Precision in Facial Measurements: Comparative Analysis Between a Digital 3D Scanner and an Analog Instrument
Abstract
1. Introduction
2. Materials and Methods
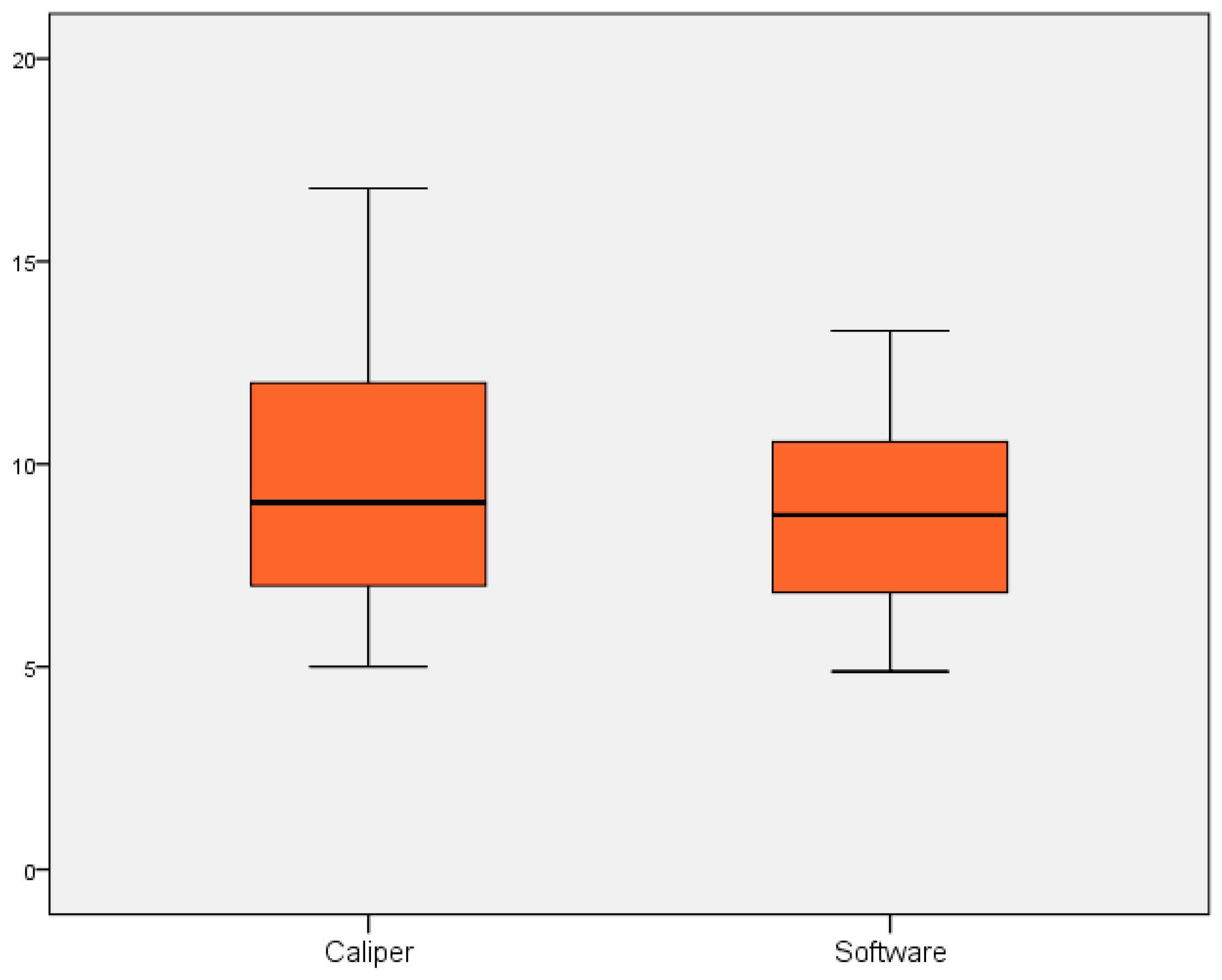
3. Results
4. Discussion
4.1. Clinical Implications
4.2. Technical Issues
4.3. Future Applications and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gahl, W.A.; Mulvihill, J.J.; Toro, C.; Markello, T.C.; Wise, A.L.; Ramoni, R.B.; Adams, D.R.; Tifft, C.J. The NIH Undiagnosed Diseases Program and Network: Applications to modern medicine. Mol. Genet. Metab. 2016, 117, 393–400. [Google Scholar] [CrossRef]
- Kesterke, M.J.; Raffensperger, Z.D.; Heike, C.L.; Cunningham, M.L.; Hecht, J.T.; Kau, C.H.; Nidey, N.L.; Moreno, L.M.; Wehby, G.L.; Marazita, M.L.; et al. Using the 3D Facial Norms Database to investigate craniofacial sexual dimorphism in healthy children, adolescents, and adults. Biol. Sex Differ. 2016, 7, 23. [Google Scholar] [CrossRef]
- Claes, P.; Liberton, D.K.; Daniels, K.; Rosana, K.M.; Quillen, E.E.; Pearson, L.N.; McEvoy, B.; Bauchet, M.; Zaidi, A.A.; Yao, W.; et al. Modeling 3D facial shape from DNA. PLoS Genet 2014, 10, e1004224. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Ferrario, V.F. Soft-tissue facial anthropometry in three dimensions: From anatomical landmarks to digital morphology in research, clinics and forensic anthropology. J. Anthropol. Sci. 2006, 84, 97–124. [Google Scholar]
- Jones, T.; Leary, S.; Atack, N.; Chawla, O.; Ness, A.; Ireland, T.; Sandy, J. Are photographs a suitable alternative to dental study casts when assessing primary surgical outcome in children born with unilateral cleft lip and palate? Eur. J. Orthod. 2016, 38, 341–344. [Google Scholar] [CrossRef][Green Version]
- Nollet, P.J.; Katsaros, C.; van ‘t Hof, M.A.; Bongaarts, C.A.; Semb, G.; Shaw, W.C.; Kuijpers-Jagtman, A.M. Photographs of study casts: An alternative medium for rating dental arch relationships in unilateral cleft lip and palate. Cleft. Palate Craniofac. J. 2004, 41, 646–650. [Google Scholar] [CrossRef]
- Baheti, M.J.; Soni, U.N.; Gharat, N.V.; Mahagaonkar, P.; Khokhani, R.; Dash, S. Intra-oral Scanners: A New Eye in Dentistry. Austin. J. Orthopade Rheumatol. 2015, 2, 1021. [Google Scholar]
