2.1. Treatment Parameters
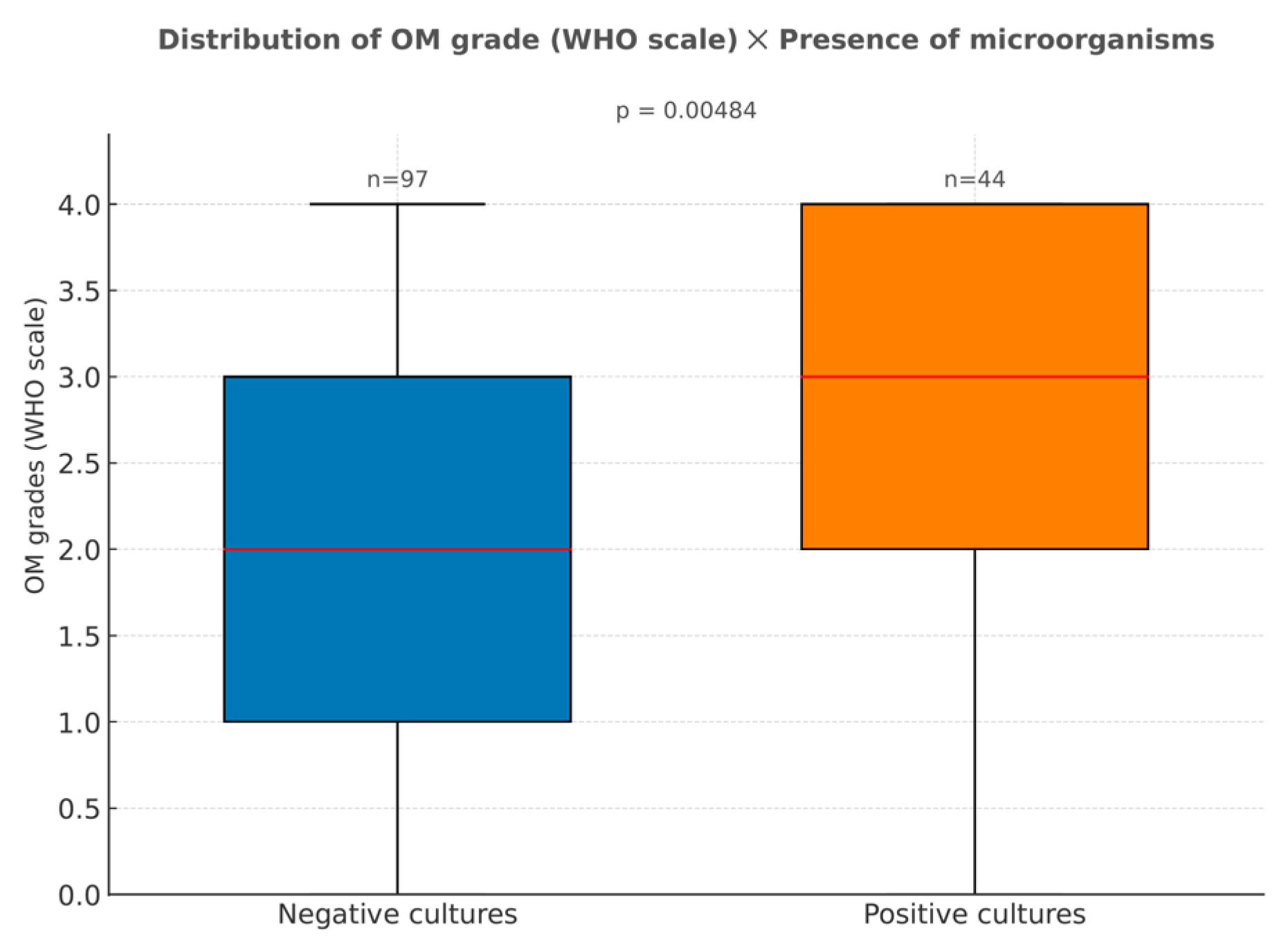
3.2.2. Presence of Microorganisms in Cultures
3.2.3. Laboratorial Parameters
3.2.4. Engraftment Times
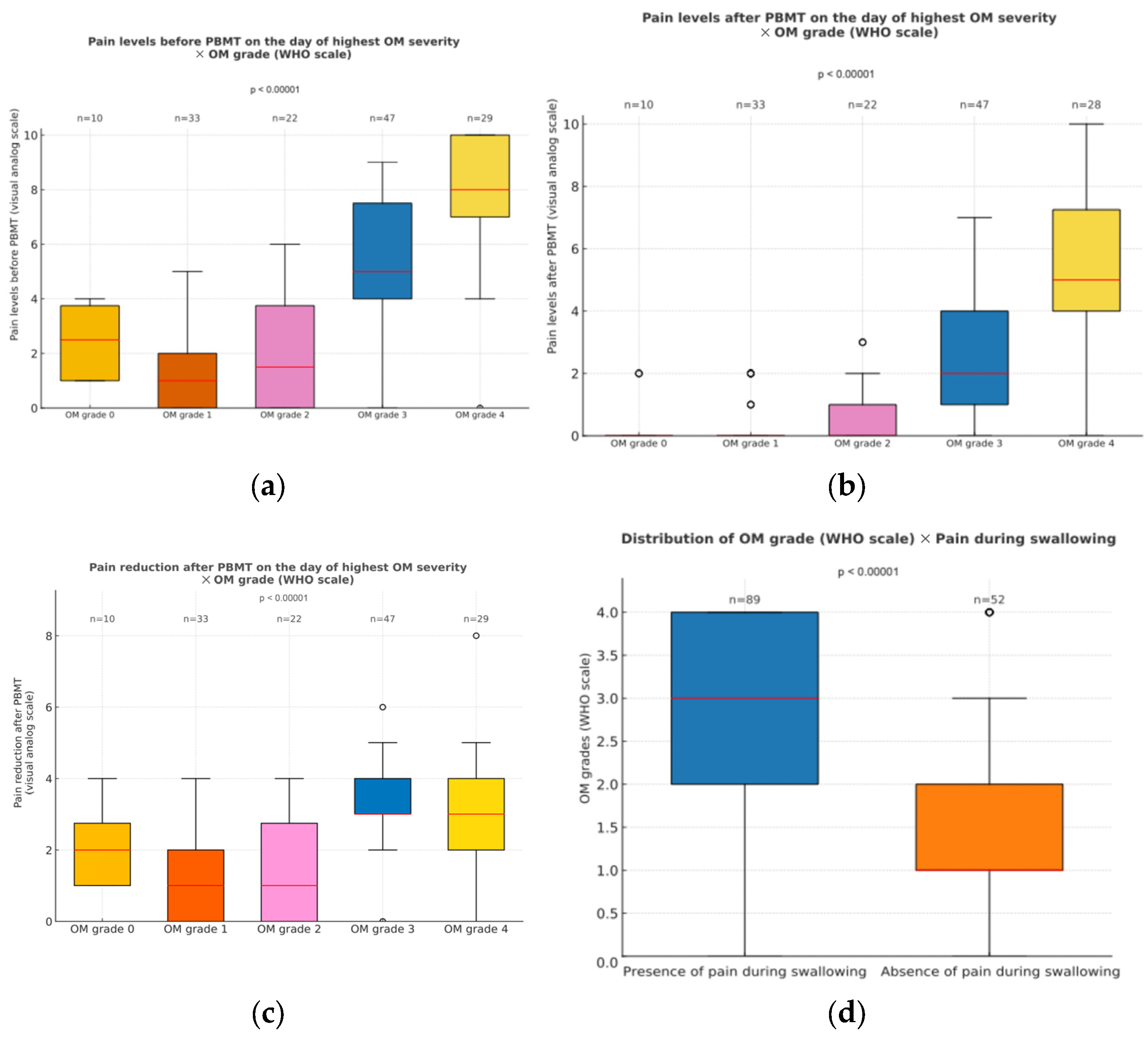
3.2.5. Pain Levels When Oral Mucositis Was at Its Peak
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OM | Oral mucositis |
| HSCT | Hematopoietic Stem Cell Transplantation |
| PBMT | Photobiomodulation therapy |
| MTX | Methotrexate |
| CRP | C-reactive protein |
| OGT | Oxalacetic glutamic transaminase |
| PGT | Pyruvic glutamic transaminase |
| MASCC | Multinational Association of Supportive Care in Cancer |
| WHO | World Health Organization |
| NCI | National Cancer Institute |
| IBM SPSS | International Business Machines Corporation |
| BEAC | Carmustine, etoposide, cytarabine, and cyclophosphamide |
| BEAM | Carmustine, etoposide, cytarabine, and melphalan |
| Bu | Busulfan |
| Cy | Cyclophosphamide |
| Flu | Fludarabine |
| Mel | Melphalan |
| TBI | Total body irradiation |
| GVHD | Graft-versus-host disease |
References
- Balassa, K.; Danby, R.; Rocha, V. Haematopoietic Stem Cell Transplants: Principles and Indications. Br. J. Hosp. Med. 2018, 80, 33–39. [Google Scholar] [CrossRef]
- Kanate, A.S.; Majhail, N.S.; Savani, B.N.; Bredeson, C.; Champlin, R.E.; Crawford, S.; Giralt, S.A.; LeMaistre, C.F.; Marks, D.I.; Omel, J.L.; et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2020, 26, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Kröger, N. Dose Intensity for Conditioning in Allogeneic Hematopoietic Cell Transplantation: Can We Recommend “When and for Whom” in 2021? Haematologica 2021, 106, 1794–1804. [Google Scholar] [CrossRef]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning Regimens for Hematopoietic Cell Transplantation: One Size Does Not Fit All. Blood 2014, 124, 344. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The Broadening Scope of Oral Mucositis and Oral Ulcerative Mucosal Toxicities of Anticancer Therapies. CA Cancer J. Clin. 2022, 72, 57–77. [Google Scholar] [CrossRef]
- Barkokebas, A.; Silva, I.H.M.; de Andrade, S.C.; Carvalho, A.A.T.; Gueiros, L.A.M.; Paiva, S.M.; Leão, J.C. Impact of Oral Mucositis on Oral-Health-Related Quality of Life of Patients Diagnosed with Cancer. J. Oral Pathol. Med. 2015, 44, 746–751. [Google Scholar] [CrossRef]
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.L.; Barasch, A.; Tishler, R.B.; Canty, T.P.; Kudrimoti, M.K.; Vera-Llonch, M.; et al. Patient-Reported Measurements of Oral Mucositis in Head and Neck Cancer Patients Treated with Radiotherapy with or without Chemotherapy. Cancer 2008, 113, 2704–2713. [Google Scholar] [CrossRef]
- Potrich, A.R.; Só, B.B.; Schuch, L.F.; Wagner, V.P.; Silveira, F.M.; de Abreu Alves, F.; Prado-Ribeiro, A.C.; Santos-Silva, A.R.; Treister, N.S.; Martins, M.D.; et al. Impact of Photobiomodulation for Prevention of Oral Mucositis on the Quality of Life of Patients with Head and Neck Cancer: A Systematic Review. Lasers Med. Sci. 2023, 39, 1. [Google Scholar] [CrossRef]
- McCann, S.; Schwenkglenks, M.; Bacon, P.; Einsele, H.; D’Addio, A.; Maertens, J.; Niederwieser, D.; Rabitsch, W.; Roosaar, A.; Ruutu, T.; et al. The Prospective Oral Mucositis Audit: Relationship of Severe Oral Mucositis with Clinical and Medical Resource Use Outcomes in Patients Receiving High-Dose Melphalan or BEAM-Conditioning Chemotherapy and Autologous SCT. Bone Marrow Transplant. 2009, 43, 141–147. [Google Scholar] [CrossRef]
