Four-Week Evaluation of the Interaction Pattern Among Saccharibacteria, Nitrate-Reducing Bacteria, and Periodontopathogens in Orthodontic Miniscrew Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
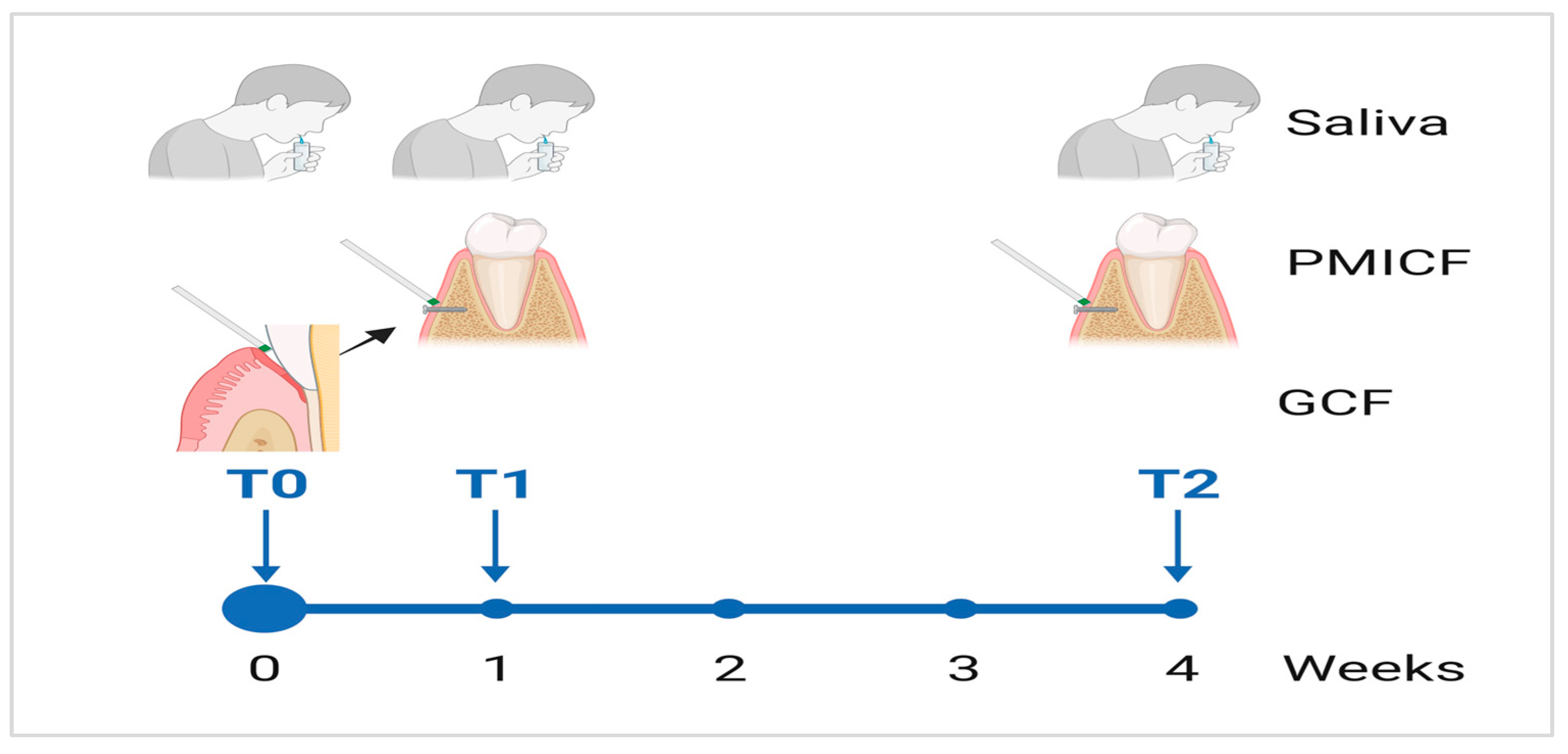
2.2. Collection of Saliva, Dental Gingival Crevicular Fluid, and Peri-Mini Implant Crevicular Fluid Samples
2.3. DNA Extraction, Sequencing and Real Time-PCR
2.4. Data Processing and Analysis
3. Results
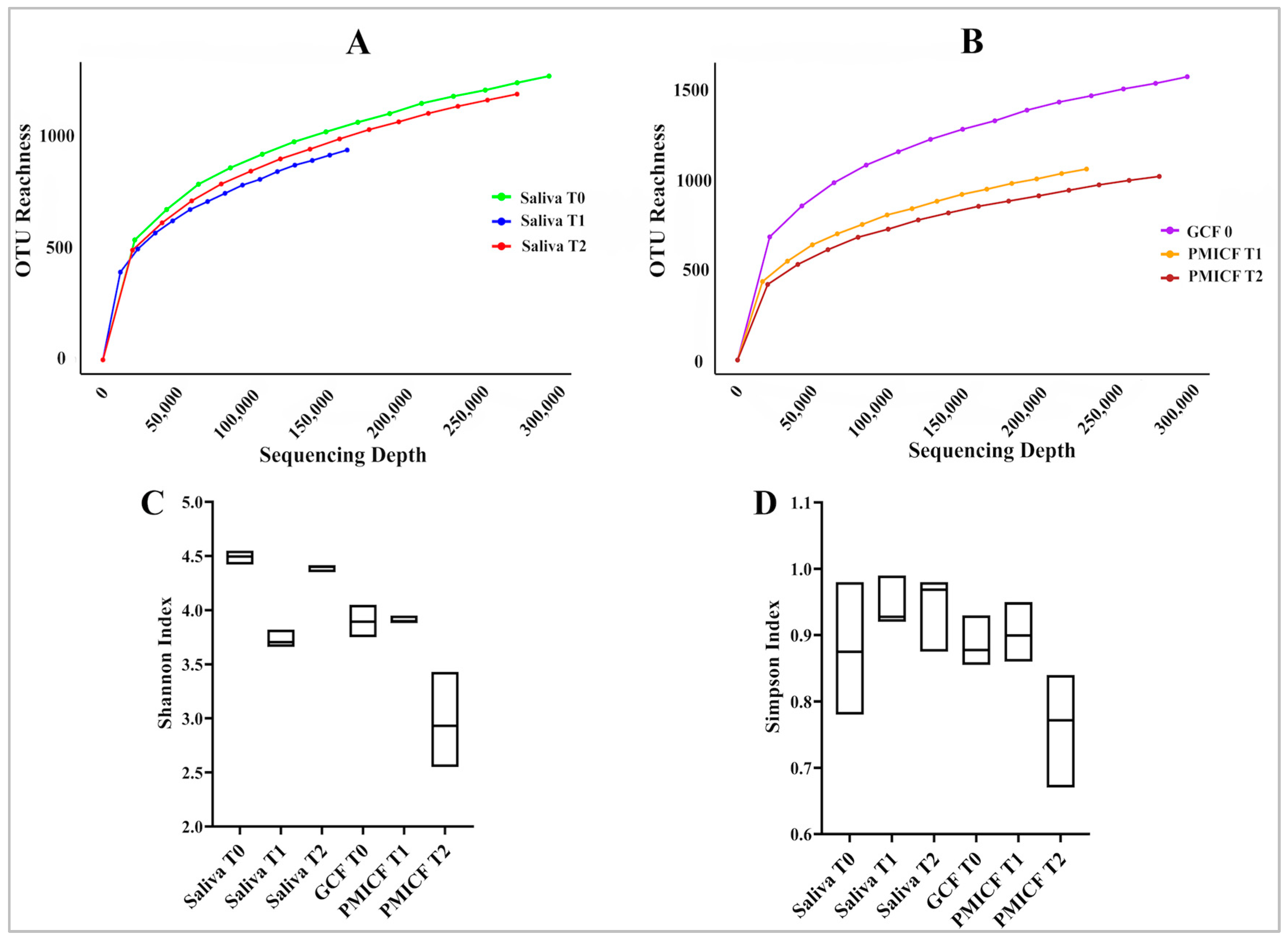
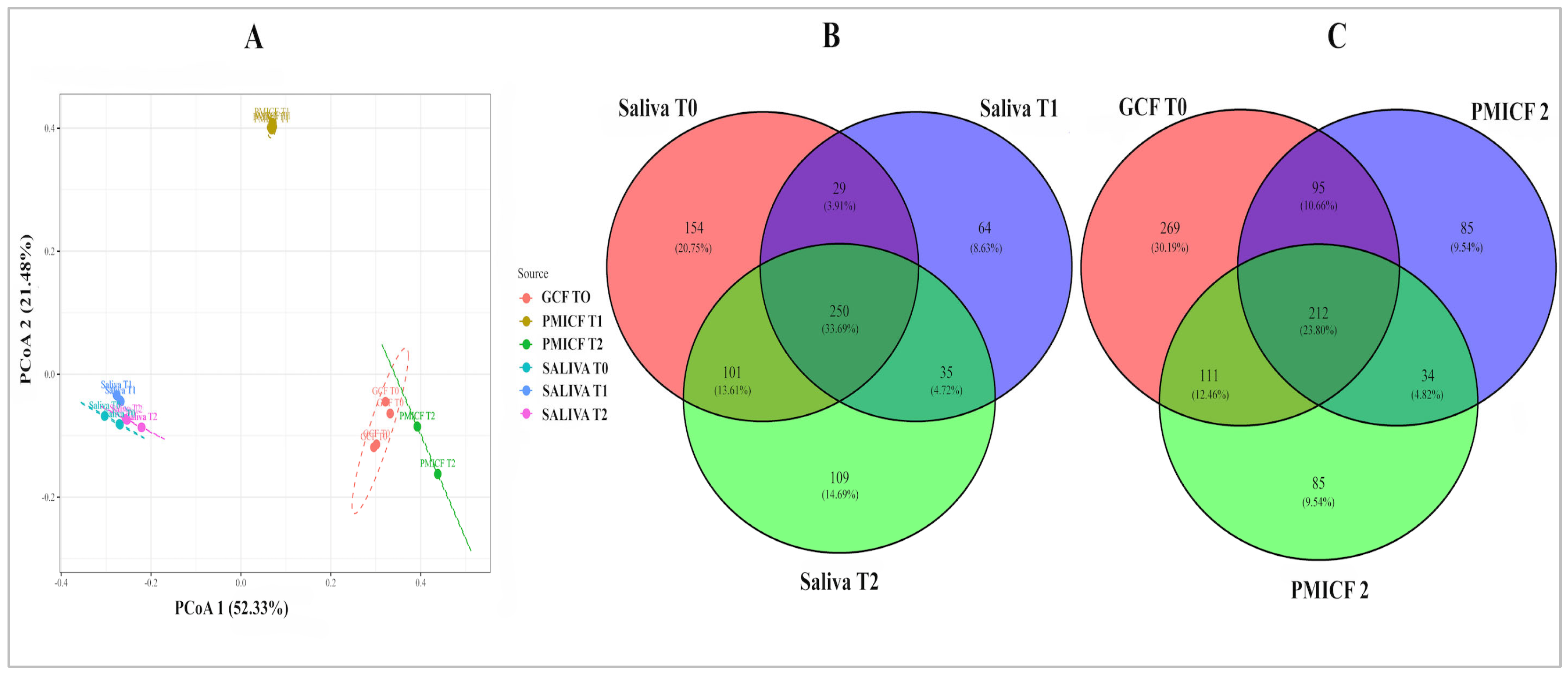
3.1. Diversity and Evolution of Microbes over Time
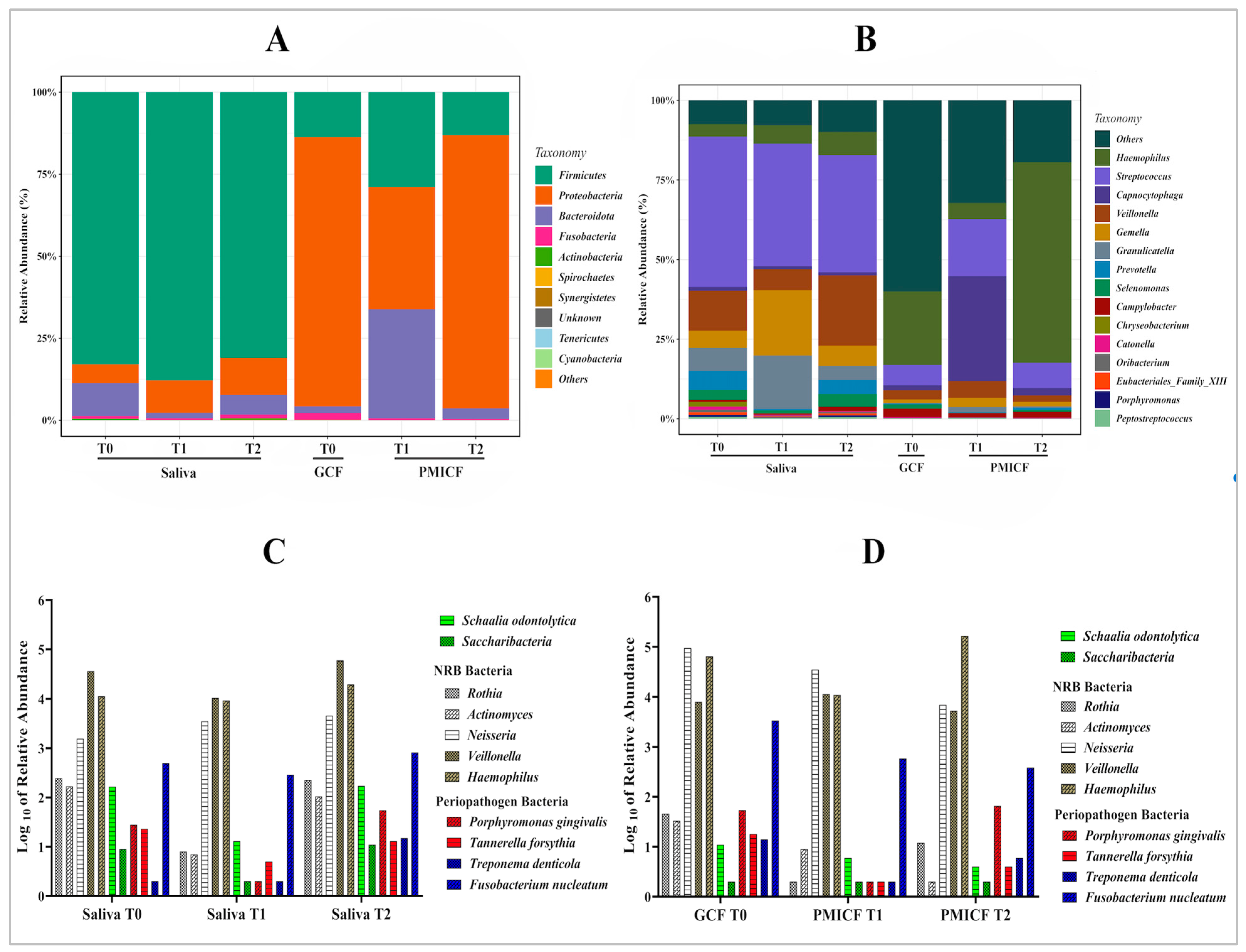
3.2. Overall Taxonomic Alteration and Microbial Composition at Phylum and Genus Level
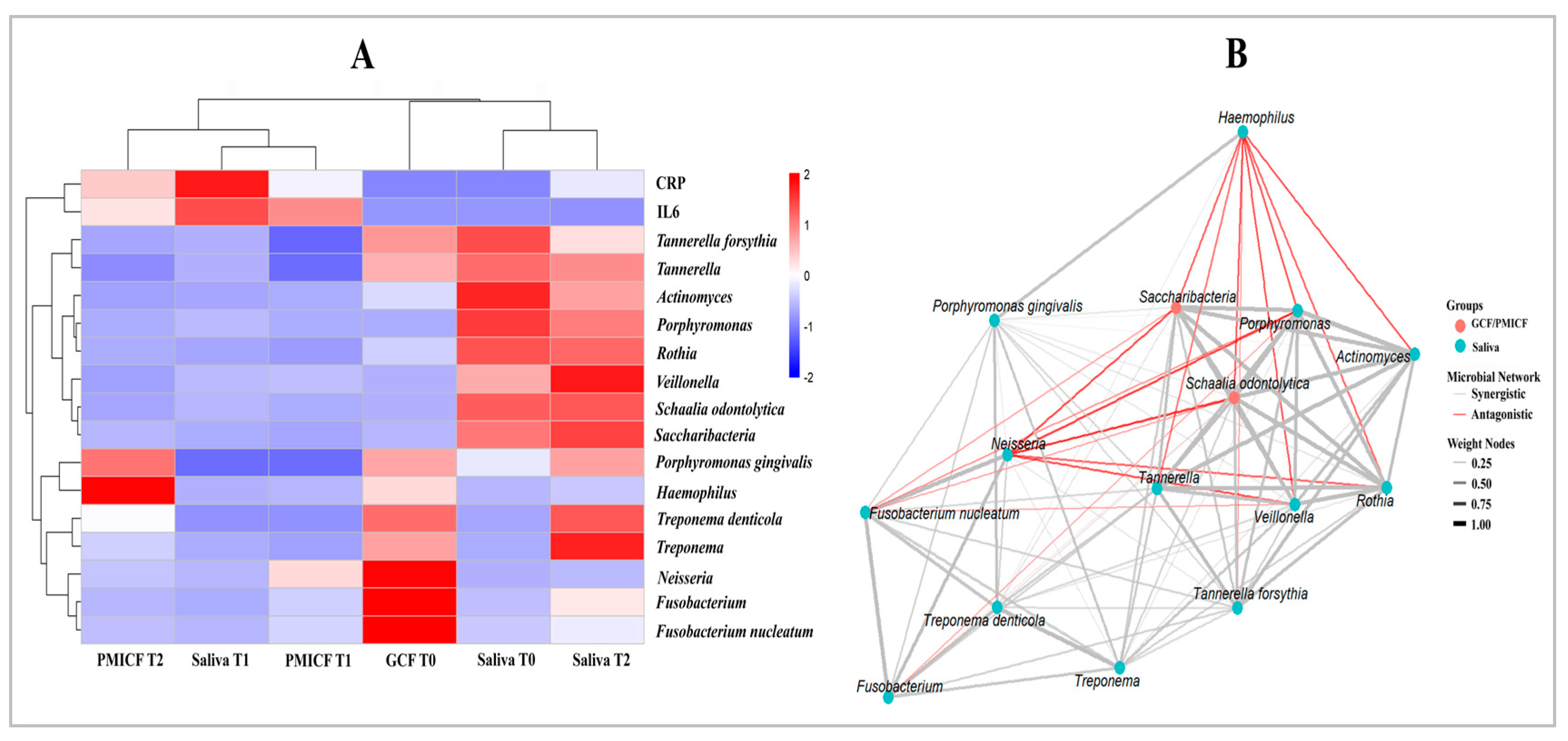
3.3. Saccharibacteria, Nitrate-Reducing Bacteria, and Periodontopathogens
3.4. Microbial Interaction and Functional Relationships
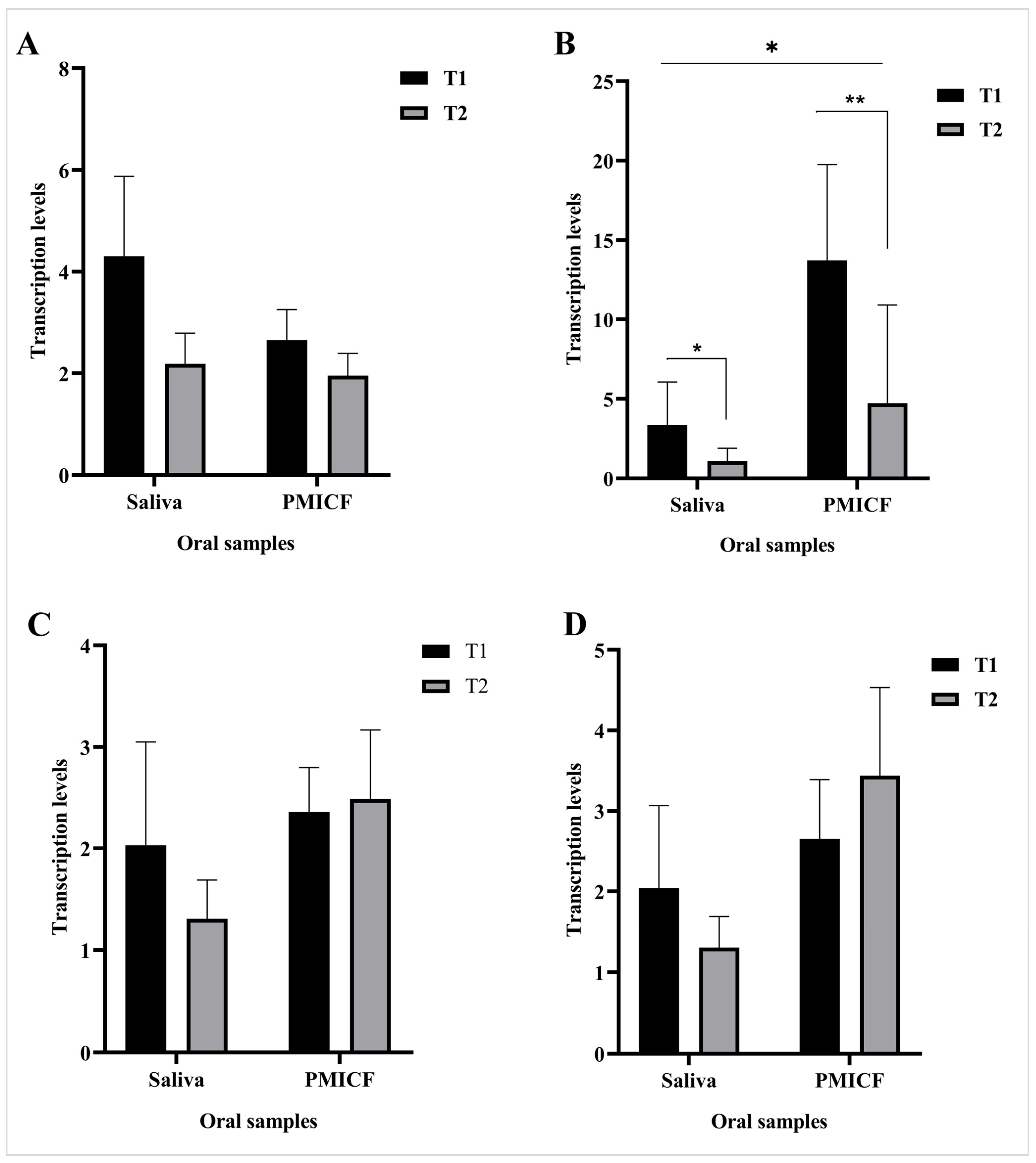
3.5. Transcription Patterns of CRP and IL-6
4. Discussion
4.1. Microbial Diversity and Stability Post-Mini Implant Placement
4.2. Stability of Saccharibacteria, Nitrate-Reducing Bacteria, and Periodontopathogens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GCF | Gingival Crevicular |
| PMICF | Peri mini-Implant Crevicular Fluids |
| NRB | Nitrate-Reducing Bacteria |
| OHIs | Oral Hygiene Index simplified |
| OUT | Operational Taxonomic Unit |
| CRP | C-reactive protein |
| IL-6 | Interleukin-6 |
References
- Schwiertz, A. Microbiota of the Human Body: Implications in Health and Disease. Preface. Adv. Exp. Med. Biol. 2016, 902, 5. [Google Scholar]
- Otake, T.; Washio, J.; Ezoe, K.; Sato, S.; Abiko, Y.; Igarashi, K.; Takahashi, N. The Effect of Environmental Factors on the Nitrate and Nitrite Metabolism of Oral Actinomyces and Schaalia Species. Mol. Oral Microbiol. 2025, 40, 104–115. [Google Scholar] [CrossRef]
- Akhi, R.; Lavrinienko, A.; Hakula, M.; Tjaderhane, L.; Hindstrom, R.; Nissinen, A.; Wang, C.; Auvinen, J.; Kullaa, A.M.; Ylostalo, P.; et al. Oral microbiome diversity associates with carotid intima media thickness in middle-aged male subjects. Commun. Med. 2025, 5, 66. [Google Scholar] [CrossRef]