- Weinberg, S.M.; Naidoo, S.; Govier, D.P.; Martin, R.A.; Kane, A.A.; Marazita, M.L. Anthropometric precision and accuracy of digital three-dimensional photogrammetry: Comparing the Genex and 3dMD imaging systems with one another and with direct anthropometry. J. Craniofac. Surg. 2006, 17, 477–483. [Google Scholar] [CrossRef]
- Hong, C.; Choi, K.; Kachroo, Y.; Kwon, T.; Nguyen, A.; McComb, R.; Moon, W. Evaluation of the 3dMDface system as a tool for soft tissue analysis. Orthod. Craniofac. Res. 2017, 20 (Suppl. 1), 119–124. [Google Scholar] [CrossRef]
- Gibelli, D.; Codari, M.; Rosati, R.; Dolci, C.; Tartaglia, G.M.; Cattaneo, C.; Sforza, C. A Quantitative Analysis of Lip Aesthetics: The Influence of Gender and Aging. Aesthetic. Plast. Surg. 2015, 39, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Codari, M.; Pucciarelli, V.; Pisoni, L.; Sforza, C. Laser scanner compared with stereophotogrammetry for measurements of area on nasal plaster casts. Br. J. Oral. Maxillofac. Surg. 2015, 53, 769–770. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Mehl, A.; Mormann, W.H.; Reich, S. Intraoral scanning systems—A current overview. Int. J. Comput. Dent. 2015, 18, 101–129. [Google Scholar]
- Alghazzawi, T.F. Advancements in CAD/CAM technology: Options for practical implementation. J. Prosthodont. Res. 2016, 60, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, E.C.P.; Fusco, N.D.S.; Carrara, C.F.C.; Bergamo, M.T.; Neto, N.L.; Cruvinel, T.; Rios, D.; Almeida, A.; Soares, S.; Machado, M.; et al. Digital Volumetric Monitoring of Palate Growth in Children With Cleft Lip and Palate. J. Craniofac. Surg. 2022, 33, e143–e145. [Google Scholar] [CrossRef] [PubMed]
- Rando, G.M.; Ambrosio, E.C.P.; Jorge, P.K.; Prado, D.Z.A.; Falzoni, M.M.M.; Carrara, C.F.C.; Soares, S.; Machado, M.; Oliveira, T.M. Anthropometric Analysis of the Dental Arches of Five-Year-Old Children With Cleft Lip and Palate. J. Craniofac. Surg. 2018, 29, 1657–1660. [Google Scholar] [CrossRef]
- Gomez-Polo, M.; Gomez-Polo, C.; Del Rio, J.; Ortega, R. Stereophotogrammetric impression making for polyoxymethylene, milled immediate partial fixed dental prostheses. J. Prosthet. Dent. 2018, 119, 506–510. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, M.; Ceron-Zapata, A.M.; Lopez-Palacio, A.M.; Mapelli, A.; Pisoni, L.; Sforza, C. Evaluation of a Three-Dimensional Stereophotogrammetric Method to Identify and Measure the Palatal Surface Area in Children With Unilateral Cleft Lip and Palate. Cleft. Palate Craniofac. J. 2016, 53, 16–21. [Google Scholar] [CrossRef]