- McCullough, R.W. US Oncology-Wide Incidence, Duration, Costs and Deaths from Chemoradiation Mucositis and Antimucositis Therapy Benefits. Future Oncol. 2017, 13, 2823–2852. [Google Scholar] [CrossRef] [PubMed]
- Bezinelli, L.M.; de Paula Eduardo, F.; da Graça Lopes, R.M.; Biazevic, M.G.H.; de Paula Eduardo, C.; Correa, L.; Hamerschlak, N.; Michel-Crosato, E. Cost-Effectiveness of the Introduction of Specialized Oral Care with Laser Therapy in Hematopoietic Stem Cell Transplantation. Hematol. Oncol. 2014, 32, 31–39. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Robinson, P.D.; Baggott, C.; Gibson, P.; Ljungman, G.; Massey, N.; Ottaviani, G.; Phillips, R.; Revon-Rivière, G.; Treister, N.; et al. Clinical Practice Guideline for the Prevention of Oral and Oropharyngeal Mucositis in Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients: 2021 Update. Eur. J. Cancer 2021, 154, 92–101. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; Porta, C.L.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral Mucositis: The Hidden Side of Cancer Therapy. J. Exp. Clin. Cancer Res. CR 2020, 39, 210. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation and Oral Mucositis: A Systematic Review. Dent. J. 2020, 8, 87. [Google Scholar] [CrossRef]
- Lins, R.D.A.U.; Dantas, E.M.; Lucena, K.C.R.; Catão, M.H.C.V.; Granville-Garcia, A.F.; Carvalho Neto, L.G. Biostimulation Effects of Low-Power Laser in the Repair Process. An. Bras. Dermatol. 2010, 85, 849–855. [Google Scholar] [CrossRef]
- de Sales, P.V.A.; Godói, I.P.D.; Brito, G.A.d.C.; Leitão, R.C.; de Araújo, A.A.; de Medeiros, C.A.C.X. Mechanisms of Photobiomodulation Therapy in Treating and Preventing Antineoplastic-Induced Oral Mucositis: A Systematic Review. Acta Cirúrgica Bras. 2025, 40, e403125. [Google Scholar] [CrossRef] [PubMed]
- de Pauli Paglioni, M.; Alves, C.G.B.; Fontes, E.K.; Lopes, M.A.; Ribeiro, A.C.P.; Brandão, T.B.; Migliorati, C.A.; Santos-Silva, A.R. Is Photobiomodulation Therapy Effective in Reducing Pain Caused by Toxicities Related to Head and Neck Cancer Treatment? A Systematic Review. Support. Care Cancer 2019, 27, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of Oral Mucositis in Patients with Cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef]
- Robijns, J.; Nair, R.G.; Lodewijckx, J.; Arany, P.; Barasch, A.; Bjordal, J.M.; Bossi, P.; Chilles, A.; Corby, P.M.; Epstein, J.B.; et al. Photobiomodulation Therapy in Management of Cancer Therapy-Induced Side Effects: WALT Position Paper 2022. Front. Oncol. 2022, 12, 927685. [Google Scholar] [CrossRef]
- Simões, A.; Eduardo, F.P.; Luiz, A.C.; Campos, L.; Sá, P.H.R.N.; Cristófaro, M.; Marques, M.M.; Eduardo, C.P. Laser Phototherapy as Topical Prophylaxis against Head and Neck Cancer Radiotherapy-Induced Oral Mucositis: Comparison between Low and High/Low Power Lasers. Lasers Surg. Med. 2009, 41, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Paiva, D.L.; Oliveira, V.R.; Bagnato, V.S.; Simões, A. Long-Term Survival of Cancer Patients after Photobiomodulation Therapy for Prevention and Treatment of Oral Mucositis. Photodiagn. Photodyn. Ther. 2024, 48, 104248. [Google Scholar] [CrossRef]