- Choi, D.S.; Cha, B.K.; Jost-Brinkmann, P.G.; Lee, S.Y.; Chang, B.S.; Jang, I.; Song, J.S. Microbiologic changes in subgingival plaque after removal of fixed orthodontic appliances. Angle Orthod. 2009, 79, 1149–1155. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, D.S.; Jang, I.; Cha, B.K.; Jost-Brinkmann, P.G.; Song, J.S. Microbiologic changes in subgingival plaque before and during the early period of orthodontic treatment. Angle Orthod. 2012, 82, 254–260. [Google Scholar] [CrossRef]
- van Gastel, J.; Quirynen, M.; Teughels, W.; Coucke, W.; Carels, C. Longitudinal changes in microbiology and clinical periodontal parameters after removal of fixed orthodontic appliances. Eur. J. Orthod. 2011, 33, 15–21. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, Q.; Guo, Y.; Cui, S.; Tian, Y.; Zhou, Y.; Wang, X. Analysis of oral microbiome on temporary anchorage devices under different periodontal conditions. Prog. Orthod. 2023, 24, 42. [Google Scholar] [CrossRef]
- Sanders, N.L. Evidence-based care in orthodontics and periodontics: A review of the literature. J. Am. Dent. Assoc. 1999, 130, 521–527. [Google Scholar] [CrossRef]
- Maret, D.; Marchal-Sixou, C.; Vergnes, J.N.; Hamel, O.; Georgelin-Gurgel, M.; Van Der Sluis, L.; Sixou, M. Effect of fixed orthodontic appliances on salivary microbial parameters at 6 months: A controlled observational study. J. Appl. Oral Sci. 2014, 22, 38–43. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T.; Ericsson, I.; Lindhe, J. Spontaneous progression of experimentally induced periimplantitis. J. Clin. Periodontol. 2004, 31, 845–849. [Google Scholar] [CrossRef]
- Mishra, G.; Kharbanda, O.P.; Chaudhry, R.; Duggal, R. A pattern of microbiological colonization of orthodontic miniscrew implants. Am. J. Orthod. Dentofac. Orthop. 2023, 164, 554–566. [Google Scholar] [CrossRef]
- Kononen, E.; Paju, S.; Pussinen, P.J.; Hyvonen, M.; Di Tella, P.; Suominen-Taipale, L.; Knuuttila, M. Population-based study of salivary carriage of periodontal pathogens in adults. J. Clin. Microbiol. 2007, 45, 2446–2451. [Google Scholar] [CrossRef]