- Penarrocha-Diago, M.; Balaguer-Marti, J.C.; Penarrocha-Oltra, D.; Balaguer-Martinez, J.F.; Penarrocha-Diago, M.; Agustin-Panadero, R. A combined digital and stereophotogrammetric technique for rehabilitation with immediate loading of complete-arch, implant-supported prostheses: A randomized controlled pilot clinical trial. J. Prosthet. Dent. 2017, 118, 596–603. [Google Scholar] [CrossRef]
- Deli, R.; Gioia, E.D.; Galantucci, L.M.; Percoco, G. Accurate facial morphologic measurements using a 3-camera photogrammetric method. J. Craniofac. Surg. 2011, 22, 54–59. [Google Scholar] [CrossRef]
- Plooij, J.M.; Maal, T.J.; Haers, P.; Borstlap, W.A.; Kuijpers-Jagtman, A.M.; Berge, S.J. Digital three-dimensional image fusion processes for planning and evaluating orthodontics and orthognathic surgery. A systematic review. Int. J. Oral. Maxillofac. Surg. 2011, 40, 341–352. [Google Scholar] [CrossRef]
- Gwilliam, J.R.; Cunningham, S.J.; Hutton, T. Reproducibility of soft tissue landmarks on three-dimensional facial scans. Eur. J. Orthod. 2006, 28, 408–415. [Google Scholar] [CrossRef]
- Menendez Lopez-Mateos, M.L.; Carreno-Carreno, J.; Palma, J.C.; Alarcon, J.A.; Menendez Lopez-Mateos, C.; Menendez-Nunez, M. Three-dimensional photographic analysis of the face in European adults from southern Spain with normal occlusion: Reference anthropometric measurements. BMC Oral Health 2019, 19, 196. [Google Scholar] [CrossRef]
- Coachman, C.; Georg, R.; Bohner, L.; Rigo, L.C.; Sesma, N. Chairside 3D digital design and trial restoration workflow. J. Prosthet. Dent. 2020, 124, 514–520. [Google Scholar] [CrossRef]
- Richert, R.; Goujat, A.; Venet, L.; Viguie, G.; Viennot, S.; Robinson, P.; Farges, J.C.; Fages, M.; Ducret, M. Intraoral Scanner Technologies: A Review to Make a Successful Impression. J. Healthc. Eng. 2017, 2017, 8427595. [Google Scholar] [CrossRef]
- Goracci, C.; Franchi, L.; Vichi, A.; Ferrari, M. Accuracy, reliability, and efficiency of intraoral scanners for full-arch impressions: A systematic review of the clinical evidence. Eur. J. Orthod. 2016, 38, 422–428. [Google Scholar] [CrossRef]