- Biala, M. Low-Level Laser Therapy: A Literature Review of the Prevention and Reduction of Oral Mucositis in Patients Undergoing Stem Cell Transplantation. Clin. J. Oncol. Nurs. 2022, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Calarga, C.C.; Cotomácio, C.C.; Simões, A. Photobiomodulation for Oral Mucositis Management in Pediatric Patients: A Systematic Review. Lasers Med. Sci. 2024, 39, 272. [Google Scholar] [CrossRef] [PubMed]
- Stocker, N.; Baltes, V.; Bellaiche, S.; Brouillard, F.; Belmoufid, N.; Rousseau, C.; Bonnin, A.; Van de Wyngaert, Z.; Ricard, L.; Banet, A.; et al. Photobiomodulation: A Promising Innovative Approach for Preventing Oral Mucositis in Patients Undergoing Hematopoietic Stem Cell Transplantation. Support. Care Cancer 2022, 30, 8211–8216. [Google Scholar] [CrossRef]
- Khalil, M.; Hamadah, O.; Saifo, M. Preconditioning with Photobiomodulation as an Effective Method in Preventing Chemotherapy-Induced Oral Mucositis: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2023, 41, 597–607. [Google Scholar] [CrossRef]
- Magee, K.; Robins, J.; Staton, S.; Llaurador, G.; Stevens, A.M. Implementation of a Light Therapy Team to Administer Photobiomodulation Therapy: A Standardized Protocol to Prevent and Treat Oral Mucositis in the Pediatric Hematopoietic Stem Cell Transplant Population. Pediatr. Blood Cancer 2024, 71, e30966. [Google Scholar] [CrossRef]
- Cruz, A.R.; Minicucci, E.M.; Betini, M.; Almeida-Lopes, L.; Tieghi Neto, V.; Cataneo, A.J.M. Efficacy of Photobiomodulation in the Treatment of Oral Mucositis in Patients Undergoing Antineoplastic Therapy: Systematic Review and Meta-Analysis. Support. Care Cancer 2023, 31, 645. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Gianini, C.A.; Pereira, R.B.; Silvani, G.; Bagnato, V.S.; Panhóca, V.H. Photobiomodulation Therapy for the Management of Oral Mucositis: A Clinical Case. In Proceedings of the Imaging, Therapeutics, and Advanced Technology in Head and Neck Surgery and Otolaryngology 2024, San Francisco, CA, USA, 27 January–1 February 2024; SPIE: Washington, DC, USA, 2024; Volume 12818, pp. 19–22. [Google Scholar]
- Cotomacio, C.C.; Campos, L.; Nesadal de Souza, D.; Arana-Chavez, V.E.; Simões, A. Dosimetric Study of Photobiomodulation Therapy in 5-FU-Induced Oral Mucositis in Hamsters. J. Biomed. Opt. 2017, 22, 18003. [Google Scholar] [CrossRef]
- de Castro, J.R.; da Silva Pereira, F.; Chen, L.; Arana-Chavez, V.E.; Ballester, R.Y.; DiPietro, L.A.; Simões, A. Improvement of Full-Thickness Rat Skin Wounds by Photobiomodulation Therapy (PBMT): A Dosimetric Study. J. Photochem. Photobiol. B 2020, 206, 111850. [Google Scholar] [CrossRef]
- Mendez, T.M.T.V.; Pinheiro, A.L.B.; Pacheco, M.T.T.; Nascimento, P.M.; Ramalho, L.M.P. Dose and Wavelength of Laser Light Have Influence on the Repair of Cutaneous Wounds. J. Clin. Laser Med. Surg. 2004, 22, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Mamani, M.; da Silva, B.M.; da Silva Pinto, A.C.; Rubira-Bullen, I.R.F.; Honório, H.M.; Rubira, C.M.F.; da Silva Santos, P.S. Low-Level Laser Therapy Dosimetry Most Used for Oral Mucositis Due to Radiotherapy for Head and Neck Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2019, 138, 14–23. [Google Scholar] [CrossRef]