- Rosier, B.T.; Takahashi, N.; Zaura, E.; Krom, B.P.; MartInez-Espinosa, R.M.; van Breda, S.G.J.; Marsh, P.D.; Mira, A. The Importance of Nitrate Reduction for Oral Health. J. Dent. Res. 2022, 101, 887–897. [Google Scholar] [CrossRef]
- Shaiber, A.; Willis, A.D.; Delmont, T.O.; Roux, S.; Chen, L.X.; Schmid, A.C.; Yousef, M.; Watson, A.R.; Lolans, K.; Esen, O.C.; et al. Functional and genetic markers of niche partitioning among enigmatic members of the human oral microbiome. Genome Biol. 2020, 21, 292. [Google Scholar] [CrossRef]
- Jaffe, A.L.; Thomas, A.D.; He, C.; Keren, R.; Valentin-Alvarado, L.E.; Munk, P.; Bouma-Gregson, K.; Farag, I.F.; Amano, Y.; Sachdeva, R.; et al. Patterns of Gene Content and Co-occurrence Constrain the Evolutionary Path toward Animal Association in Candidate Phyla Radiation Bacteria. mBio 2021, 12, e0052121. [Google Scholar] [CrossRef]
- McLean, J.S.; Bor, B.; Kerns, K.A.; Liu, Q.; To, T.T.; Solden, L.; Hendrickson, E.L.; Wrighton, K.; Shi, W.; He, X. Acquisition and Adaptation of Ultra-small Parasitic Reduced Genome Bacteria to Mammalian Hosts. Cell Rep. 2020, 32, 107939. [Google Scholar] [CrossRef]
- Bor, B.; Poweleit, N.; Bois, J.S.; Cen, L.; Bedree, J.K.; Zhou, Z.H.; Gunsalus, R.P.; Lux, R.; McLean, J.S.; He, X.; et al. Phenotypic and Physiological Characterization of the Epibiotic Interaction Between TM7x and Its Basibiont Actinomyces. Microb. Ecol. 2016, 71, 243–255. [Google Scholar] [CrossRef]
- Naud, S.; Valles, C.; Abdillah, A.; Abou Chacra, L.; Mekhalif, F.Z.; Ibrahim, A.; Caputo, A.; Baudoin, J.P.; Gouriet, F.; Bittar, F.; et al. Preliminary landscape of Candidatus Saccharibacteria in the human microbiome. Front. Cell. Infect Microbiol. 2023, 13, 1195679. [Google Scholar] [CrossRef]
- Cross, K.L.; Campbell, J.H.; Balachandran, M.; Campbell, A.G.; Cooper, C.J.; Griffen, A.; Heaton, M.; Joshi, S.; Klingeman, D.; Leys, E.; et al. Targeted isolation and cultivation of uncultivated bacteria by reverse genomics. Nat. Biotechnol. 2019, 37, 1314–1321. [Google Scholar] [CrossRef]