- Lawson, N.C.; Burgess, J.O. Clinicians reaping benefits of new concepts in impressioning. Compend. Contin. Educ. Dent. 2015, 36, 152–153. [Google Scholar]
- Aragon, M.L.; Pontes, L.F.; Bichara, L.M.; Flores-Mir, C.; Normando, D. Validity and reliability of intraoral scanners compared to conventional gypsum models measurements: A systematic review. Eur. J. Orthod. 2016, 38, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, R.; Lizio, A.; Cervino, G.; Fabiana, N.; Francesco, P.; Ausiello, P.; Cicciu, M. The Horizontal Root Fractures. Diagnosis, Clinical Management and Three-Year Follow-Up. Open Dent. J. 2018, 12, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, R.; Galletti, C.; Tribst, J.P.M.; Melenchón, L.P.; Matarese, M.; Miniello, A.; Cucinotta, F.; Salmeri, F. In Vivo Analysis of Intraoral Scanner Precision Using Open-Source 3D Software. Prosthesis 2022, 4, 554–563. [Google Scholar] [CrossRef]
- Amornvit, P.; Sanohkan, S. The Accuracy of Digital Face Scans Obtained from 3D Scanners: An In Vitro Study. Int. J. Environ. Res. Public Health 2019, 16, 5061. [Google Scholar] [CrossRef]
- Awad, D.; Reinert, S.; Kluba, S. Accuracy of Three-Dimensional Soft-Tissue Prediction Considering the Facial Aesthetic Units Using a Virtual Planning System in Orthognathic Surgery. J. Pers. Med. 2022, 12, 1379. [Google Scholar] [CrossRef]
- Sabri, H.; Tehranchi, A.; Sarkarat, F. 3-dimensional analysis of nasal soft tissue alterations following maxillary Lefort I advancement with and without impaction using 3D photogrammetry scanner. Oral. Maxillofac. Surg. 2024, 28, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Tartaro, G.; Nicoletti, G.F.; Fragola, R.; Lo Giudice, G.; Santagata, M. Alar cinch sutures in orthognathic surgery: Scoping review and proposal of a classification. Int. J. Oral. Maxillofac. Surg. 2022, 51, 643–650. [Google Scholar] [CrossRef]
- Krijt, L.L.; Kapetanovic, A.; Sijmons, W.J.L.; Bruggink, R.; Baan, F.; Berge, S.J.; Noverraz, R.R.M.; Xi, T.; Schols, J. What is the impact of miniscrew-assisted rapid palatal expansion on the midfacial soft tissues? A prospective three-dimensional stereophotogrammetry study. Clin. Oral. Investig. 2023, 27, 5343–5351. [Google Scholar] [CrossRef]
