Maxillary Expansion in the Management of Obstructive Sleep Apnea: A Comprehensive Review
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Study Quality Assessment
3. Rapid Maxillary Expansion
3.1. Indications and Patient Selection
3.2. The Mechanism of Action
3.3. Clinical Outcomes and Evidence
3.4. Limitations and Considerations
4. Surgically Assisted Rapid Palatal Expansion
4.1. Indications for Surgical Intervention
4.2. Mechanism of Action
4.3. Clinical Outcomes and Evidence
4.4. Limitations and Considerations
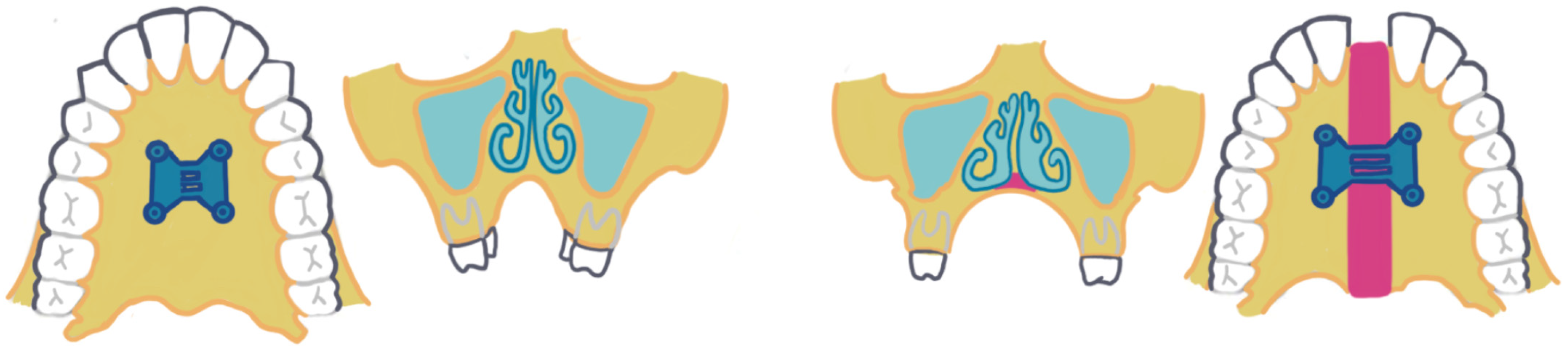
5. Mini-Screw-Assisted Rapid Palatal Expansion
5.1. Indications
5.1.1. Advantages over RME
5.1.2. Patient Selection
Influence of Suture Ossification Stage in Young Adults
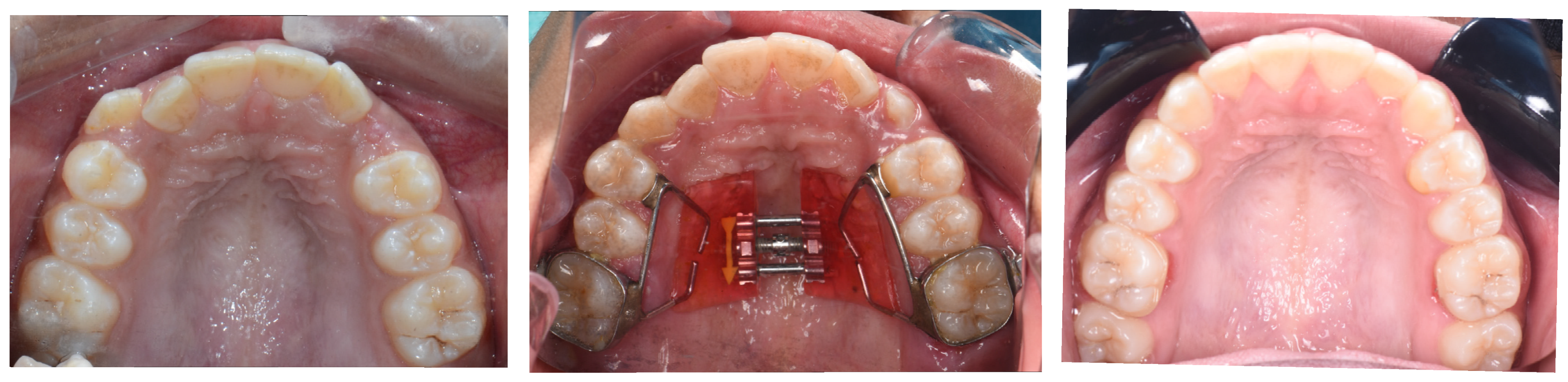
5.2. MARPE Step by Step
5.2.1. Appliance Fabrication
5.2.2. Appliance Fitting and Mini-Screw Insertion
5.2.3. Activation Protocol
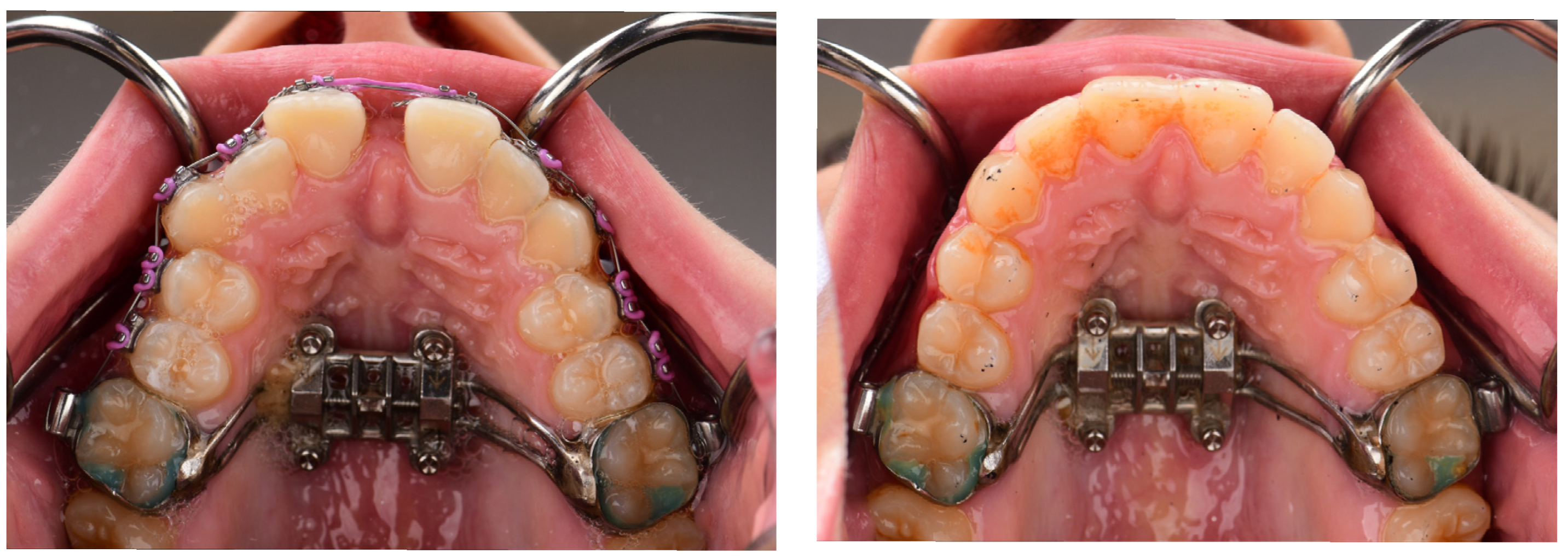
5.2.4. Monitoring and Maintenance
5.2.5. Retention Phase
5.3. Mechanism of Action
5.3.1. Airway Volume Changes in Adults
5.3.2. Systematic Review on Upper Airway Volume
5.3.3. Immediate vs. Long-Term Airway Changes (11–15-Year-Olds)
5.3.4. Tooth-Borne vs. Tooth-Bone-Borne RME in Growing Patients
5.4. Clinical Outcomes and Evidence
Impact of MARPE on Adult OSA and QOL
5.5. Limitations and Considerations
- Complications and Side Effects
5.5.1. Patient Related Factors
- Skeletal maturity, age, and suture ossification: Non-surgical expansion of the maxilla using orthopedic appliances relies on the mid-palatal maturation stage to assess treatment success. The predictability of suture separation declines with age, even when using TADs [27]. Obliteration of the mid-palatal suture varies amongst individuals; hence, determining the precise timing remains challenging [25]. In adults and late adolescents, the skeletal expansion associated with MARPE is limited due to mid-palatal suture interdigitation [31]. Previous research suggests that chronological age is a key factor influencing the success of maxillary expansion, as the maturation and fusion of the mid-palatal and surrounding circummaxillary sutures progress with age [25]. Maturation of the mid-palatal