1. Introduction
Dental diseases like caries and periodontal conditions such as gingivitis and periodontitis are some of the most common chronic health conditions worldwide [
1]. Dental caries, more commonly known as tooth decay, is a highly prevalent disease that affects almost everyone at some point. It is the most common chronic disease of childhood [
2] and is signified by the breakdown of enamel on the surface of teeth as the result of acid production by bacteria. Periodontal (gum) diseases are inflammatory conditions that begin with microbial accumulation on tooth surfaces that begin to invade the tissues that hold teeth in place. Gingivitis is an early, reversible form of periodontal disease characterized by red, swollen gums, while periodontitis is a chronic condition that leads to irreversible destruction of periodontal ligament fibers and supporting bone loss. Nearly half of adults in the United States over the age of 30 have some degree of periodontitis, showing that it is not only a dental health problem but also a public health concern [
3]. Alongside dental caries, these diseases are the leading causes of tooth loss and can significantly impact systemic health if left untreated.
Prevention of dental caries and periodontal disease is primarily achieved through physical removal of bacteria from tooth surfaces through routine oral hygiene practices such as brushing, flossing, and professional dental prophylactic cleaning [
4]. Usage of mouthrinses can provide additional preventative benefit by killing oral microbes, reducing dental plaque biofilm, or providing fluoride, which in turn reduces the likelihood of developing periodontal disease or caries [
5,
6]. While mouthrinses are not designed to be an alternative to brushing and flossing, they can be used as an additional line of defense to protect the oral cavity, being particularly effective in areas that may be missed by mechanical removal techniques [
7,
8].
Mouthrinses vary in composition and intended usage or benefit, which can usually be determined based on their active ingredients. Over-the-counter mouthrinses may contain antiseptic agents, natural compounds, essential oils, and/or fluoride. Oral rinses are also available and aim to help control dry mouth or oral sores [
9]. Studies have shown that mouthrinses can be effective in the control of bacteria, plaque, and dental disease [
5,
6,
9,
10]. In addition to controlling bacteria, several oral rinses have shown clinical benefit in freshening breath, reducing gingivitis, and reducing the risk of cavities [
11]. For example, mouthrinses that contain chlorhexidine as an active ingredient used consistently over a four-to-six-week period can show significant reduction in both plaque and gingivitis [
9].
A mouthrinse that is advertised as antiseptic should be able to kill oral microorganisms. An additional desirable quality of an antiseptic mouthrinse is the ability to kill bacteria protected in a biofilm, since dental diseases generally arise from biofilm-associated bacteria [
12].
Streptococcus mutans, a cariogenic bacterial pathogen that is highly associated with initiation of dental caries [
13], provides a model target for determining the antibacterial and antibiofilm activity of mouthrinses.
S. mutans is a key contributor to the caries initiation process due to its ability to produce acid in the presence of dietary sugars and form biofilms on the surface of teeth (dental plaque).
S. mutans can metabolize carbohydrates, resulting in acid production that leads to the demineralization of tooth enamel at a pH lower than 5.5 [
14].
S. mutans can also produce soluble and insoluble extracellular polysaccharides (EPS), which make up the protective matrix of the biofilm and also allow for other bacteria to join the biofilm, resulting in the development of multispecies biofilms on the surface of the tooth [
15,
16].
Commercial mouthrinses are marketed for various uses in oral hygiene and some have formulations that have not been assessed for antibacterial and antibiofilm activity. One such brand is TheraBreath™, which has marketed mouthrinses for various applications, yet no studies have investigated their antibacterial and antibiofilm efficacy. Therefore, in this study, we aimed to determine the in vitro bactericidal and antibiofilm activity of five TheraBreath™ mouthrinses. Bactericidal activity was assessed by exposing planktonic S. mutans and saliva-derived microbial communities to the various oral rinses, while the antibiofilm activity of TheraBreath™ mouthrinses was assessed using monospecies S. mutans in vitro biofilms as a model of cariogenic plaque biofilm.