- Sforza, C.; Ulaj, E.; Gibelli, D.M.; Allevi, F.; Pucciarelli, V.; Tarabbia, F.; Ciprandi, D.; Dell’Aversana Orabona, G.; Dolci, C.; Biglioli, F. Three-dimensional superimposition for patients with facial palsy: An innovative method for assessing the success of facial reanimation procedures. Br. J. Oral. Maxillofac. Surg. 2018, 56, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Mohamed, A.S.; Habumugisha, J.; Guo, Y.; Zou, R.; Wang, F. A Study of the Facial Soft Tissue Morphology in Nasal- and Mouth-Breathing Patients. Int. Dent. J. 2023, 73, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Olmos, M.; Matta, R.; Buchbender, M.; Jaeckel, F.; Nobis, C.P.; Weber, M.; Kesting, M.; Lutz, R. 3D assessment of the nasolabial region in cleft models comparing an intraoral and a facial scanner to a validated baseline. Sci. Rep. 2023, 13, 12216. [Google Scholar] [CrossRef]
- Bertazzo, T.L.; D’Ornellas, M.C. Protocol for capturing 3D facial meshes for rhinoseptoplasty planning. Braz. J. Otorhinolaryngol. 2023, 89, 101289. [Google Scholar] [CrossRef]
- Lin, W.S.; Harris, B.T.; Phasuk, K.; Llop, D.R.; Morton, D. Integrating a facial scan, virtual smile design, and 3D virtual patient for treatment with CAD-CAM ceramic veneers: A clinical report. J. Prosthet. Dent. 2018, 119, 200–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, B.; Wang, F.; Wu, Y. Integrating a mouth opening assessment of virtual patients to prevent intraoperative challenges during treatment. J. Prosthet. Dent. 2023. [Google Scholar] [CrossRef]
- Natale, M.; Soardi, C.M.; Saleh, M.H.A.; Ponzi, A.; Tagliaferri, D.; Filannino, F.M.; Fontana, F.; Decker, A.; Marinotti, F.; d’Ambrosio, A.; et al. Immediate Implant Placement Using the Socket Shield Technique: Clinical, Radiographic, and Volumetric Results Using 3D Digital Techniques-A Case Series. Int. J. Periodontics Restorative Dent. 2024, 44, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gibelli, D.; Pucciarelli, V.; Poppa, P.; Cummaudo, M.; Dolci, C.; Cattaneo, C.; Sforza, C. Three-dimensional facial anatomy evaluation: Reliability of laser scanner consecutive scans procedure in comparison with stereophotogrammetry. J. Craniomaxillofac. Surg. 2018, 46, 1807–1813. [Google Scholar] [CrossRef]
- Marmulla, R.; Hassfeld, S.; Luth, T.; Muhling, J. Laser-scan-based navigation in cranio-maxillofacial surgery. J. Craniomaxillofac. Surg. 2003, 31, 267–277. [Google Scholar] [CrossRef]