suture begins posteriorly, exhibiting individual variability in interdigitation, obliteration, and ossification patterns [25].The timing of complete ossification may not respond directly to the chronological age [25]. Hence, suture maturation could be an alternative method to assess eligibility to achieve orthopedic maxillary expansion [25]. Although histological examination remains the most definitive method for evaluation of midpalatal suture maturation, it is not feasible in living patients [25]. Consequently, conventional computed tomography (CT) imaging falls short of providing a reliable microscopic-level assessment of suture obliteration, highlighting the challenge in accurately predicting the optimal timing for orthopedic expansion [25].A prospective study examining age-dependent effects on MARPE revealed that expansion could be achieved with greater ease in patients younger than 20 years in comparison to those aged 20 years and above [24]. As chronological age increases, skeletal-to-screw expansion ratio declines [24]. In this study, patients were divided into four groups based on age: early adolescents (11–14 years), late adolescents (15–19 years), young adults (20–24), and old adults (25–34.1 years), with suture separation success rates of 100%, 100%, 88.2%, and 85.7%, respectively [24]. In this study, two young males failed to demonstrate suture separation, which could be linked to the marked acceleration in suture obliteration after the age of 20 [24].In a retrospective study, 31% of patients did not achieve sufficient expansion; hence, a second attempt was required, in which 38% failed to achieve adequate expansion [26]. Thus, patients should be made aware of the potential of treatment failure. In this study, most failures were in patients above 25 years of age. Among patients with failures to achieve successful suture separation, males accounted for 67.7% of failures, while females accounted for 32.2% [26]. Additional treatment may be necessary if the suture split fails, and causes of failure may be due to failure of bone integration with micro-implants, osteoporosis, poor oral hygiene, tobacco consumption, excessive alcohol intake, and inadequate follow-up [26].