- Duran-Pinedo, A.E.; Chen, T.; Teles, R.; Starr, J.R.; Wang, X.; Krishnan, K.; Frias-Lopez, J. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. 2014, 8, 1659–1672. [Google Scholar] [CrossRef]
- Hugenholtz, P.; Tyson, G.W.; Webb, R.I.; Wagner, A.M.; Blackall, L.L. Investigation of candidate division TM7, a recently recognized major lineage of the domain Bacteria with no known pure-culture representatives. Appl. Environ. Microbiol. 2001, 67, 411–419. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Chipashvili, O.; Utter, D.R.; Bedree, J.K.; Ma, Y.; Schulte, F.; Mascarin, G.; Alayyoubi, Y.; Chouhan, D.; Hardt, M.; Bidlack, F.; et al. Episymbiotic Saccharibacteria suppresses gingival inflammation and bone loss in mice through host bacterial modulation. Cell Host Microbe 2021, 29, 1649–1662.e7. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol 2009, 9, 259. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Huang, M.; Wang, M.; Ming, Y.; Chen, W.; Chen, Y.; Tang, Z.; Jia, B. From nitrate to, N.O. potential effects of nitrate-reducing bacteria on systemic health and disease. Eur. J. Med. Res. 2023, 28, 425. [Google Scholar] [CrossRef]
- Komiya, S.; Matsuo, Y.; Nakagawa, S.; Morimoto, Y.; Kryukov, K.; Okada, H.; Hirota, K. MinION, a portable long-read sequencer, enables rapid vaginal microbiota analysis in a clinical setting. BMC Med. Genom. 2022, 15, 68. [Google Scholar] [CrossRef]
- Bachtiar, B.M.; Theodorea, C.F.; Tahapary, D.L.; Astrella, C.; Bachtiar, E.W. A pilot study of red complex and three genera subgingival microbiome in periodontitis subjects with and without diabetes, evaluated by MinION platform. F1000Research 2021, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Nasir, S. Comparative evaluation of oral hygiene status by using oral hygiene index, simplified oral hygiene index, and modified oral hygiene index: Revalidation of modified oral hygiene index. J. Indian. Soc. Periodontol. 2024, 28, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Eggert, F.M.; Levin, L. Biology of teeth and implants: Host factors—Pathology, regeneration, and the role of stem cells. Quitessence Int. 2018, 49, 497–509. [Google Scholar]