- de Menezes, M.; Rosati, R.; Ferrario, V.F.; Sforza, C. Accuracy and reproducibility of a 3-dimensional stereophotogrammetric imaging system. J. Oral Maxillofac. Surg. 2010, 68, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Gibelli, D.; Pucciarelli, V.; Cappella, A.; Dolci, C.; Sforza, C. Are Portable Stereophotogrammetric Devices Reliable in Facial Imaging? A Validation Study of VECTRA H1 Device. J. Oral Maxillofac. Surg. 2018, 76, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Liberton, D.K.; Mishra, R.; Beach, M.; Raznahan, A.; Gahl, W.A.; Manoli, I.; Lee, J.S. Comparison of Three-Dimensional Surface Imaging Systems Using Landmark Analysis. J. Craniofac. Surg. 2019, 30, 1869–1872. [Google Scholar] [CrossRef]
- Puleio, F.; Tosco, V.; Pirri, R.; Simeone, M.; Monterubbianesi, R.; Lo Giudice, G.; Lo Giudice, R. Augmented Reality in Dentistry: Enhancing Precision in Clinical Procedures-A Systematic Review. Clin. Pract. 2024, 14, 2267–2283. [Google Scholar] [CrossRef]

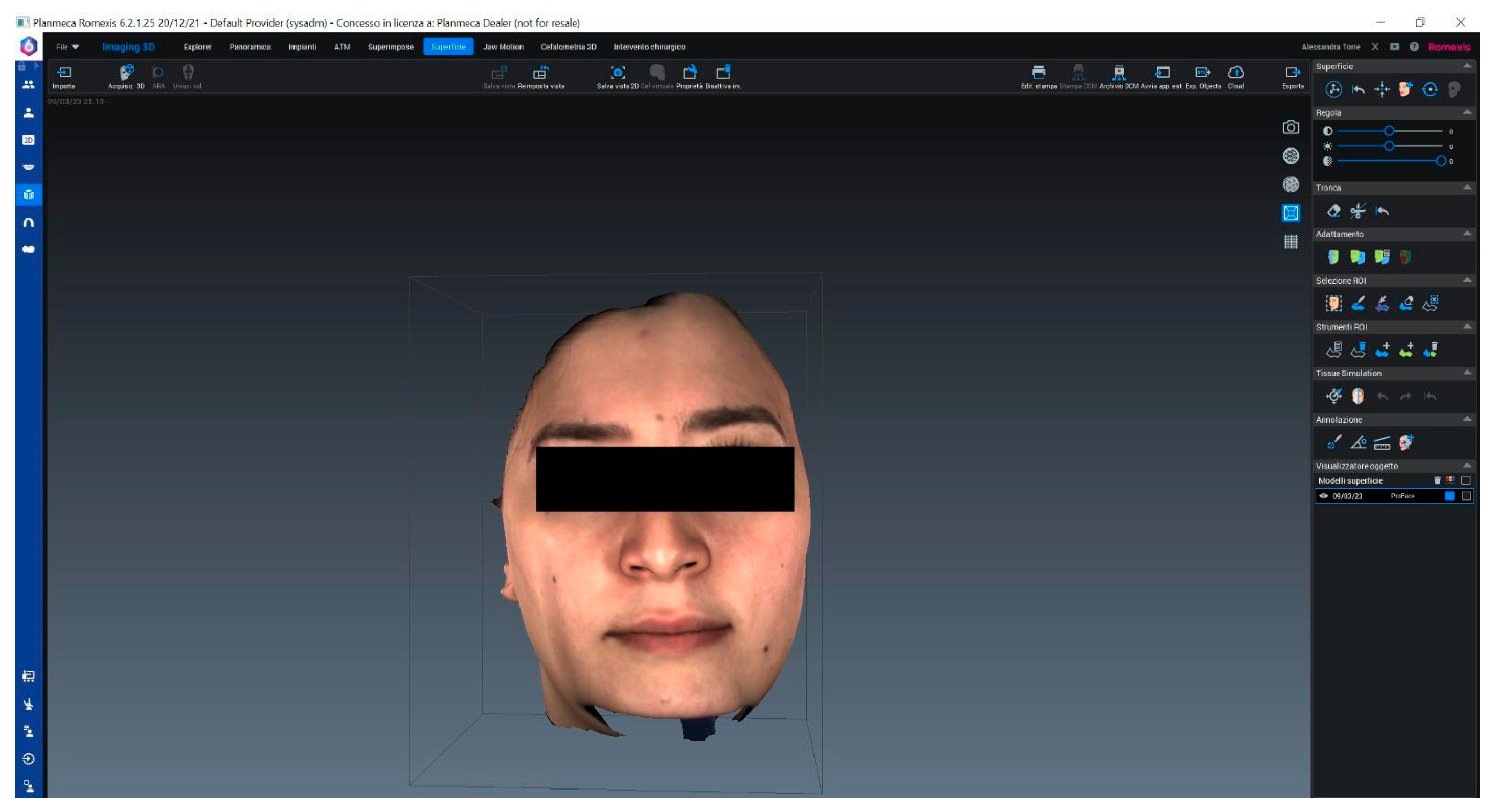
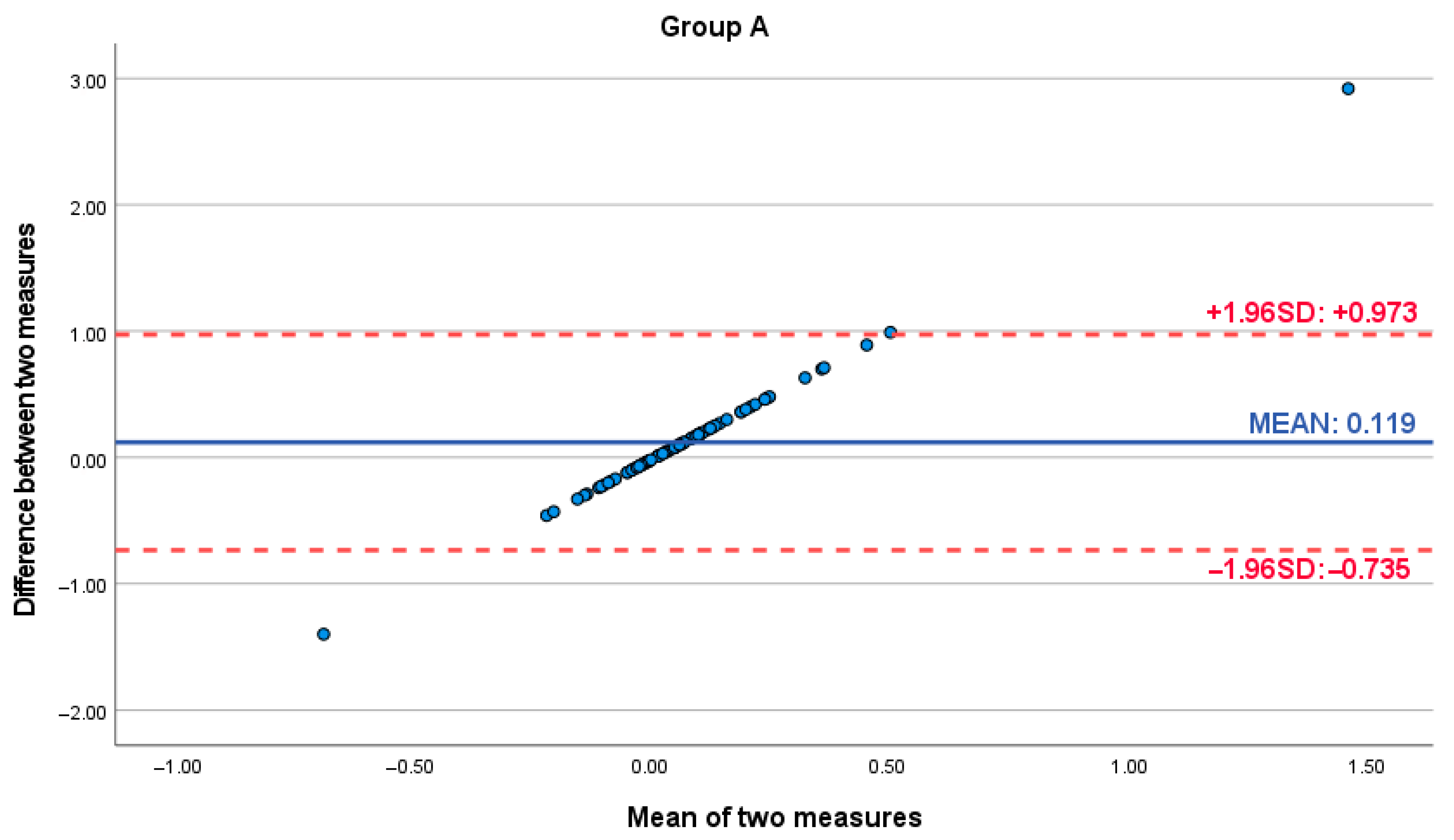
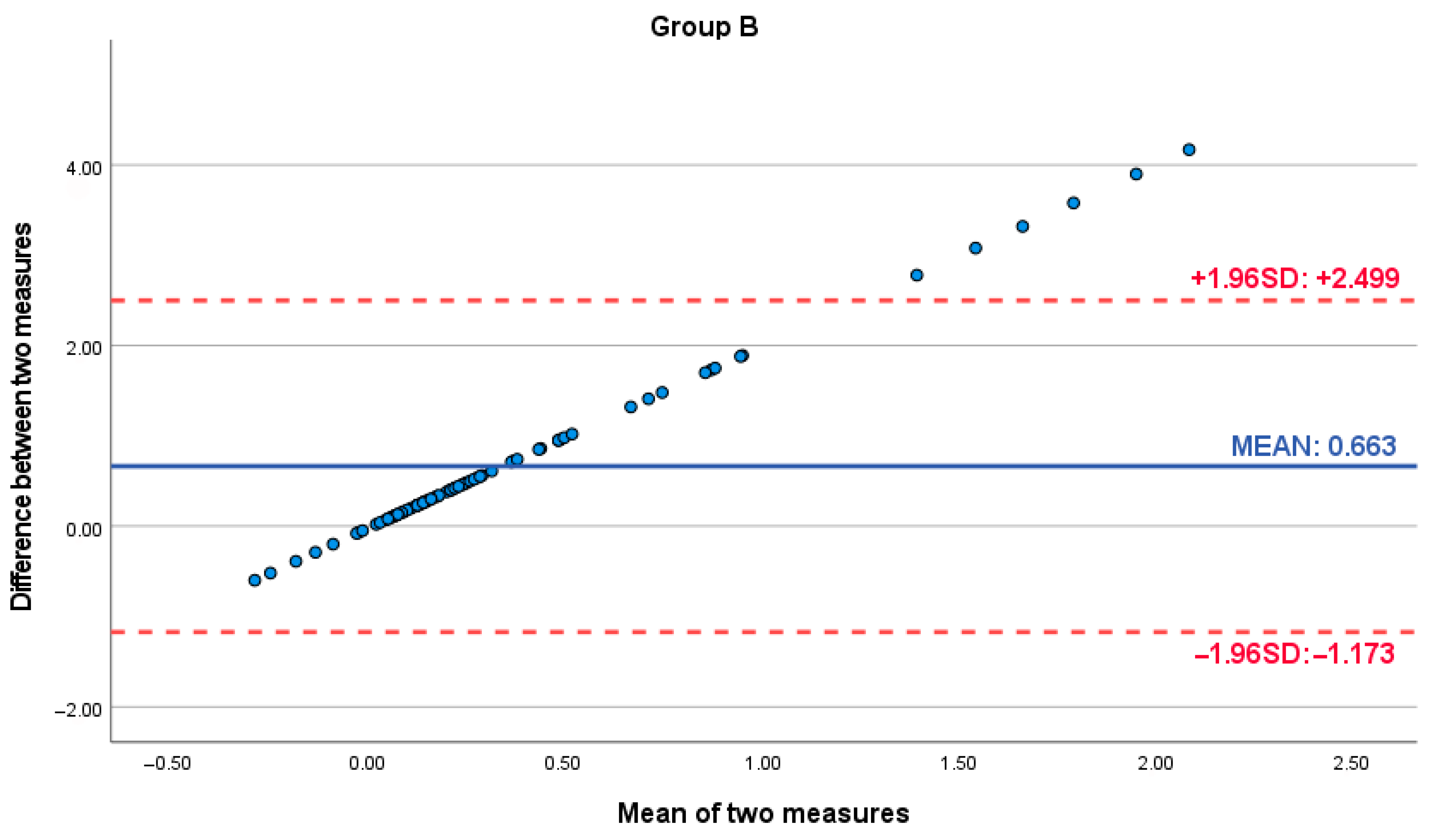
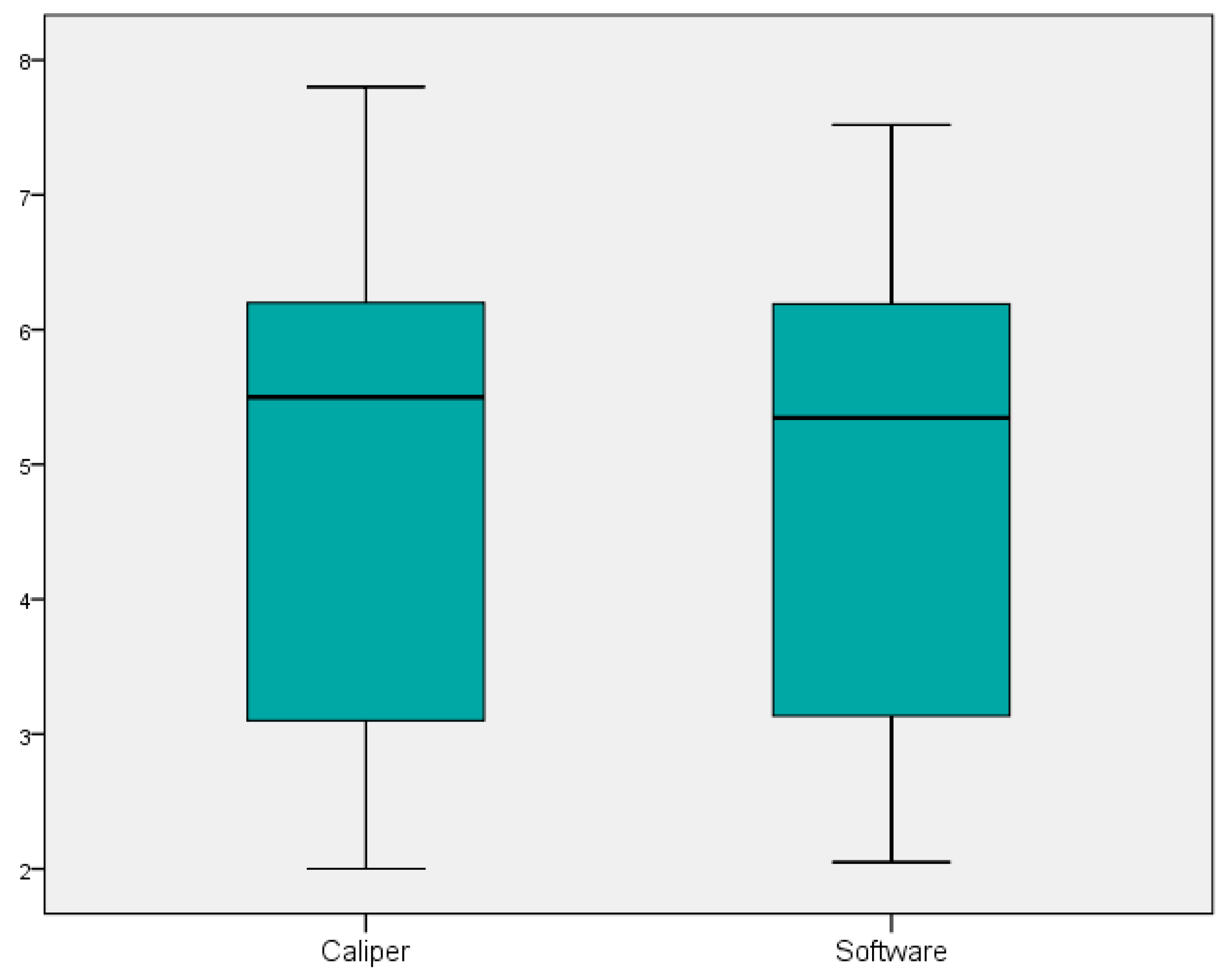
| Differences in Pairs—Group A | Paired t-Test | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean Difference | SD | 95% Confidence Interval | |||||
| Lower | Upper | |||||||
| Pair | Caliper-Software Romexis v.62 | 12 | 0.24 | 0.9 | 0.016 | 0.464 | 2.335 | 0.016 |
| Differences in Pairs—Group B | Paired t-Test | p-Value | ||||||
| N | Mean Difference | SD | 95% Confidence Interval | |||||
| Lower | Upper | |||||||
| Pair | Caliper-Software | 12 | 0.71 | 2.8 | 0.248 | 1.172 | 3.688 | 0.001 |
| Measurement Name | Caliper Mean | Caliper Std | Caliper Min | Caliper Max | Digital Mean | Digital Std | Digital Min | Digital Max |
|---|---|---|---|---|---|---|---|---|
| AB | 3.39 | 0.43 | 2.40 | 4.00 | 3.26 | 0.34 | 2.40 | 3.41 |
| AC | 7.21 | 1.14 | 6.00 | 8.20 | 6.26 | 0.50 | 6.00 | 7.31 |
| AD | 11.07 | 1.41 | 9.00 | 13.00 | 9.42 | 1.27 | 8.45 | 11.11 |
| AE | 14.04 | 1.48 | 12.00 | 16.20 | 11.05 | 1.07 | 10.30 | 12.30 |
| AF | 2.54 | 0.59 | 2.00 | 3.50 | 2.57 | 0.48 | 2.05 | 3.31 |
| AI | 6.24 | 0.19 | 6.00 | 6.50 | 6.26 | 0.27 | 6.18 | 6.54 |
| AL | 9.29 | 1.07 | 8.40 | 10.00 | 9.37 | 1.12 | 8.14 | 10.17 |
| AN | 9.14 | 0.38 | 8.50 | 10.00 | 9.25 | 0.23 | 9.05 | 9.28 |