2. Materials and Methods
2.1. Liquid Killing Assay for Antibacterial Activity
Streptomycin-resistant
Streptococcus mutans (ATCC
® 25175) was grown in BHI broth (Becton Dickinson and Company, Sparks, MD, USA) overnight at 37 °C/5% CO
2. Optical density at 600 nm (OD
600) was measured and cultures were centrifuged and adjusted to an OD
600 = 0.5 in 1X phosphate-buffered saline (PBS) (~5.0 × 10
8 CFU/mL). This culture was diluted 1:10 in PBS and 50 µL was added to 200 µL of PBS or mouthrinse (final concentration of 80% mouthrinse). Mixtures were incubated with rotation at 37 °C for 30 min or 2 min.
S. mutans mixtures were plated on BHI agar (Becton Dickinson and Company, Sparks, MD, USA) containing 500 µg/mL streptomycin and grown for 48 h at 37 °C/5% CO
2. Surviving bacteria were enumerated by colony counting. Percent survival was calculated by the number of surviving colonies in the treatment condition/PBS control × 100. Strains were tested a minimum of 3 times in separate experiments. Mouthrinses used in this study, along with their ingredients, are listed in
Table 1.
2.2. Collection and Treatment of Human Saliva Samples
This study was reviewed and approved by the University of Detroit Mercy Institutional Review Board (IRB No. 24-25-42). Following informed consent, ten volunteers were provided with orthodontic wax and instructed to chew on it for a period of five minutes to stimulate salivation and were asked to provide the sample in sterile collection cups. Each collected sample was immediately divided for treatment with PBS, TheraBreath™ Whitening Fresh Breath, TheraBreath™ Healthy Gums, or Listerine® Naturals. Then, 100 µL of stimulated saliva was added to a tube containing 900 µL of mouthrinse or PBS (90% final mouthrinse concentration). The sample was mixed by inversion for 30 s followed by immediate dilution in PBS. Treatments with the remaining mouthrinses were completed one by one. Post treatment, samples were plated onto BHI agar supplemented with 5% sheep blood (HemoStat Laboratories, Dixon, CA, USA), 0.5 µg/mL vitamin K1 (Sigma Aldrich, St. Louis, MO, USA), and 5 µg/mL hemin (Thermo Scientific, Fair Lawn, NJ, USA) and Mitis Salivarius agar (Becton Dickinson and Company, Sparks, MD, USA) supplemented with 1% potassium tellurite (Sigma Aldrich, St. Louis, MO, USA) for oral streptococci. These plates were subsequently incubated at 37 °C/5% CO2. Surviving bacteria were enumerated by colony counting. Percent survival was calculated by the number of surviving colonies in the treatment condition/PBS control × 100.
2.3. Biofilm Setup and Treatment
Overnight broth cultures of streptomycin-resistant Streptococcus mutans ATCC® 25175 (American Type Culture Collection, Manassas, VA, USA) were prepared in BHI broth with 500 µg/mL streptomycin from colonies and incubated for 16 h at 37 °C/5% CO2. Overnight cultures were adjusted to an OD600 of 0.1. Then, tissue culture-treated 24-well plates containing Brain Heart Infusion (BHI) broth, 1% sucrose, 500 µg/mL streptomycin, and 1 µM Cascade Blue-conjugated 10,000 MW dextran (Life Technologies Corporation, Eugene, OR, USA) were inoculated with S. mutans to a final OD600 = 0.01 and grown for 24 h at 37 °C/5% CO2. After 24 h of growth, biofilm supernatants containing non-adherent and loosely adherent bacteria were removed using a Vacusip (NTEGRA Biosciences Corp., Hudson, NH, USA). Wells were washed twice with 1 mL of PBS by swirling gently. Following, 500 µL of mouthrinses was added to each biofilm well and shaken at 150 rpm for 15 min on an orbital shaker. Then, treatments were removed by Vacusip and washed 2 times with 1 mL of PBS. Biofilm killing was analyzed via fluorescent Live/Dead staining using SYTO9 (Life Technologies Corporation, Eugene, OR, USA) and propidium iodide (Sigma Aldrich, St. Louis, MO, USA). Extracellular polysaccharide (EPS) content of the biofilm was measured via Cascade Blue-conjugated Dextran. Cascade blue-labeled dextran was added during biofilm growth to assess the extracellular polysaccharide content of the biofilms after treatment. The fluorescently labeled dextran acts as an acceptor for glucosidic bonds, becoming incorporated into the biofilm matrix during biofilm development.