- Sonnenburg, J.L.; Fischbach, M.A. Community health care: Therapeutic opportunities in the human microbiome. Sci. Transl. Med. 2011, 3, 78ps12. [Google Scholar] [CrossRef]
- Bachtiar, E.; Bachtiar, B.M.; Kusumaningrum, A.; Sunarto, H.; Soeroso, Y.; Sulijaya, B.; Apriyanti, E.; Theodorea, C.F.; Putra Pratomo, I.; Yudhistira, Y.; et al. The utility of salivary CRP and IL-6 as a non-invasive measurement evaluated in patients with COVID-19 with and without diabetes. F1000Research 2023, 12, 419. [Google Scholar] [CrossRef]
- Klein, Y.; David, E.; Pinto, N.; Khoury, Y.; Barenholz, Y.; Chaushu, S. Breaking a dogma: Orthodontic tooth movement alters systemic immunity. Prog. Orthod. 2024, 25, 38. [Google Scholar] [CrossRef]
- Shetty, N.; Patil, A.K.; Ganeshkar, S.V.; Hegde, S. Comparison of the effects of ibuprofen and acetaminophen on PGE2 levels in the GCF during orthodontic tooth movement: A human study. Prog. Orthod. 2013, 14, 6. [Google Scholar] [CrossRef]
- Groeger, S.; Zhou, Y.; Ruf, S.; Meyle, J. Pathogenic Mechanisms of Fusobacterium nucleatum on Oral Epithelial Cells. Front. Oral Health 2022, 3, 831607. [Google Scholar] [CrossRef]
- Apel, S.; Apel, C.; Morea, C.; Tortamano, A.; Dominguez, G.C.; Conrads, G. Microflora associated with successful and failed orthodontic mini-implants. Clin. Oral Implant. Res. 2009, 20, 1186–1190. [Google Scholar] [CrossRef]
- Lopez-Oliva, I.; Paropkari, A.D.; Saraswat, S.; Serban, S.; Yonel, Z.; Sharma, P.; de Pablo, P.; Raza, K.; Filer, A.; Chapple, I.; et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Paropkari, A.D.; Leblebicioglu, B.; Christian, L.M.; Kumar, P.S. Smoking, pregnancy and the subgingival microbiome. Sci. Rep. 2016, 6, 30388. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.M.; Dabdoub, S.M.; Nagaraja, H.N.; Scott, M.L.; Pamulapati, S.; Berman, M.L.; Shields, P.G.; Wewers, M.E.; Kumar, P.S. Adverse effects of electronic cigarettes on the disease-naive oral microbiome. Sci. Adv. 2020, 6, eaaz0108. [Google Scholar] [CrossRef]