- Al-Jamaei, A.A.H.; Epstein, J.B.; de Visscher, J.G.A.M.; Spielberger, R.T.; Nakamura, R.; Raber-Durlacher, J.E. Comparing the Risk of Severe Oral Mucositis Associated with Methotrexate as Graft-versus Host-Disease Prophylaxis to Other Immunosuppressive Prophylactic Agents in Hematopoietic Cell Transplantation: A Systematic Review and Meta-Analysis. Support. Care Cancer 2024, 32, 519. [Google Scholar] [CrossRef]
- Curra, M.; Gabriel, A.F.; Ferreira, M.B.C.; Martins, M.A.T.; Brunetto, A.T.; Gregianin, L.J.; Martins, M.D. Incidence and Risk Factors for Oral Mucositis in Pediatric Patients Receiving Chemotherapy. Support. Care Cancer 2021, 29, 6243–6251. [Google Scholar] [CrossRef]
- Wardill, H.R.; Sonis, S.T.; Blijlevens, N.M.A.; Van Sebille, Y.Z.A.; Ciorba, M.A.; Loeffen, E.A.H.; Cheng, K.K.F.; Bossi, P.; Porcello, L.; Castillo, D.A.; et al. Prediction of Mucositis Risk Secondary to Cancer Therapy: A Systematic Review of Current Evidence and Call to Action. Support. Care Cancer 2020, 28, 5059–5073. [Google Scholar] [CrossRef]
- Bardellini, E.; Schumacher, F.; Conti, G.; Porta, F.; Campus, G.; Majorana, A. Risk Factors for Oral Mucositis in Children Receiving Hematopoietic Cell Transplantation for Primary Immunodeficiencies: A Retrospective Study. Pediatr. Transplant. 2013, 17, 492–497. [Google Scholar] [CrossRef]
- Tebbutt, N.C.; Norman, A.R.; Cunningham, D.; Allen, M.; Chau, I.; Oates, J.; Hill, M. Analysis of the Time Course and Prognostic Factors Determining Toxicity Due to Infused Fluorouracil. Br. J. Cancer 2003, 88, 1510–1515. [Google Scholar] [CrossRef]
- Eduardo, F.d.P.; Bezinelli, L.; Luiz, A.C.; Correa, L.; Vogel, C.; Eduardo, C.d.P. Severity of Oral Mucositis in Patients Undergoing Hematopoietic Cell Transplantation and an Oral Laser Phototherapy Protocol: A Survey of 30 Patients. Photomed. Laser Surg. 2009, 27, 137–144. [Google Scholar] [CrossRef]
- Palmer, M.K. WHO Handbook for Reporting Results of Cancer Treatment. Br. J. Cancer 1982, 45, 484–485. [Google Scholar] [CrossRef]
- Rotz, S.J.; Bhatt, N.S.; Hamilton, B.K.; Duncan, C.; Aljurf, M.; Atsuta, Y.; Beebe, K.; Buchbinder, D.; Burkhard, P.; Carpenter, P.A.; et al. International Recommendations for Screening and Preventative Practices for Long-Term Survivors of Transplantation and Cellular Therapy: A 2023 Update. Transplant. Cell. Ther. 2024, 30, 349–385. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Kasi, A. Oral Mucositis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Parulekar, W.; Mackenzie, R.; Bjarnason, G.; Jordan, R.C.K. Scoring Oral Mucositis. Oral Oncol. 1998, 34, 63–71. [Google Scholar] [CrossRef]
- Barlas, T.; İnci, K.; Aygencel, G.; Türkoğlu, M.; Tunçcan, Ö.G.; Can, F.; Aydın Kaynar, L.; Özkurt, Z.N.; Yeğin, Z.A.; Yağcı, M. Infections in Hematopoietic Stem Cell Transplant Patients Admitted to Hematology Intensive Care Unit: A Single-Center Study. Hematol. Amst. Neth. 2021, 26, 328–339. [Google Scholar] [CrossRef]
- Nouér, S.A.; Nucci, M.; Anaissie, E. Tackling Antibiotic Resistance in Febrile Neutropenia: Current Challenges with and Recommendations for Managing Infections with Resistant Gram-Negative Organisms. Expert Rev. Hematol. 2015, 8, 647–658. [Google Scholar] [CrossRef]