- Gender differences: A retrospective study examining suture separation success rates amongst the genders revealed a 61.05% success in males and a 94.17% success in females [25]. This suggests that gender may play a role in determining the success of suture separation and, ultimately, MARPEs’ success in achieving orthopedic expansion and managing OSA. The success of suture separation amongst genders, particularly in 21–25 year olds, was examined, and it was determined that the odds of suture separation were 22-fold greater in females than in males. The study revealed that participants older than 15 years experienced a decline in success rates, reaching a success rate of 53.85% in males and 92.59% in females, with an overall rate of 73.58% [25]. The success rate of females above 15 years exceeded that of males within every age subgroup [25]. In participants above 20 years of age, a statistically significant association was found between gender and successful suture separation [25]. The likelihood of suture non-separation with progressing age had a statistically significant increase in males, and success was not achieved in any male patient aged over 30 years [25]. This may be linked to stiffness of the skeletal complex and maturation in the craniofacial region [25]. Hence, treatment, especially for males, at a young age is associated with more successful outcomes, including skeletal expansion.
- Bone density: Patient-related factors such as palatal bone morphology may influence MARPE’s outcomes [30]. Increased palatal cortical bone thickness and flatter palatal planes have been associated with enhanced stability [30]. These findings underscore the need for careful case selection to maximize treatment success.
- Compliance: Patient compliance plays a critical role in the success of MARPE treatment. Consistent appliance activation according to the orthodontist’s instructions is essential to achieving timely and controlled suture separation. Poor compliance can result in asymmetrical expansion or undercorrection. Compliance with follow-up appointments is equally important, particularly during the retention phase, where premature appliance removal may increase the risk of relapse. Therefore, evaluating the patient’s willingness to adhere to treatment protocols should be an integral part of case selection and treatment planning.
- Oral Hygiene: It is challenging for patients to maintain good oral hygiene; therefore, the most common complication is inflammation around the appliance, which is managed with chlorhexidine rinses along with oral hygiene instructions if mild [26]. If the inflammation was severe, premature removal and antibiotics may be required [26]. The design of the appliance can be altered to prevent palatal tissue compression and facilitate better cleaning [26]. This shows the important role of good oral hygiene in achieving treatment success.
5.5.2. Non-Patient Related Factors
- Appliance design considerations: Numerous designs of the MARPE appliance exist. There are variations based on the jackscrews’ type, position, and the size of the mini-implants. The biomechanical design of the MARPE appliance influences its skeletal impact and subsequent airway response. MARPE can be categorized into three groups based on method of anchorage: bone-borne (BB), TBB, and tissue-bone-borne [24].The systematic review by Abu Arqub et al. [29] included studies with TBB and BB expanders. TBB appliances were associated with a statistically significant improvement in airflow of the nasal cavity and reduction in nasal resistance [29]. However, a major limitation of the systematic review is that it only examined three studies. Beyond the choice of anchorage method, the configuration and number of mini-implants may also influence skeletal expansion outcomes. Superior skeletal expansion is achieved by using a MARPE with four mini-screws in comparison to two mini-screws [24]. In adult patients with OSA, achieving mid-palatal suture separation is a prerequisite for skeletal expansion. While Choi et al. [34] showed that longer mini-screws resulted in greater skeletal expansion, the study also noted that longer screw length alone did not guarantee successful suture separation. Thus, successful suture separation is an important consideration in OSA patients who may require maximal skeletal change to achieve meaningful airway improvements.In addition to the anchorage method and the number of mini-implants, anatomical constraints must also be considered when selecting the appliance. Patients with a transverse deficiency greater than 8 mm often present a challenge when selecting an appropriate expander. They may have inadequate palatal width to place a maxillary expander greater than 8 mm; hence, a second expander might be required for patients with a narrow maxillary arch [24].
- Retention phase and long-term stability: One consideration when interpreting MARPE’s effects on airway volume is the role of the retention phase. Anéris et al. [23] reported that the upper airway volume continued to increase for up to 4 months post-expansion, suggesting that retention may play a key role in achieving full skeletal and soft tissue adaptation, regardless of suture ossification level. Therefore, appropriate retention protocols should be put in place to ensure maximum benefit. Figure 3. MARPE expander before and after diastema closure and retention of the MARPE to ensure maximum benefit.