- de Freitas, A.O.; Alviano, C.S.; Alviano, D.S.; Siqueira, J.F., Jr.; Nojima, L.I.; Nojima Mda, C. Microbial colonization in orthodontic mini-implants. Braz. Dent. J. 2012, 23, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Reichert, S.; Grollmitz, J.; Friebe, L.; Kohnert, M.; Hofmann, B.; Schaller, H.G.; Klawonn, F.; Shi, R. The role of Saccharibacteria (TM7) in the subginival microbiome as a predictor for secondary cardiovascular events. Int. J. Cardiol. 2021, 331, 255–261. [Google Scholar] [CrossRef]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162 2Pt A, 22–38. [Google Scholar] [CrossRef]
- Cugini, C.; Klepac-Ceraj, V.; Rackaityte, E.; Riggs, J.E.; Davey, M.E. Porphyromonas gingivalis: Keeping the pathos out of the biont. J. Oral Microbiol. 2013, 5, 19804. [Google Scholar] [CrossRef]
- Szafranski, S.P.; Deng, Z.L.; Tomasch, J.; Jarek, M.; Bhuju, S.; Meisinger, C.; Kuhnisch, J.; Sztajer, H.; Wagner-Dobler, I. Functional biomarkers for chronic periodontitis and insights into the roles of Prevotella nigrescens and Fusobacterium nucleatum: A metatranscriptome analysis. npj Biofilms Microbiomes 2015, 1, 15017. [Google Scholar] [CrossRef]
- Abusleme, L.; Hoare, A.; Hong, B.Y.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.B.; Wang, B.; Zhang, X.; Yin, Y.L.; Bai, H. Alterations of the oral microbiome in patients treated with the Invisalign system or with fixed appliances. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 633–640. [Google Scholar] [CrossRef]
- Bor, B.; Bedree, J.K.; Shi, W.; McLean, J.S.; He, X. Saccharibacteria (TM7) in the Human Oral Microbiome. J. Dent. Res. 2019, 98, 500–509. [Google Scholar] [CrossRef]
- Al-Mutairi, M.A.; Al-Salamah, L.; Nouri, L.A.; Al-Marshedy, B.S.; Al-Harbi, N.H.; Al-Harabi, E.A.; Al-Dosere, H.A.; Tashkandi, F.S.; Al-Shabib, Z.M.; Altalhi, A.M. Microbial Changes in the Periodontal Environment Due to Orthodontic Appliances: A Review. Cureus 2024, 16, e64396. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Colombo, A.P.; Boches, S.K.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.; et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J. Periodontol. 2009, 80, 1421–1432. [Google Scholar] [CrossRef]