- Averbuch, D.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Orasch, C.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. Targeted Therapy against Multi-Resistant Bacteria in Leukemic and Hematopoietic Stem Cell Transplant Recipients: Guidelines of the 4th European Conference on Infections in Leukemia (ECIL-4, 2011). Haematologica 2013, 98, 1836–1847. [Google Scholar] [CrossRef]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.-A.H.; Boeckh, M.A. Guidelines for Preventing Infectious Complications among Hematopoietic Cell Transplantation Recipients: A Global Perspective. Biol. Blood Marrow Transplant. 2009, 15, 1143–1238. [Google Scholar] [CrossRef]
- Mendes, E.T.; Dulley, F.; Basso, M.; Batista, M.V.; Coracin, F.; Guimarães, T.; Shikanai-Yasuda, M.A.; Levin, A.S.; Costa, S.F. Healthcare-Associated Infection in Hematopoietic Stem Cell Transplantation Patients: Risk Factors and Impact on Outcome. Int. J. Infect. Dis. 2012, 16, e424–e428. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.M.; Tofani, A.; Santos, C.F.; Morassi, C.V.; Costa, D.C.R.; Ito, F.T.; Gato, M.I.R.; Castro, P.F.; Villa, P.R.; Macedo, R.S. Transplante de Células-Tronco Hematopoéticas Introdução para Farmacêuticos; Segmento Farma Editores Ltda.: São Paulo, SP, Brazil, 2018; Volume II, pp. 30–33. [Google Scholar]
- da Silva, B.M.; Prosdócimo, M.L.; Gasparini, L.R.; da Silva, B.M.; de Araujo, M.R.; Amenábar, J.M. Most Used Photobiomodulation Dosimetry Parameters to Treat Oral Mucositis after Preconditioning for Hematopoietic Stem Cell Transplantation: Systematic Review and Meta-Analysis. Support. Care Cancer 2022, 30, 3721–3732. [Google Scholar] [CrossRef]
- Selestin Raja, I.; Kim, C.; Oh, N.; Park, J.-H.; Hong, S.W.; Kang, M.S.; Mao, C.; Han, D.-W. Tailoring Photobiomodulation to Enhance Tissue Regeneration. Biomaterials 2024, 309, 122623. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.C.; de Sousa, M.V.P.; Ferreira, N.L.; Braga, N.A.; Aldred, A.; Gomes, G.; Freire, G.M.G.; Ashmawi, H.A.; Chacur, M. Customized Photobiomodulation Modulates Pain and Alters Thermography Pattern in Patients with Knee Osteoarthritis: A Randomized Double-Blind Pilot Study. Photobiomodul. Photomed. Laser Surg. 2022, 40, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-L.; Huang, X.-J. Conditioning Regimen Options. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2025; Volume 1475, pp. 41–55. [Google Scholar] [CrossRef]
- Chaudhry, H.M.; Bruce, A.J.; Wolf, R.C.; Litzow, M.R.; Hogan, W.J.; Patnaik, M.S.; Kremers, W.K.; Phillips, G.L.; Hashmi, S.K. The Incidence and Severity of Oral Mucositis among Allogeneic Hematopoietic Stem Cell Transplantation Patients: A Systematic Review. Biol. Blood Marrow Transplant. 2016, 22, 605–616. [Google Scholar] [CrossRef]
- Michniacki, T.F.; Choi, S.W.; Peltier, D.C. Immune Suppression in Allogeneic Hematopoietic Stem Cell Transplantation. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2022; Volume 272, pp. 209–243. [Google Scholar] [CrossRef]
- Amanam, I.; Otoukesh, S.; Al Malki, M.M.; Salhotra, A. Chronic GVHD: Review Advances in Prevention, Novel Endpoints, and Targeted Strategies. Hematol. Am. Soc. Hematol. Educ. Program 2023, 2023, 164–170. [Google Scholar] [CrossRef]
- Watkins, B.; Williams, K.M. Controversies and Expectations for the Prevention of GVHD: A Biological and Clinical Perspective. Front. Immunol. 2022, 13, 1057694. [Google Scholar] [CrossRef]