- Variability in response: Expansion of the maxilla may improve AHI scores since widening of the nasal cavity may result in a reduction in airflow resistance [3]. The expansion also allows the tongue to sit in a more forward position, which helps stretch the soft palate muscles and enhances their tone and functional activity [3]. Due to the multifactorial nature of OSA, the therapeutic response to MARPE may vary significantly among individuals [3]. It is suggested that patients with greater pharyngeal obstructions, particularly at the level of the nasopharynx, are more likely to experience notable improvements following expansion [3]. These findings highlight the importance of individualized assessment in predicting outcomes, as anatomical site and severity of airway obstruction may significantly influence the therapeutic response to MARPE.
6. Distraction Osteogenesis Maxillary Expansion
6.1. Indications and Patient Selection
6.2. Step-by-Step Technique of DOME
6.2.1. Expander Installation
6.2.2. DOME Surgery
6.2.3. Expander Activation
6.2.4. Orthodontic Treatment
6.2.5. Bone Consolidation Phase
6.3. Mechanism of Action
6.3.1. Structural Widening and Airflow Resistance
6.3.2. Functional and Pressure-Based Changes
6.3.3. Stability and Tongue Position
6.3.4. Radiographic Findings and Targeted Areas of Expansion
6.4. Clinical Outcomes and Evidence
6.4.1. Improvements in AHI and Nasal Dimensions
6.4.2. Airway Patency and Breathing Efficiency
6.4.3. Objective and Subjective Treatment Outcomes
6.4.4. Consistency Across Studies
6.4.5. Anatomical Variability and Predictors of Efficacy
6.4.6. Measurement of Treatment Success
Objective Measures
- AHI
- Quantifies the number of apneic and hypopneic events per hour of sleep used to assess OSA severity [38].
- 2.
- Oxygen Saturations
- Measures mean and minimum nocturnal oxygen levels to reflect hypoxemia severity.
- 3.
- ODI
- Captures the frequency of oxygen desaturation events per hour of sleep, indicating intermittent hypoxemia [39].
- 4.
- Airflow Velocity
- Evaluates the speed of airflow during respiration to assess improvements in airway patency after expansion.
- 5.
- Nasal Airflow Resistance
- Nasal airflow resistance is the pressure difference between the nostrils and the back of the nasal cavity, expressed in pascals, divided by the airflow rate in millimeters per second [40]. It measures the resistance to airflow through the nasal passages, indicating how well expansion relieves upper airway obstruction.
- 6.
- REM Sleep percentage
- Assesses the proportion of sleep spent in REM, which can reflect improved sleep quality and architecture post-treatment.
Subjective Measures
- 1.
- ESS
- 2.
- QSQ
- 3.
- NOSE Score
- Patient-reported measure of nasal obstruction severity and its effect on QoL [42].
6.5. Limitations, Risks, and Considerations
6.5.1. Potential Risks and Complications
6.5.2. Variability in Treatment Response
6.5.3. Postoperative Considerations and Counselling
6.6. Summary of Maxillary Expansion Techniques
7. Age-Specific Clinical Protocols for Maxillary Expansion
7.1. Childhood and Early Adolescence Protocols (6–11 Years)
- Childhood (6–9 years): Early Mixed Dentition: The recommended appliance for this age group is RPE since expansion is the easiest during this age due to minimal resistance from the mid-palatal suture [43]. The direction of nasomaxillary complex growth is primarily horizontal at this stage, and the response to expansion is favorable [43]. RPE is usually effective; however, if greater skeletal change is needed, TADs may be added for anchorage [43]. This is a useful treatment modality for children with narrow arches or airway concerns, especially those with adenotonsillar hypertrophy [43].
- Early Adolescence (10–11 years): Late Mixed Dentition: At this stage, transverse maxillary growth is largely complete, and further maxillary development occurs primarily in the vertical direction [43]. However, if the nasomaxillary complex still requires forward growth, MARPE may be a suitable option, and it can be used in conjunction with facemask therapy [43]. This allows forces from the mini-implants to be applied to the nasomaxillary complex, allowing forward midface skeletal development [43].