2.4. Live/Dead Fluorescent Vitality Staining
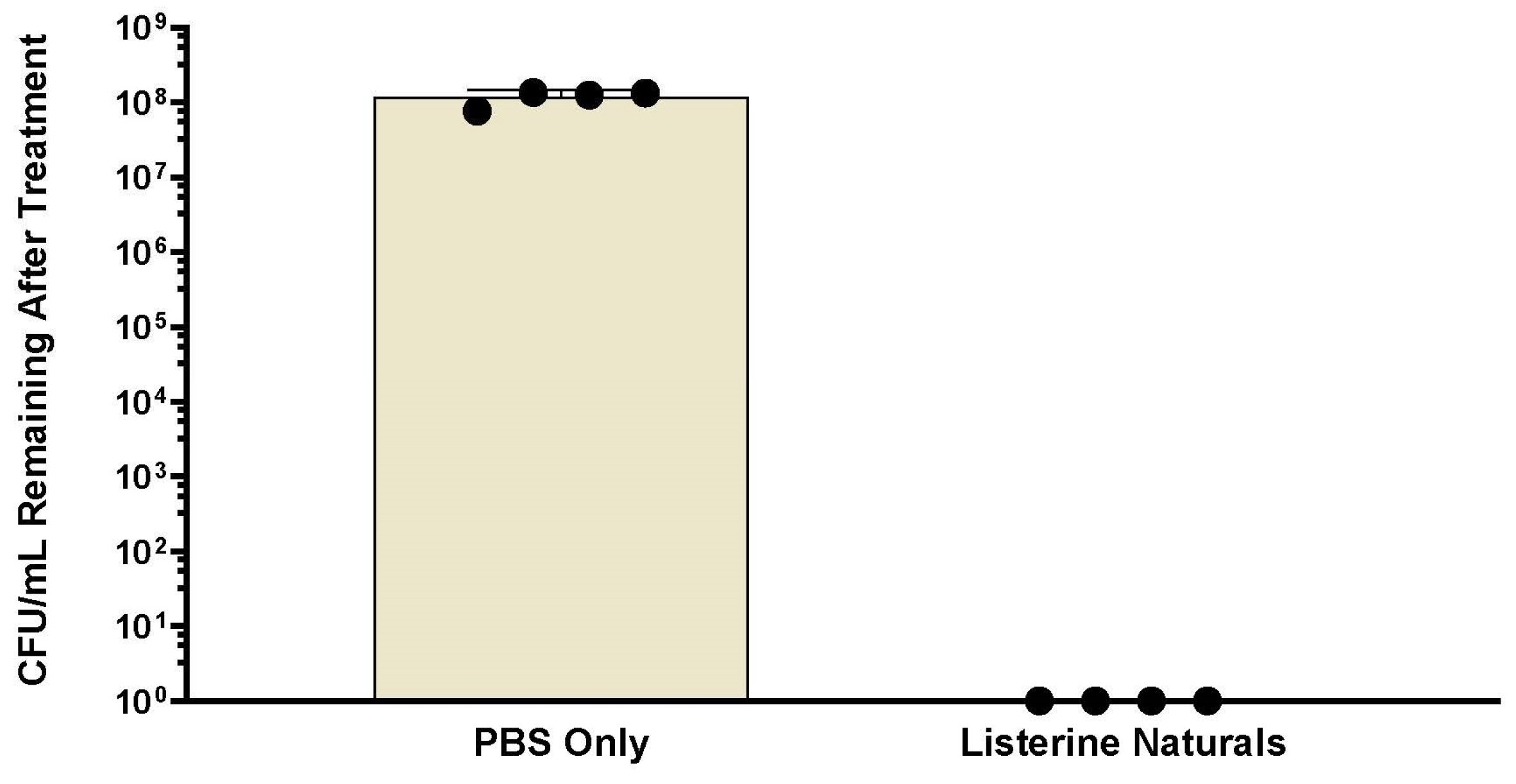
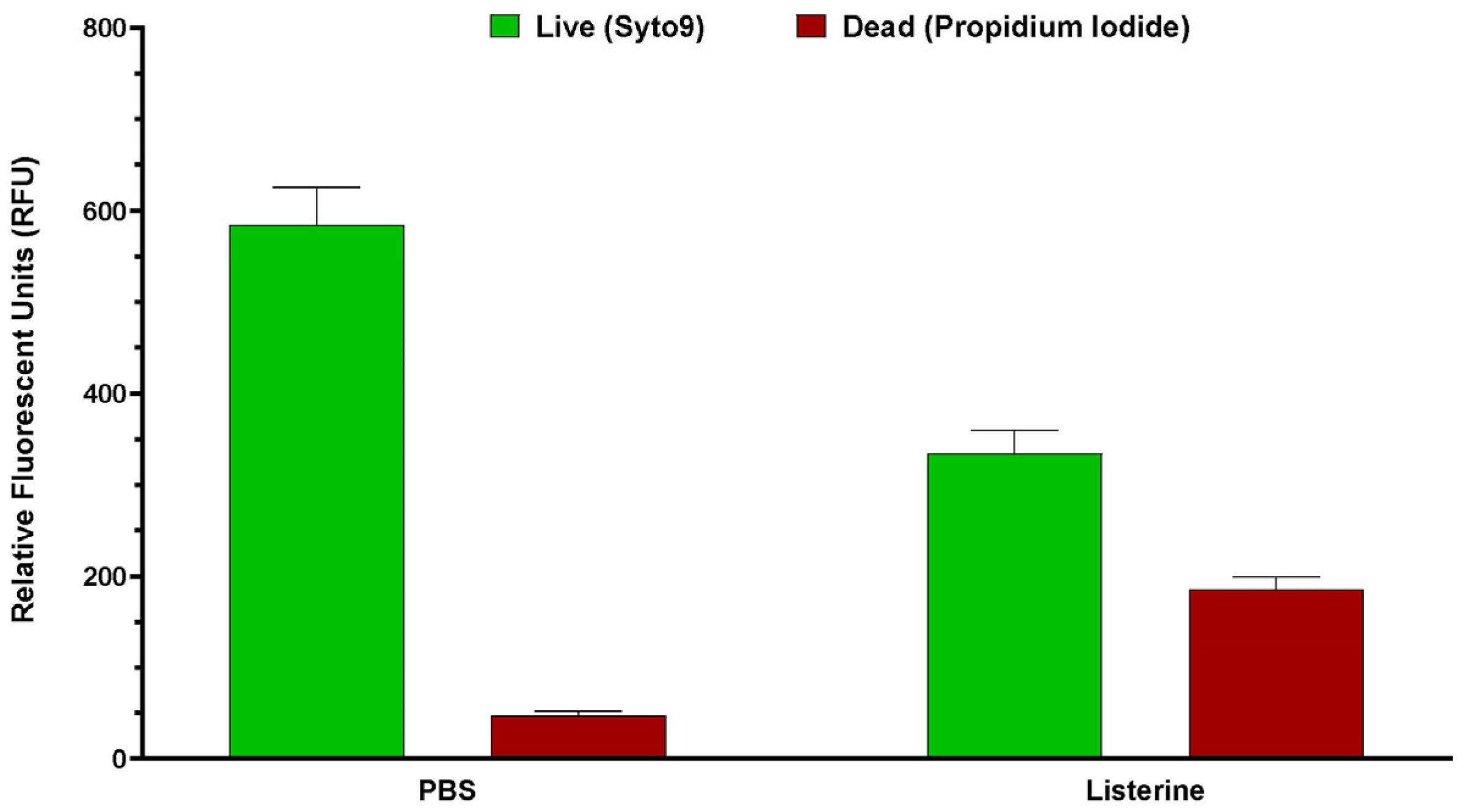
Post-treatment biofilm vitality was assessed using 250 µL of Live/Dead stain consisting of 2 µM SYTO9 and 20 µM propidium iodide. Biofilms were incubated in the stain for 15 min in the dark. Fluorescence was measured using a Spark microplate reader (TECAN, Grodig, Austria) at five spots within the biofilm: SYTO9 (ex. 485 nm, em. 530 nm), propidium iodide (ex. 485 nm, em. 630 nm), and Dextran (ex. 400 nm, em. 420 nm). The average fluorescent intensity of the five readings was used for each well of the biofilm plate. Experiments were conducted at least three times. Previous experiments have shown that Listerine-treated biofilms produce no surviving bacteria on colony count (
Appendix A,
Figure A1,
Figure A2 and
Figure A3). Therefore, for each experiment, the background SYTO9 fluorescent intensity measured in the Listerine
®-treated biofilms was subtracted from the SYTO9 intensity of all other treatments. If this led to a number less than zero, it was set to zero. Live (SYTO9) and Dextran fluorescent intensities from each experiment were normalized to fluorescent intensities from the PBS-treated biofilms, while the dead fluorescent intensities in each experiment were normalized as a percentage of the respective intensities of the Listerine
®-treated biofilms. Live/Dead ratios were calculated for each treated biofilm for each experiment by dividing the average live fluorescent intensity for each well by the average dead fluorescent intensity of the well. Live/Dead ratios are displayed as a percentage of the Live/Dead ratio of PBS-treated biofilms.