- Lazarevic, V.; Whiteson, K.; Hernandez, D.; Francois, P.; Schrenzel, J. Study of inter- and intra-individual variations in the salivary microbiota. BMC Genom. 2010, 11, 523. [Google Scholar] [CrossRef]
- Lourenco, T.G.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P. Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036. [Google Scholar] [CrossRef]
- Lee, Y.; Hong, Y.; Kim, B.; Lee, D.; Kim, S.; Rhyu, I.C. Efficacy of salivary versus subgingival bacterial sampling for the detection and quantification of periodontal pathogens. J. Periodontal Implant. Sci. 2020, 50, 358–367. [Google Scholar] [CrossRef]
- Haririan, H.; Andrukhov, O.; Bertl, K.; Lettner, S.; Kierstein, S.; Moritz, A.; Rausch-Fan, X. Microbial analysis of subgingival plaque samples compared to that of whole saliva in patients with periodontitis. J. Periodontol. 2014, 85, 819–828. [Google Scholar] [CrossRef]
- Brinig, M.M.; Lepp, P.W.; Ouverney, C.C.; Armitage, G.C.; Relman, D.A. Prevalence of bacteria of division TM7 in human subgingival plaque and their association with disease. Appl. Environ. Microbiol. 2003, 69, 1687–1694. [Google Scholar] [CrossRef]
- Nowicki, E.M.; Shroff, R.; Singleton, J.A.; Renaud, D.E.; Wallace, D.; Drury, J.; Zirnheld, J.; Colleti, B.; Ellington, A.D.; Lamont, R.J.; et al. Microbiota and Metatranscriptome Changes Accompanying the Onset of Gingivitis. mBio 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Liu, B.; Faller, L.L.; Klitgord, N.; Mazumdar, V.; Ghodsi, M.; Sommer, D.D.; Gibbons, T.R.; Treangen, T.J.; Chang, Y.C.; Li, S.; et al. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS ONE 2012, 7, e37919. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Dongari-Bagtzoglou, A. Dysbiosis revisited: Understanding the role of the oral microbiome in the pathogenesis of gingivitis and periodontitis: A critical assessment. J. Periodontol. 2021, 92, 1071–1078. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nunez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Szalai, A.J.; van Ginkel, F.W.; Dalrymple, S.A.; Murray, R.; McGhee, J.R.; Volanakis, J.E. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J. Immunol. 1998, 160, 5294–5299. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Zhu, B.; Wei, W.; Sheng, R. The Contribution of Nitrate Dissimilation to Nitrate Consumption in narG- and napA-Containing Nitrate Reducers with Various Oxygen and Nitrate Supplies. Microbiol. Spectr. 2022, 10, e0069522. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Qu, Y.; Wang, X.; An, W.; Li, Z.; Han, Z.; Qin, L. Nitrate partially inhibits lipopolysaccharide-induced inflammation by maintaining mitochondrial function. J. Int. Med. Res. 2020, 48, 300060520902605. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlstrom, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachtiar, B.M.; Bachtiar, E.W.; Jakubovics, N.S.; Fath, T.; Sumardi, S.; Ismah, N.; Haerani, N.; Tadjoedin, F.M.; Radzi, Z. Four-Week Evaluation of the Interaction Pattern Among Saccharibacteria, Nitrate-Reducing Bacteria, and Periodontopathogens in Orthodontic Miniscrew Implants. Dent. J. 2025, 13, 405. https://doi.org/10.3390/dj13090405
Bachtiar BM, Bachtiar EW, Jakubovics NS, Fath T, Sumardi S, Ismah N, Haerani N, Tadjoedin FM, Radzi Z. Four-Week Evaluation of the Interaction Pattern Among Saccharibacteria, Nitrate-Reducing Bacteria, and Periodontopathogens in Orthodontic Miniscrew Implants. Dentistry Journal. 2025; 13(9):405. https://doi.org/10.3390/dj13090405
Chicago/Turabian StyleBachtiar, Boy M., Endang W. Bachtiar, Nicholas S. Jakubovics, Turmidzi Fath, Sariesendy Sumardi, Nada Ismah, Natalina Haerani, Fatimah Maria Tadjoedin, and Zamri Radzi. 2025. "Four-Week Evaluation of the Interaction Pattern Among Saccharibacteria, Nitrate-Reducing Bacteria, and Periodontopathogens in Orthodontic Miniscrew Implants" Dentistry Journal 13, no. 9: 405. https://doi.org/10.3390/dj13090405
APA StyleBachtiar, B. M., Bachtiar, E. W., Jakubovics, N. S., Fath, T., Sumardi, S., Ismah, N., Haerani, N., Tadjoedin, F. M., & Radzi, Z. (2025). Four-Week Evaluation of the Interaction Pattern Among Saccharibacteria, Nitrate-Reducing Bacteria, and Periodontopathogens in Orthodontic Miniscrew Implants. Dentistry Journal, 13(9), 405. https://doi.org/10.3390/dj13090405