7.2. Middle Adolescence Protocols (12–15 Years)
7.3. Late Adolescence Protocols (16–18 Years)
7.4. Young Adults Protocols (19–25 Years)
7.5. Adult Protocols (>25 Years)
8. Discussion
8.1. How Maxillary Expansion Complements Other OSA Treatments
8.2. Limitations of the Review
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSA | Obstructive Sleep Apnea |
| QoL | Quality of Life |
| RME | Rapid Maxillary Expansion |
| MARPE | Mini-screw-Assisted Rapid Palatal Expansion |
| SARPE | Surgically-Assisted Rapid Palatal Expansion |
| DOME | Distraction Osteogenesis Maxillary Expansion |
| NC | Neck Circumference |
| CBCT | Cone-beam Computed Tomography |
| AHI | Apnea-hypopnea Index |
| RPE | Rapid Palatal Expander |
| TB | Tooth-borne |
| TBB | Tooth-bone-borne |
| BB | Bone-borne |
| TAD | Temporary Anchorage Device |
| ESS | Epworth Sleepiness Scale |
| QSQ | Quebec Sleep Questionnaire |
| CT | Computed Tomography |
| BMI | Body Mass Index |
| ODI | Oxygen Desaturation Index |
| NOSE | Nasal Obstruction Symptom Evaluation |
| REM | Rapid Eye Movement |
| SBD | Sleep Disordered Breathing |
| PAP | Positive Airway Pressure |
| OAT | Oral Appliance Therapy |
| MAA | Mandibular Advancement Appliances |
| TRD | Tongue Retaining Devices |
| MMA | Maxillomandibular Advancement |
References
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, D.P.; Moschik, C.E.; Dominguez-Mompell, R.; Jaria, E.; Sant’aNna, E.F.; Moon, W. Mini-implant assisted rapid palatal expansion (MARPE) effects on adult obstructive sleep apnea (OSA) and quality of life: A multi-center prospective controlled trial. Prog. Orthod. 2022, 23, 1–11. [Google Scholar] [CrossRef]
- Batool-Anwar, S.; Goodwin, J.L.; Kushida, C.A.; Walsh, J.A.; Simon, R.D.; Nichols, D.A.; Quan, S.F. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA). J. Sleep Res. 2016, 25, 731–738. [Google Scholar] [CrossRef]
- Chang, H.-P.; Chen, Y.-F.; Du, J.-K. Obstructive sleep apnea treatment in adults. Kaohsiung J. Med. Sci. 2020, 36, 7–12. [Google Scholar] [CrossRef]
- Santana, D.M.; Nogueira, V.S.; Lima, S.A.; Fernandes, L.P.; Weber, S.A. The effect of rapid maxillary expansion in children: A meta-analysis. Braz. J. Otorhinolaryngol. 2022, 88, 907–916. [Google Scholar] [CrossRef]
- Liu, S.Y.; Guilleminault, C.; Huon, L.; Yoon, A. Distraction Osteogenesis Maxillary Expansion (DOME) for Adult Obstructive Sleep Apnea Patients with High Arched Palate. Otolaryngol. Neck Surg. 2017, 157, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.; Kim, T.K.; Abdelwahab, M.; Nguyen, M.; Suh, H.Y.; Park, J.; Oh, H.; Pirelli, P.; Liu, S.Y.-C. What changes in maxillary morphology from distraction osteogenesis maxillary expansion (DOME) correlate with subjective and objective OSA measures? Sleep Breath. 2023, 27, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Garrocho-Rangel, A.; Rosales-Berber, M.Á.; Ballesteros-Torres, A.; Hernández-Rubio, Z.; Flores-Velázquez, J.; Yáñez-González, E.; Ruiz-Rodríguez, S.; Pozos-Guillén, A. Rapid maxillary expansion and its consequences on the nasal and oropharyngeal anatomy and breathing function of children and adolescents: An umbrella review. Int. J. Pediatr. Otorhinolaryngol. 2023, 171, 111633. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Ferrara, I.; Viapiano, F.; Netti, A.; Campanelli, M.; Buongiorno, S.; Latini, G.; Carpentiere, V.; Ciocia, A.M.; Ceci, S.; et al. Rapid Maxillary Expansion on the Adolescent Patient: Systematic Review and Case Report. Children 2022, 9, 1046. [Google Scholar] [CrossRef]
- Marañón-Vásquez, G.A.; de Andrade, A.C.D.V.; Maia, L.C.; dos Santos, R.L.; Tanaka, O.M.; Paranhos, L.R.; Oliveira, D.D.; Pithon, M.M. Effect of treatment of transverse maxillary deficiency using rapid palatal expansion on oral health-related quality of life in children: Complementary results for a controlled clinical trial. Clin. Oral Investig. 2024, 28, 1–10. [Google Scholar] [CrossRef]
- Evangelista, K.; Ferrari-Piloni, C.; Barros, L.A.N.; Avelino, M.A.G.; Soares Cevidanes, L.H.; Ruellas, A.C.D.O.; Valladares-Neto, J.; Silva, M.A.G. Three-dimensional assessment of craniofacial asymmetry in children with transverse maxillary deficiency after rapid maxillary expansion: A prospective study. Orthod. Craniofacial Res. 2020, 23, 300–312. [Google Scholar] [CrossRef]
- Pithon, M.M.; Marañón-Vásquez, G.A.; da Silva, L.P.; Coqueiro, R.d.S.; dos Santos, R.L.; Tanaka, O.M.; Maia, L.C. Effect of treatment of transverse maxillary deficiency using rapid palatal expansion on oral health-related quality of life in children: A randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2022, 161, 172–181. [Google Scholar] [CrossRef]