2.5. Crystal Violet Staining
After fluorescent analysis, the stain was removed, and biofilms were heat-fixed at 80 °C for 30 min prior to crystal violet staining for determination of total biofilm remaining after treatment. Briefly, 1 mL of 0.5% crystal violet was added to each well and incubated for 15 min at room temperature. Crystal violet was then removed, and wells were rinsed gently with water until rinses were clear of excess stain. To solubilize biofilm-associated crystal violet, 1 mL of 33% acetic acid was added and incubated for 15 min at room temperature with shaking at 100 rpm. The solubilized stain was transferred to a 96-well plate, serial 1:2 dilutions were made using water, and absorbance at 570 nm was measured using a microplate reader. Absorbance readings were normalized as a percentage of the PBS-treated biofilm for each experiment.
2.6. Statistical Analysis
Analysis was completed using GraphPad Prism version 10.4.0 for Windows (GraphPad Software, Boston, MA, USA,
www.graphpad.com). Datasets were analyzed to test the assumption of normal distribution. Significance was determined using Kruskal–Wallis followed by Dunn’s multiple comparisons test comparing all mean ranks. A
p value lower than 0.05 was considered statistically significant.
4. Discussion
The objective of this study was to determine the bactericidal and antibiofilm activity of TheraBreath™ oral rinses against
S. mutans and saliva-derived microbial communities. Bactericidal activity was assessed by exposing planktonic and biofilm-associated bacteria to various oral rinses. Even though microorganisms, like
S. mutans, cause issues like caries from within a protective biofilm, it is important to study antibacterial activity against planktonic
S. mutans to establish which formulations have any measurable level of antibacterial activity because bacteria in liquid, like saliva, can potentially colonize tooth surfaces and form biofilms that are significantly more tolerant to treatments than planktonic cells [
17].
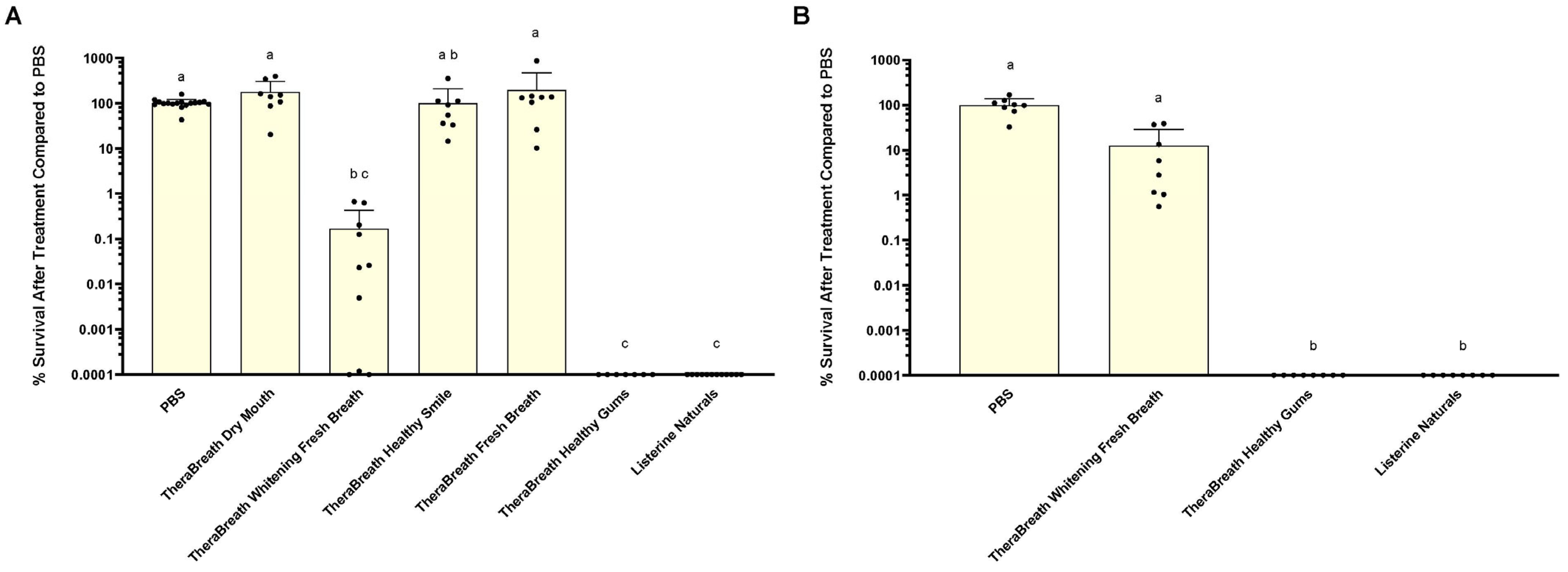
The results from this study indicate that, of the TheraBreath™ oral rinses tested, only Whitening Fresh Breath and Healthy Gums demonstrated any level of antibacterial activity against
S. mutans. Healthy Gums showed the greatest level of antibacterial activity compared to other TheraBreath™ mouthrinses based on the results for 30 and 2 min treatments of