- Hong, J.; Moon, S.M.; Ahn, H.K.; Sym, S.J.; Park, Y.S.; Park, J.; Cho, Y.K.; Cho, E.K.; Shin, D.B.; Lee, J.H. Comparison of Characteristics of Bacterial Bloodstream Infection between Adult Patients with Allogeneic and Autologous Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2013, 19, 994–999. [Google Scholar] [CrossRef][Green Version]
- Nakagaki, M.; Kennedy, G.A.; Gavin, N.C.; Clavarino, A.; Whitfield, K. The Incidence of Severe Oral Mucositis in Patients Undergoing Different Conditioning Regimens in Haematopoietic Stem Cell Transplantation. Support. Care Cancer 2022, 30, 9141–9149. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.; Li, S.; Kim, H.T.; Laglenne, P.; Szeto, K.C.; Hoffmeister, L.; Harrison, M.J.; Ho, V.; Alyea, E.; Lee, S.J.; et al. Mucositis after Allogeneic Hematopoietic Stem Cell Transplantation: A Cohort Study of Methotrexate- and Non-Methotrexate-Containing Graft-versus-Host Disease Prophylaxis Regimens. Biol. Blood Marrow Transplant. 2005, 11, 383–388. [Google Scholar] [CrossRef]
- Heil, S.G. Genetics of High-Dose Methotrexate-Induced Oral Mucositis: Current Perspectives. Pharmacogenomics 2019, 20, 621–623. [Google Scholar] [CrossRef]
- Matsukawa, T.; Hashimoto, D.; Sugita, J.; Nakazawa, S.; Matsushita, T.; Kashiwazaki, H.; Goto, H.; Onozawa, M.; Kahata, K.; Fujimoto, K.; et al. Reduced-Dose Methotrexate in Combination with Tacrolimus Was Associated with Rapid Engraftment and Recovery from Oral Mucositis without Affecting the Incidence of GVHD. Int. J. Hematol. 2016, 104, 117–124. [Google Scholar] [CrossRef]
- Eduardo, F.P.; Bezinelli, L.M.; Gobbi, M.; Rosin, F.C.P.; Carvalho, D.L.C.; Ferreira, M.H.; da Silva, C.C.; Hamerschlak, N.; Corrêa, L. Retrospective Study of the Digestive Tract Mucositis Derived from Myeloablative and Non-Myeloablative/Reduced-Intensity Conditionings with Busulfan in Hematopoietic Cell Transplantation Patient. Support. Care Cancer 2019, 27, 839–848. [Google Scholar] [CrossRef]
- Ogura, S.; Soga, Y.; Fujiwara, H.; Miura, R.; Matsuoka, K.; Maeda, Y.; Kuboki, T. Characteristics of Oral Mucositis in Patients Undergoing Haploidentical Stem Cell Transplantation with Posttransplant Cyclophosphamide: Marked Difference between Busulfan and Melphalan Regimens. Support. Care Cancer 2025, 33, 252. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.O.; Monaco, A.P.; Russell, J.A.; Lebranchu, Y.; Mohty, M. Rabbit Antithymocyte Globulin (Thymoglobulin®). Drugs 2010, 70, 691–732. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Bacigalupo, A.; Saliba, F.; Zuckermann, A.; Morelon, E.; Lebranchu, Y. New Directions for Rabbit Antithymocyte Globulin (Thymoglobulin®) in Solid Organ Transplants, Stem Cell Transplants and Autoimmunity. Drugs 2014, 74, 1605–1634. [Google Scholar] [CrossRef]
- Johansson, J.-E.; Bratel, J.; Hardling, M.; Heikki, L.; Mellqvist, U.-H.; Hasséus, B. Cryotherapy as Prophylaxis against Oral Mucositis after High-Dose Melphalan and Autologous Stem Cell Transplantation for Myeloma: A Randomised, Open-Label, Phase 3, Non-Inferiority Trial. Bone Marrow Transpl. 2019, 54, 1482–1488. [Google Scholar] [CrossRef]
- Riley, P.; McCabe, M.G.; Glenny, A.-M. Oral Cryotherapy for Preventing Oral Mucositis in Patients Receiving Cancer Treatment. JAMA Oncol. 2016, 2, 1365–1366. [Google Scholar] [CrossRef]