- Kim, K.B.; Doyle, R.E.; Araújo, E.A.; Behrents, R.G.; Oliver, D.R.; Thiesena, G. Long-term stability of maxillary and mandibular arch dimensions when using rapid palatal expansion and edgewise mechanotherapy in growing patients. Korean J. Orthod. 2019, 49, 89–96. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Kolokitha, O.-E. Comparing Airway Analysis in Two-Time Points after Rapid Palatal Expansion: A CBCT Study. J. Clin. Med. 2023, 12, 4686. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, T.; Angelopoulos, C.; Papadopoulos, M.A.; Kolokitha, O.-E. Three-Dimensional Cone-Beam Computed Tomography Evaluation of Changes in Naso-Maxillary Complex Associated with Rapid Palatal Expansion. Diagnostics 2023, 13, 1322. [Google Scholar] [CrossRef]
- Giudice, A.L.; Spinuzza, P.; Rustico, L.; Messina, G.; Nucera, R. Short-term treatment effects produced by rapid maxillary expansion evaluated with computed tomography: A systematic review with meta-analysis. Korean J. Orthod. 2020, 50, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.; Junior, O.H.; Piccoli, V.; da Rosa, B.; de Oliveira, R.; de Menezes, L. Effects of minimally invasive surgical and miniscrew-assisted rapid palatal expansion (MISMARPE) on the nasal cavity and upper airway: A comparative cohort study. Int. J. Oral Maxillofac. Surg. 2024, 53, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Sverzut, C.E.; Trivellato, A.E.; Saraiva, M.C.P.; Nguyen, T.T. Surgically assisted rapid palatal expansion (SARPE): Three-dimensional superimposition on cranial base. Clin. Oral Investig. 2022, 26, 3885–3897. [Google Scholar] [CrossRef]
- Alagoz, E.; Unver, T.; Seker, E.D.; Kurt, G.; Senturk, E.; Ozdem, A.; Dolanmaz, D. Evaluating the changes in nasal airway volume and nasal airflow after surgically assisted rapid maxillary expansion. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 533–542. [Google Scholar] [CrossRef]
- Vidalón, J.A.; Loú-Gómez, I.; Quiñe, A.; Diaz, K.T.; Duran, C.L.; Lagravère, M.O. Periodontal effects of maxillary expansion in adults using non-surgical expanders with skeletal anchorage vs. surgically assisted maxillary expansion: A systematic review. Head Face Med. 2021, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Przywitowski, S.; Golusiński, P.; Olejnik, A.; Zawiślak, E. Complications of Surgically Assisted Rapid Maxillary/Palatal Expansion (SARME/SARPE)—A Retrospective Analysis of 185 Cases Treated at a Single Center. J. Clin. Med. 2024, 13, 2053. [Google Scholar] [CrossRef]
- Anéris, F.F.; El Haje, O.; Rosário, H.D.; de Menezes, C.C.; Franzini, C.M.; Custodio, W. The effects of miniscrew-assisted rapid palatal expansion on the upper airway of adults with midpalatal suture in the last two degrees of ossification. J. World Fed. Orthod. 2023, 12, 150–155. [Google Scholar] [CrossRef]
- Jia, H.; Zhuang, L.; Zhang, N.; Bian, Y.; Li, S. Age-dependent effects of transverse maxillary deficiency treated by microimplant-assisted rapid palatal expansion: A prospective cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2022, 161, 557–573. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Choi, S.-H.; Chung, C.J.; Lee, K.-J. The success and effectiveness of miniscrew-assisted rapid palatal expansion are age- and sex-dependent. Clin. Oral Investig. 2021, 26, 2993–3003. [Google Scholar] [CrossRef]
- Yoon, A.; Payne, J.; Suh, H.; Phi, L.; Chan, A.; Oh, H. A retrospective analysis of the complications associated with miniscrew-assisted rapid palatal expansion. AJO-DO Clin. Companion 2022, 2, 423–430. [Google Scholar] [CrossRef]
- Yoon, A.; Guilleminault, C.; Zaghi, S.; Liu, S.Y.-C. Distraction Osteogenesis Maxillary Expansion (DOME) for adult obstructive sleep apnea patients with narrow maxilla and nasal floor. Sleep Med. 2020, 65, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Wang, D.; Kuo, C.-L.; Mu, J.; Vich, M.L.; Allareddy, V.; Tadinada, A.; Yadav, S. Long-term effects of mini-screw–assisted rapid palatal expansion on airway: A three-dimensional cone-beam computed tomography study. Angle Orthod. 2020, 91, 195–205. [Google Scholar] [CrossRef]
- Abu Arqub, S.; Mehta, S.; Iverson, M.G.; Yadav, S.; Upadhyay, M.; Almuzian, M. Does Mini Screw Assisted Rapid Palatal Expansion (MARPE) have an influence on airway and breathing in middle-aged children and adolescents? A systematic review. Int. Orthod. 2021, 19, 37–50. [Google Scholar] [CrossRef]
- Labunet, A.; Iosif, C.; Kui, A.; Vigu, A.; Sava, S. Miniscrew-Assisted Rapid Palatal Expansion: A Scoping Review of Influencing Factors, Side Effects, and Soft Tissue Alterations. Biomedicines 2024, 12, 2438. [Google Scholar] [CrossRef]