S. mutans in liquid. After 30 min treatments of liquid
S. mutans, colony counts revealed that Healthy Gums killed at levels comparable to Listerine
® Naturals, and Whitening Fresh Breath killed at statistically comparable levels; however, Whitening Fresh Breath did not always kill 100% of input bacteria like Healthy Gums and Listerine
® Naturals. Healthy Gums was the only TheraBreath™ formulation to exhibit complete killing in 2 min of exposure. Measured antibacterial activity against the cariogenic bacterium
S. mutans is an important quality for an antiseptic mouthrinse due to its association with the initiation of caries. Reduced levels of
S. mutans in the oral cavity, especially in primary caretakers, has been shown to lower the risk of early childhood caries in their children [
18,
19]. Based on these results, we can argue for the use of Healthy Gums as an antiseptic mouthrinse for the prevention/control of caries in individuals at high risk of
S. mutans-mediated dental disease.
While
S. mutans is a pathogen that is associated with caries, it is not the only pathogen associated with the disease, nor is caries the only dental disease caused by microbes. The oral cavity consists of over 700 bacterial species of bacteria, comprising commensal, opportunistic, and pathogenic microorganisms, and thus, antibacterial activity of mouthrinses against a broad range of microbes should also be assessed [
20]. Therefore, we expanded our study to consider other microorganisms that could impact the clinical efficacy of the various oral mouthrinses by testing them against salivary samples. After 30 s treatments of saliva samples, Healthy Gums exhibited comparable antibacterial activity to Listerine
® Naturals against saliva-derived microbes that grow aerobically on blood agar and Mitis Salivarius agar (
Figure 2). While the microbiome of saliva may differ based on multiple factors, a core microbial community comprising 68 operational taxonomic units has been described in human saliva of healthy individuals [
21]. Therefore, it can be inferred that saliva samples from multiple donors represent a defined range of diverse members of the saliva microbiome, many of which are aerobic or facultative, and that TheraBreath™ Healthy Gums has a broader range of antibacterial activity, beyond