- de Paula Eduardo, F.; Bezinelli, L.M.; da Graça Lopes, R.M.; Nascimento Sobrinho, J.J.; Hamerschlak, N.; Correa, L. Efficacy of Cryotherapy Associated with Laser Therapy for Decreasing Severity of Melphalan-Induced Oral Mucositis during Hematological Stem-Cell Transplantation: A Prospective Clinical Study. Hematol. Oncol. 2015, 33, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, R.M.; Sanfilippo, N.; Paster, B.J.; Kerr, A.R.; Li, Y.; Ramalho, L.; Queiroz, E.L.; Smith, B.; Sonis, S.T.; Corby, P.M. Host-Microbiome Cross-Talk in Oral Mucositis. J. Dent. Res. 2016, 95, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Blijlevens, N.M.A.; de Mooij, C.E.M. Mucositis and Infection in Hematology Patients. Int. J. Mol. Sci. 2023, 24, 9592. [Google Scholar] [CrossRef] [PubMed]
- Stiff, P. Mucositis Associated with Stem Cell Transplantation: Current Status and Innovative Approaches to Management. Bone Marrow Transpl. 2001, 27 (Suppl. 2), S3–S11. [Google Scholar] [CrossRef]
- van der Velden, W.J.F.M.; Herbers, A.H.E.; Netea, M.G.; Blijlevens, N.M.A. Mucosal Barrier Injury, Fever and Infection in Neutropenic Patients with Cancer: Introducing the Paradigm Febrile Mucositis. Br. J. Haematol. 2014, 167, 441–452. [Google Scholar] [CrossRef]
- Lelubre, C.; Anselin, S.; Zouaoui Boudjeltia, K.; Biston, P.; Piagnerelli, M. Interpretation of C-Reactive Protein Concentrations in Critically Ill Patients. BioMed Res. Int. 2013, 2013, 124021. [Google Scholar] [CrossRef]
- Volanakis, J.E. Human C-Reactive Protein: Expression, Structure, and Function. Mol. Immunol. 2001, 38, 189–197. [Google Scholar] [CrossRef]
- Ki, Y.; Kim, W.; Nam, J.; Kim, D.; Park, D.; Kim, D. C-Reactive Protein Levels and Radiation-Induced Mucositis in Patients with Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Tvedt, T.H.; Lie, S.A.; Reikvam, H.; Rye, K.P.; Lindås, R.; Gedde-Dahl, T.; Ahmed, A.B.; Bruserud, Ø. Pretransplant Levels of CRP and Interleukin-6 Family Cytokines; Effects on Outcome after Allogeneic Stem Cell Transplantation. Int. J. Mol. Sci. 2016, 17, 1823. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.F.; Poon, I.; Zhang, L.; Elliott, L.; Hodson, I.D.; Sagar, S.M.; Wright, J. Acute-Phase Response Reactants as Objective Biomarkers of Radiation-Induced Mucositis in Head and Neck Cancer. Head Neck 2012, 34, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Strasser, S.I.; Sullivan, K.M.; Myerson, D.; Spurgeon, C.L.; Storer, B.; Schoch, H.G.; Murakami, C.S.; McDonald, G.B. Cirrhosis of the Liver in Long-Term Marrow Transplant Survivors. Blood 1999, 93, 3259–3266. [Google Scholar] [CrossRef]
- Farthing, M.J.; Clark, M.L.; Sloane, J.P.; Powles, R.L.; McElwain, T.J. Liver Disease after Bone Marrow Transplantation. Gut 1982, 23, 465–474. [Google Scholar] [CrossRef]
- Sawinski, D. The Kidney Effects of Hematopoietic Stem Cell Transplantation. Adv. Chronic Kidney Dis. 2014, 21, 96–105. [Google Scholar] [CrossRef]
- Abudayyeh, A.; Wanchoo, R. Kidney Disease Following Hematopoietic Stem Cell Transplantation. Adv. Chronic Kidney Dis. 2022, 29, 103–115.e1. [Google Scholar] [CrossRef]
- Renaghan, A.D.; Jaimes, E.A.; Malyszko, J.; Perazella, M.A.; Sprangers, B.; Rosner, M.H. Acute Kidney Injury and CKD Associated with Hematopoietic Stem Cell Transplantation. Clin. J. Am. Soc. Nephrol. 2020, 15, 289–297. [Google Scholar] [CrossRef]