- Li, Q.; Tang, H.; Liu, X.; Luo, Q.; Jiang, Z.; Martin, D.; Guo, J. Comparison of dimensions and volume of upper airway before and after mini-implant assisted rapid maxillary expansion. Angle Orthod. 2020, 90, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhai, M.; Wang, M.; Cui, S.; Cheng, C.; Wang, J.; Wei, F. Three-Dimensional Evaluation Effects of Microimplant-Assisted Rapid Palatal Expansion on the Upper Airway Volume: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1790. [Google Scholar] [CrossRef]
- Bazargani, F.; Knode, V.; Plaksin, A.; Magnuson, A.; Ludwig, B. Three-dimensional comparison of tooth-borne and tooth-bone-borne RME appliances: A randomized controlled trial with 5-year follow-up. Eur. J. Orthod. 2023, 45, 690–702. [Google Scholar] [CrossRef]
- Choi, E.-H.A.; Lee, K.-J.; Choi, S.-H.; Jung, H.-D.; Ahn, H.-J.; Deguchi, T.; Cha, J.-Y. Skeletal and dentoalveolar effects of miniscrew-assisted rapid palatal expansion based on the length of the miniscrew: A randomized clinical trial. Angle Orthod. 2023, 93, 390–397. [Google Scholar] [CrossRef]
- Iwasaki, T.; Yoon, A.; Guilleminault, C.; Yamasaki, Y.; Liu, S.Y. How does distraction osteogenesis maxillary expansion (DOME) reduce severity of obstructive sleep apnea? Sleep Breath. 2019, 24, 287–296. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Yoon, A.; Okland, T.; Poomkonsarn, S.; Gouveia, C.; Liu, S.Y. Impact of Distraction Osteogenesis Maxillary Expansion on the Internal Nasal Valve in Obstructive Sleep Apnea. Otolaryngol. Neck Surg. 2019, 161, 362–367. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yoon, A.; Abdelwahab, M.; Yu, M.S. Feasibility of distraction osteogenesis maxillary expansion in patients with persistent nasal obstruction after septoplasty. Int. Forum Allergy Rhinol. 2021, 12, 868–871. [Google Scholar] [CrossRef]
- Alqudah, A.M.; Elwali, A.; Kupiak, B.; Hajipour, F.; Jacobson, N.; Moussavi, Z. Obstructive sleep apnea detection during wakefulness: A comprehensive methodological review. Med. Biol. Eng. Comput. 2024, 62, 1277–1311. [Google Scholar] [CrossRef]
- Blekic, N.; Bold, I.; Mettay, T.; Bruyneel, M. Impact of Desaturation Patterns versus Apnea–Hypopnea Index in the Development of Cardiovascular Comorbidities in Obstructive Sleep Apnea Patients. Nat. Sci. Sleep 2022, 14, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Moreddu, E.; Meister, L.; Philip-Alliez, C.; Triglia, J.-M.; Medale, M.; Nicollas, R. Computational Fluid Dynamics in the assessment of nasal obstruction in children. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Arab, M.; Kim, E.Y.; Vaughan, M.; Park, J.; Yoon, A. Screening sleep-disordered breathing (SDB) in the everyday dental office—Pediatric and adult patients. Semin. Orthod. 2025, 31, 490–503. [Google Scholar] [CrossRef]
- Floyd, E.M.; Ho, S.; Patel, P.; Rosenfeld, R.M.; Gordin, E. Systematic Review and Meta-analysis of Studies Evaluating Functional Rhinoplasty Outcomes with the NOSE Score. Otolaryngol. Neck Surg. 2017, 156, 809–815. [Google Scholar] [CrossRef]
- Yoon, A.; Gozal, D.; Kushida, C.; Pelayo, R.; Liu, S.; Faldu, J.; Hong, C. A roadmap of craniofacial growth modification for children with sleep-disordered breathing: A multidisciplinary proposal. Sleep 2023, 46, zsad095. [Google Scholar] [CrossRef] [PubMed]
- Alrejaye, N.S.; Al-Jahdali, H. Dentists’ role in obstructive sleep apnea: A more comprehensive review. Sleep Epidemiol. 2024, 4, 100073. [Google Scholar] [CrossRef]
- Vale, F.; Albergaria, M.; Carrilho, E.; Francisco, I.; Guimarães, A.; Caramelo, F.; Maló, L. Efficacy of Rapid Maxillary Expansion in the Treatment of Obstructive Sleep Apnea Syndrome: A Systematic Review With Meta-analysis. J. Évid. Based Dent. Pr. 2017, 17, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bamagoos, A.A.; Cistulli, P.A.; Sutherland, K.; Madronio, M.; Eckert, D.J.; Hess, L.; Edwards, B.A.; Wellman, A.; Sands, S.A. Polysomnographic Endotyping to Select Patients with Obstructive Sleep Apnea for Oral Appliances. Ann. Am. Thorac. Soc. 2019, 16, 1422–1431. [Google Scholar] [CrossRef]
| Technique | Advantages Over Other Techniques | Indications | Outcomes | Limitations/Complications | |
|---|---|---|---|---|---|
| RME | An appliance applies lateral forces to the maxilla, opening the mid-palatal suture. Skeletal expansion occurs, and bone fills the suture separation. | No surgery is required Relatively non-invasive | Children and early-middle adolescents with transverse maxillary deficiency. | Allows proper alignment of teeth. Eliminates crossbites Enhanced breathing, speech, and overall facial aesthetics. | Pain and discomfort with temporary speech or eating difficulty during expansion. Does not correct complex skeletal discrepancies and additional treatment may be required. Relapse risk if retention protocols are not followed; a retainer is required. |