S. mutans and other oral streptococci.
It is assumed that biofilms are also significantly more tolerant of chemical disinfection than stationary-phase planktonic cells [
17]. So, if a mouthrinse has antibacterial activity against planktonic cells, it cannot be assumed that it retains this activity towards biofilm-associated cells. Since acid production that affects tooth enamel comes from bacteria within a biofilm, we tested the TheraBreath™ oral rinses for antibiofilm activity against in vitro
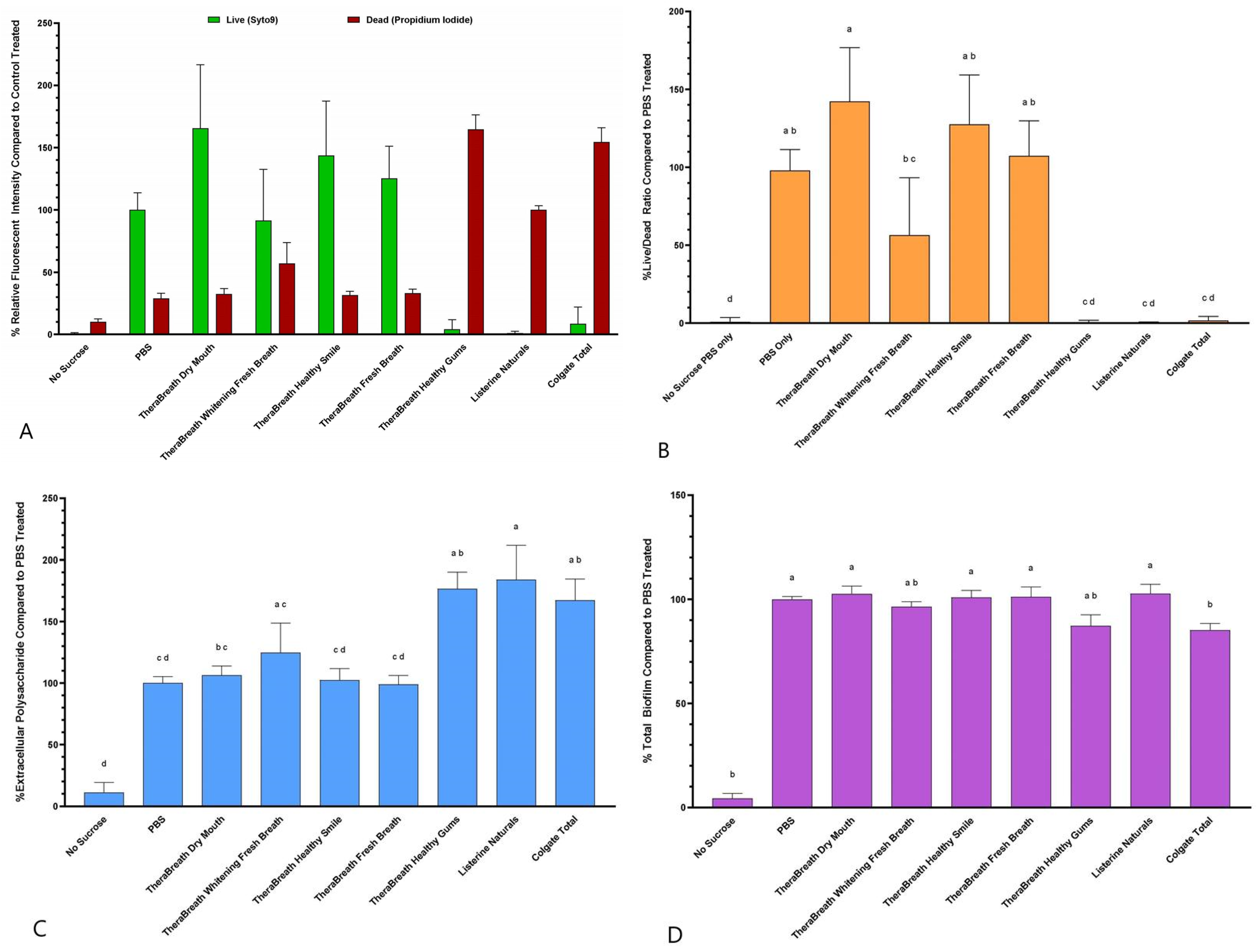
S. mutans biofilms. The Live/Dead fluorescence ratio after treatment with TheraBreath™ Healthy Gums was statistically comparable to treatment with other commercially sold mouthrinses including Listerine
® Naturals and Colgate
® Total (
Figure 3B) indicating its ability to kill bacteria protected within a biofilm. TheraBreath™ Whitening Fresh Breath showed a statistically significant decrease in biofilm vitality compared to PBS and other TheraBreath™ rinses; however, the Live/Dead ratio was significantly higher than Healthy Gums, Listerine
® Naturals, and Colgate
® Total. Of all the mouthrinses tested, there was no significant disruption of biofilm structure when assessed by fluorescently labeled dextran and crystal violet staining (
Figure 3C,D). The inability of the tested mouthrinses to reduce EPS and overall biofilm in this study reinforces the use of mouthrinses as an adjunct to physical biofilm removal, not as a replacement [
8].