| AP | 13.33 | 1.38 | 12.50 | 15.40 | 12.34 | 0.37 | 12.22 | 13.32 |
| CG | 3.23 | 0.34 | 3.20 | 4.00 | 3.17 | 0.38 | 3.05 | 4.08 |
| CL | 6.37 | 0.49 | 5.30 | 6.50 | 5.47 | 1.35 | 3.58 | 6.46 |
| EH | 3.01 | 0.32 | 2.20 | 3.00 | 3.05 | 0.34 | 2.27 | 3.24 |
| EL | 9.21 | 1.00 | 8.20 | 10.30 | 9.28 | 1.03 | 8.34 | 10.20 |
| EM | 6.07 | 0.42 | 5.50 | 6.50 | 6.13 | 0.34 | 5.53 | 6.49 |
| EO | 9.11 | 1.00 | 8.00 | 10.00 | 9.24 | 0.28 | 8.39 | 9.27 |
| EP | 13.27 | 2.02 | 12.00 | 17.20 | 12.37 | 0.53 | 12.13 | 13.29 |
| GM | 6.06 | 1.09 | 5.00 | 7.00 | 6.36 | 1.16 | 5.28 | 7.08 |
| IG | 6.00 | 1.08 | 5.20 | 7.00 | 6.14 | 1.00 | 5.09 | 7.50 |
| IL | 6.20 | 1.28 | 5.00 | 7.50 | 6.16 | 1.34 | 4.35 | 7.52 |
| IP | 7.03 | 1.03 | 6.40 | 8.30 | 7.09 | 0.57 | 6.06 | 8.28 |
| LM | 6.26 | 1.37 | 4.50 | 7.50 | 6.18 | 1.28 | 4.56 | 7.08 |
| LP | 6.56 | 0.23 | 6.30 | 7.00 | 6.26 | 0.40 | 6.05 | 7.27 |
| MP | 7.36 | 1.08 | 6.40 | 8.20 | 7.08 | 1.02 | 6.36 | 7.46 |
| NP | 4.54 | 0.49 | 4.00 | 5.50 | 4.31 | 0.48 | 4.32 | 5.32 |
| Digital Measuring Methods (e.g., Planmeca ProFace Scanner) | Analog Measuring Methods (e.g., Caliper) | |
|---|---|---|
| Advantages | -Efficiency: Faster data acquisition. -Comfort: Non-invasive, increased patient comfort. -Detail: Captures comprehensive 3D facial data, including texture and color. -Analysis: Enables complex angular, surface, and volume analysis. -Communication: Facilitates better patient communication. | -Precision: High accuracy for small, linear measurements when performed meticulously. -Simplicity: No specialized software or advanced training required beyond basic instrument use. -Cost-Effective: Generally lower initial equipment cost. |
| Disadvantages | -Accuracy Variability: Can show significant deviations for larger measurements (6.5 cm). -Soft Tissue Challenges: Simulation can be complex due to the dynamic nature of soft tissues. -Cost: Higher initial investment in equipment and software. -Learning Curve: Requires specific training for operation and software interpretation. | -Time-Consuming: Slower data acquisition. -Operator Dependent: Highly susceptible to inter-operator variability and human error. -Limited Scope: Primarily provides linear measurements, lacks 3D data for complex analysis. -Patient Discomfort: Can be invasive for certain measurements. |