- Valer, J.B.; Curra, M.; Gabriel, A.d.F.; Schmidt, T.R.; Ferreira, M.B.C.; Roesler, R.; Evangelista, J.M.C.; Martins, M.A.T.; Gregianin, L.; Martins, M.D. Oral Mucositis in Childhood Cancer Patients Receiving High-Dose Methotrexate: Prevalence, Relationship with Other Toxicities and Methotrexate Elimination. Int. J. Paediatr. Dent. 2021, 31, 238–246. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, L.; Chen, X.; Pan, B.; Mao, J.; Song, H.; Li, J.; Tang, Y. Serum Creatinine and Creatinine Clearance for Predicting Plasma Methotrexate Concentrations after High-Dose Methotrexate Chemotherapy for the Treatment for Childhood Lymphoblastic Malignancies. Cancer Chemother. Pharmacol. 2014, 73, 79–86. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-Level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Miron, I.C.; Dalvi, S.; Arany, P.; Bensadoun, R.J.; Benedicenti, S. A Systematic Review of Laser Photobiomodulation Dosimetry and Treatment Protocols in the Management of Medications-Related Osteonecrosis of the Jaws: A Rationalised Consensus for Future Randomised Controlled Clinical Trials. Pharmaceuticals 2024, 17, 1011. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, P.A.G.; Lessa, R.C.; Carraro, D.M.; Assis Pellizzon, A.C.; Jaguar, G.C.; Alves, F.A. Three Photobiomodulation Protocols in the Prevention/Treatment of Radiotherapy-Induced Oral Mucositis. Photodiagn. Photodyn. Ther. 2020, 31, 101906. [Google Scholar] [CrossRef]
- Cruz, É.d.P.d.; Campos, L.; Pereira, F.d.S.; Magliano, G.C.; Benites, B.M.; Arana-Chavez, V.E.; Ballester, R.Y.; Simões, A. Clinical, Biochemical and Histological Study of the Effect of Antimicrobial Photodynamic Therapy on Oral Mucositis Induced by 5-Fluorouracil in Hamsters. Photodiagn. Photodyn. Ther. 2015, 12, 298–309. [Google Scholar] [CrossRef]
- Joseph, B.; Mauramo, M.; Vijayakumary, B.K.; Waltimo, T.; Anil, S.; Sorsa, T. Photodynamic Therapy for Oral Mucositis in Cancer Patients- a Systematic Review and Meta-Analysis. Photodiagn. Photodyn. Ther. 2024, 50, 104424. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezende, S.B.; Campos, L.; de Souza, M.C.; Schoenmann, M.; Macedo, M.C.M.d.A.; da Silva, R.L.; Simões, A. Determinants of Severe Oral Mucositis Development Despite Photobiomodulation Therapy in Stem Cell Transplant Patients. Dent. J. 2025, 13, 411. https://doi.org/10.3390/dj13090411
Rezende SB, Campos L, de Souza MC, Schoenmann M, Macedo MCMdA, da Silva RL, Simões A. Determinants of Severe Oral Mucositis Development Despite Photobiomodulation Therapy in Stem Cell Transplant Patients. Dentistry Journal. 2025; 13(9):411. https://doi.org/10.3390/dj13090411
Chicago/Turabian StyleRezende, Sandra Bastos, Luana Campos, Maria Clara de Souza, Marcos Schoenmann, Maria Cristina Martins de Almeida Macedo, Roberto Luiz da Silva, and Alyne Simões. 2025. "Determinants of Severe Oral Mucositis Development Despite Photobiomodulation Therapy in Stem Cell Transplant Patients" Dentistry Journal 13, no. 9: 411. https://doi.org/10.3390/dj13090411
APA StyleRezende, S. B., Campos, L., de Souza, M. C., Schoenmann, M., Macedo, M. C. M. d. A., da Silva, R. L., & Simões, A. (2025). Determinants of Severe Oral Mucositis Development Despite Photobiomodulation Therapy in Stem Cell Transplant Patients. Dentistry Journal, 13(9), 411. https://doi.org/10.3390/dj13090411