Overall, Healthy Gums was the only TheraBreath™ mouthrinse tested that had similar antiseptic activity to the levels of other antiseptic-claiming commercial rinses, while Whitening Fresh Breath showed intermediate antiseptic activity in some experiments. The antibacterial activity of Healthy Gums is most likely due to the active ingredient, cetylpyridinium chloride (CPC). Products that contain CPC, like Colgate
® Total, have shown antibacterial activity in many studies against
S. mutans and other bacterial species [
5,
22,
23]. CPC, a quaternary ammonium compound, disrupts bacterial cell membranes by binding to negatively charged bacterial surfaces, leading to increased membrane permeability, leakage of intracellular contents, and ultimately cell death [
22]. In the context of biofilms, CPC is able to penetrate the extracellular polymeric matrix and reach bacteria located deeper within the biofilm structure [
22]. This characteristic, along with its described mechanism of antibacterial activity, is a plausible reason for the antibacterial effects against
S. mutans biofilms measured after exposure to TheraBreath™ Healthy Gums.
TheraBreath™ Whitening Fresh Breath generally exhibited less antibacterial activity compared to Healthy Gums but still showed intermediate levels. Unlike Healthy Gums, Whitening Fresh Breath lacks an obvious antimicrobial ingredient. To identify candidate ingredients that could account for the antibacterial activity of Whitening Fresh Breath, its ingredients were compared with other TheraBreath™ formulations that lacked antibacterial activity. After exclusion of similar ingredients, potential ingredients in Whitening Fresh Breath that may account for the intermediate antibacterial activity arose including sodium benzoate, polyvinylpolypyrrolidone, papain, glucose oxidase, and D-limonene.
Sodium benzoate is a preservative and has demonstrated antibacterial activity [
24]; however, it is also present in other TheraBreath™ mouthrinses (Dry Mouth, Healthy Smile, and Fresh Breath), which did not show any antibacterial activity (
Figure 1). Polyvinylpolypyrrolidone is a water-soluble synthetic homopolymer that is added to toothpastes and rinses to increase clearing time of hydrogen peroxide for longer whitening effects [
25].
Papain, a proteolytic enzyme from papaya, is commonly used to break down proteins. The pellicle layer, being proteinaceous, also has the ability to absorb pigments, leading to stains on teeth. While it can break down pellicle proteins, this component likely did not play a role in the breakdown of extracellular matrix in our biofilm model, since
S. mutans biofilm matrices are primarily polysaccharide [
26]. The ability to reduce pellicle accumulation is not considered to be an antiseptic function, but it is a capability that aids in the overall effectiveness of mouthwash [
27].
Glucose oxidase is an enzyme that converts glucose into gluconic acid and hydrogen peroxide, both of which could contribute to bacterial killing [
28]. This enzyme is likely to be included in the formulation to provide a source of hydrogen peroxide since the mouthrinse does not include hydrogen peroxide. While production of hydrogen peroxide may contribute to the antibacterial activity, this enzyme requires glucose as a substrate for the production, which is present in our liquid killing assays.
Limonene, a monoterpene found in citrus oils and other plants, has been shown to have antibacterial activity, particularly in its R/D-form [
29]. Also, in the presence of glucose oxidase, D-limonene is converted into the oxygenated monoterpene, carvone, which has additional antimicrobial properties [
30]. While this was not tested in this study, based on these other studies, it is likely the D-limonene component of the Whitening Fresh Breath formulation is likely providing the antiseptic activity in this oral rinse.
Limitations
While saliva-based experiments were included to better simulate clinical conditions, many of the assays were still studied in controlled in vitro settings that do not fully simulate the oral cavity or the patient. For example, some patients swish mouthrinse around very quickly, but some patients use it very slowly. Additionally, the health of the oral cavity, including saliva production and immune status, can influence how well a mouthrinse performs. Saliva composition itself can also vary between individuals due to hydration, medications, or underlying conditions, which can also impact the mouth rinse effectiveness. An additional limitation is that this study focused primarily on a single strain of Streptococcus mutans, but as stated, dental biofilms are composed of a complex, multispecies microbial community and the exposure time of biofilms in the experiments is longer than the directed usage times. While not an exact replica of oral biofilm, monospecies S. mutans biofilms are a commonly used model of oral biofilms that provides a consistent measure for initial testing of antibiofilm activity. Further research should focus on cytotoxic evaluation and the in vivo effect of TheraBreath™ mouthrinses on clinical parameters during routine usage.