| Indications | Comprehensive Facial Analysis: Ideal for detailed pre-and post-treatment evaluation in orthodontics, orthognathic surgery, and aesthetic procedures. -Treatment Planning: For complex cases requiring 3D visualization and simulation, especially for minor changes. -Patient Education: Enhances understanding of planned interventions. | -Validation: For verifying critical linear measurements, especially those where digital methods may show less precision (e.g., larger distances). -Basic Linear Measurements: For simple, direct measurements where 3D data is not critical. -Resource-Limited Settings: Where digital equipment is not available or feasible. |
| Limitations | -Precision for Large Distances: May lack sufficient precision for measurements exceeding 6.5 cm, requiring compensatory strategies. -Soft Tissue Dynamics: Challenges in accurately predicting dynamic soft tissue changes. | Inability to Capture 3D: Does not provide volumetric or surface data. -Limited Accessibility: Some facial areas may be difficult to access for direct measurement. -Replication: Difficult to replicate exact measurement points consistently across multiple sessions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puleio, F.; Lo Giudice, G.; Alibrandi, A.; Campione, I.; Di Spirito, F.; Lo Giudice, R. Precision in Facial Measurements: Comparative Analysis Between a Digital 3D Scanner and an Analog Instrument. Dent. J. 2025, 13, 395. https://doi.org/10.3390/dj13090395
Puleio F, Lo Giudice G, Alibrandi A, Campione I, Di Spirito F, Lo Giudice R. Precision in Facial Measurements: Comparative Analysis Between a Digital 3D Scanner and an Analog Instrument. Dentistry Journal. 2025; 13(9):395. https://doi.org/10.3390/dj13090395
Chicago/Turabian StylePuleio, Francesco, Giorgio Lo Giudice, Angela Alibrandi, Ilenia Campione, Federica Di Spirito, and Roberto Lo Giudice. 2025. "Precision in Facial Measurements: Comparative Analysis Between a Digital 3D Scanner and an Analog Instrument" Dentistry Journal 13, no. 9: 395. https://doi.org/10.3390/dj13090395
APA StylePuleio, F., Lo Giudice, G., Alibrandi, A., Campione, I., Di Spirito, F., & Lo Giudice, R. (2025). Precision in Facial Measurements: Comparative Analysis Between a Digital 3D Scanner and an Analog Instrument. Dentistry Journal, 13(9), 395. https://doi.org/10.3390/dj13090395