| SARPE | Requires general anesthetic for the surgical intervention. Combines selective osteotomies and mechanical expansion forces. Weakens resistance areas: mid-palatal suture, lateral nasal walls, pterygoid plates. Palatal appliance is gradually activated post-surgery; the maxilla splits, and new bone fills the expansion gap. | Provides true skeletal expansion in skeletally mature patients. Reduces dental tipping and alveolar bending risks. | Skeletally mature patients with transverse maxillary deficiency. Cases where non-surgical expansion fails. Prepares space for dental alignment, orthognathic surgery, and improves smile esthetics and function. | Dependable skeletal expansion. Expands nasal airway, improves breathing and chewing function. Enhanced facial esthetics and overall QoL. | More invasive and costly. Surgical risks: infection, bleeding, nerve injury, swelling, discomfort. Risk of harm to the nasal mucosal tissues and tooth roots. Post-operative discomfort and temporary functional limits. Requires strict retention; poor compliance may lead to relapse or asymmetry. Precise planning required for the surgical process and liaising of the orthodontist and surgeon. |
| MARPE | No osteotomies required. Expander placed intraorally on basal bones under local anesthesia. Mini-implants inserted into pre-perforated palatal bone using a bur. Active expansion for 4–6 weeks, followed by a retention period. Mid-palatal suture opens with lateral displacement of the nasal walls. | Avoids dental anchorage, minimizing root resorption and periodontal defects. Greater skeletal expansion in comparison to RME. Non-surgical alternative for improving airway function in adults. | Transverse maxillary deficiency in late adolescents and young adults. Success influenced by suture ossification stage. | Reduces nasal resistance and enhances airway patency. Improves breathing during sleep. Increase in nasopharyngeal airway volume and nasal cavity volume. | Retention required to prevent relapse. Soft tissue irritation, inflammation, tenderness, or pain around the appliance during and after expansion. Risk of appliance breakage, asymmetric expansion, or minor papillary recession. Rare complications: short-term hearing loss, swelling, numbness, paresthesia, sinus infection, severe gag reflex, discoloration of teeth, possible loss of vitality, infraorbital nerve hypoesthesia. Treatment success depends on patient compliance and the stage of suture maturation; success declines with age. |
| DOME | Maxillary expander is secured with mini-implants; minimally invasive Le Fort I osteotomy under general anesthesia Expander activated 5–7 days post-surgery, daily for approximately one month. Gap heals naturally with bone regeneration. | Less invasive osteotomy compared to SARPE. Minimizes dental tipping. | Adults with OSA and a transverse maxillary deficiency. High-arched palate, minimal soft tissue excess. Cases requiring enhanced airway volume and functional improvement. Late adolescents with failed MARPE or fully mature sutures. | Expands maxilla and nasal floor, reducing airway resistance. Substantial AHI reduction and improved oxygen saturations. Enhanced REM sleep, NOSE and ESS scores and radiographic airway widening. Overall functional airway and QoL improvement. | Requires general anesthesia with surgical risks (infection, bleeding, nerve injury). Loss of vitality of maxillary incisors. Risk of oronasal fistula, sinus infection, asymmetric expansion or malunion. Temporary or permanent paresthesia of the anterior maxilla possible; 6–8 months of retention required to ensure bone fill and prevent relapse. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrumaih, R.; Alterki, A.; Qali, M. Maxillary Expansion in the Management of Obstructive Sleep Apnea: A Comprehensive Review. Dent. J. 2025, 13, 410. https://doi.org/10.3390/dj13090410
Alrumaih R, Alterki A, Qali M. Maxillary Expansion in the Management of Obstructive Sleep Apnea: A Comprehensive Review. Dentistry Journal. 2025; 13(9):410. https://doi.org/10.3390/dj13090410
Chicago/Turabian StyleAlrumaih, Roqaya, Ali Alterki, and Mohammad Qali. 2025. "Maxillary Expansion in the Management of Obstructive Sleep Apnea: A Comprehensive Review" Dentistry Journal 13, no. 9: 410. https://doi.org/10.3390/dj13090410
APA StyleAlrumaih, R., Alterki, A., & Qali, M. (2025). Maxillary Expansion in the Management of Obstructive Sleep Apnea: A Comprehensive Review. Dentistry Journal, 13(9), 410. https://doi.org/10.3390/dj13090410






