Evaluation of Compatibility of Different Attachment Types Used in Orthodontic Clear Aligners with Electron Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Size
2.2. Specimen Preparation
2.3. Attachment Design and Production
- Rectangular attachment: 3 mm width × 5 mm length × 2 mm height;
- Optimized attachment: 3 mm width × 5 mm length × 2.5 mm height with curved surfaces.
2.4. Attachment Bonding Protocol
- Enamel etching with 37% phosphoric acid for 30 s [39];
- Application of bonding agent (Transbond XT Primer, 3M Unitek, Monrovia, CA, USA) [40];
- Light curing for 20 s using Woodpecker Dental iLed (Guilin, China);
- Composite placement (Transbond XT, 3M Unitek) in template;
- Template positioning with 50 g standardized force measured with Dentaurum force gauge;
- Light curing for 40 s [41];
- Following template removal, excess composite material was carefully removed using a scalpel blade under magnification, ensuring attachment margins were smooth without altering the designed dimensions.
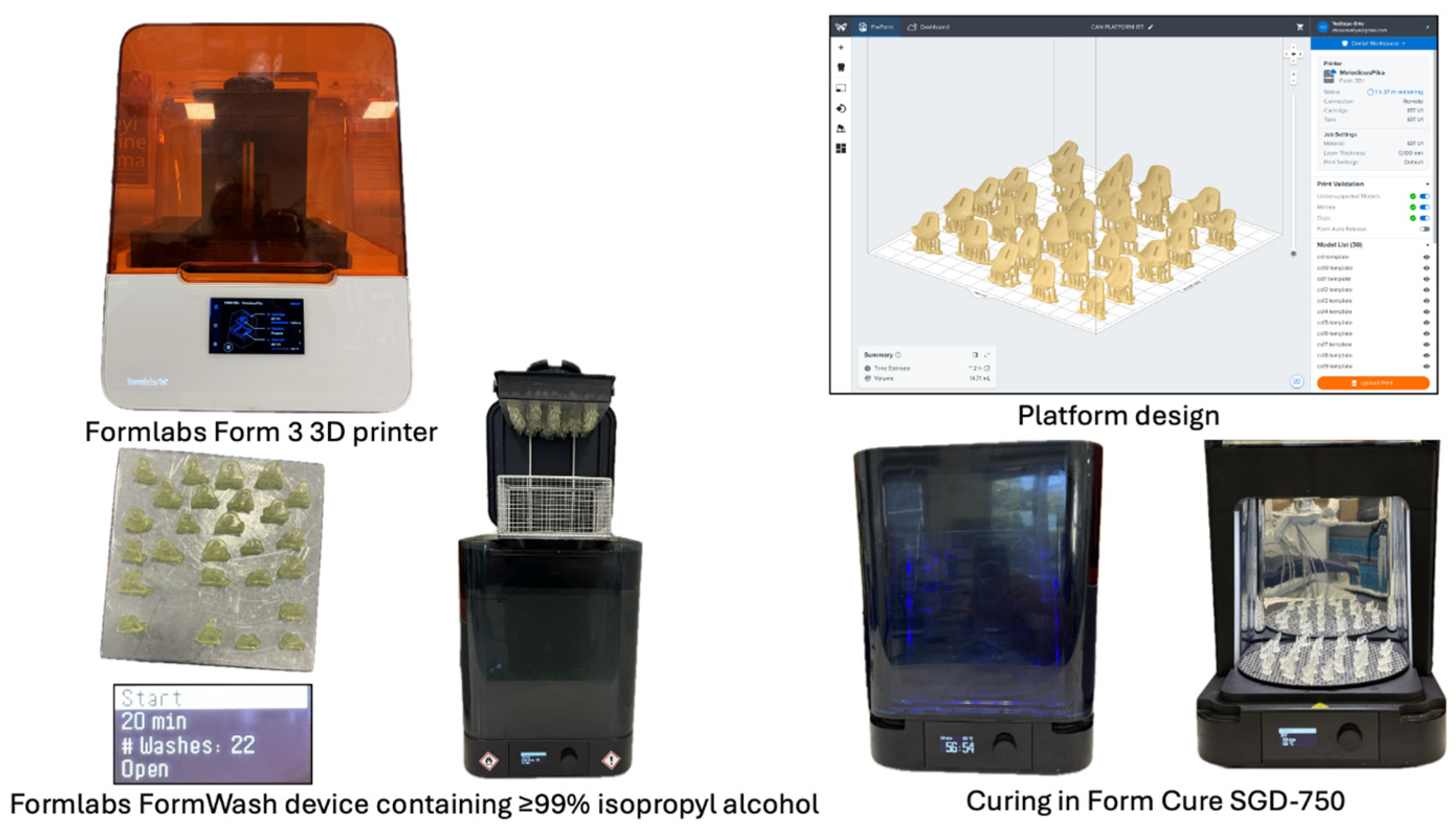
2.5. Aligner Fabrication
- Duran+ (Scheu Dental, Iserlohn, Germany): 0.76 mm single-layer PETG;
- Zendura FLX (Bay Materials, Fremont, CA, USA): 0.76 mm multi-layer polyurethane.
2.6. Scanning Electron Microscopy Analysis
- Rectangular attachments: 7 measurement points (Figure 4);
- Optimized attachments: 5 measurement points (Figure 5);
2.7. Statistical Analysis
3. Results
3.1. Material Comparison
3.2. Attachment Design Comparison
3.3. Regional Variation Patterns
- Largest gaps consistently occurred at gingival borders (67.18–91.07 µm);
- Intermediate gaps were observed at occlusal borders (38.41–47.28 µm);
- Smallest gaps were found at flat surfaces and acute angles (14.75–20.98 µm).
4. Discussion
4.1. Material Properties and Adaptation
4.2. Attachment Design Implications
4.3. Regional Variation and Clinical Significance
4.4. Comparison with Previous Studies
4.5. Clinical Implications
- More conservative staging with smaller incremental movements;
- Additional attachments to distribute forces across multiple contact points;
- Auxiliary mechanics such as elastics or temporary anchorage devices;
- Selection of multi-layer aligner materials for complex movements;
- Preference for optimized attachment designs when available.
4.6. Future Directions
- Multi-stage thermoforming with differential pressure application;
- Direct 3D printing of aligners to eliminate thermoforming limitations [38];
- Development of shape-memory materials with improved adaptation properties;
- Hybrid manufacturing combining thermoforming with selective reinforcement;
- Attachment designs specifically optimized for regional adaptation patterns.
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosvall, M.D.; Fields, H.W.; Ziuchkovski, J.; Rosenstiel, S.F.; Johnston, W.M. Attractiveness, acceptability, and value of orthodontic appliances. Am. J. Orthod. Dentofacial Orthop. 2009, 135, 276.e1–276.e12. [Google Scholar] [CrossRef]
- Azaripour, A.; Weusmann, J.; Mahmoodi, B.; Peppas, D.; Gerhold-Ay, A.; Van Noorden, C.J.; Willershausen, B. Braces versus Invisalign®: Gingival parameters and patients’ satisfaction during treatment: A cross-sectional study. BMC Oral Health 2015, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Kesling, H.D. Coordinating the predetermined pattern and tooth positioner with conventional treatment. Am. J. Orthod. Oral Surg. 1946, 32, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ponitz, R.J. Invisible retainers. Am. J. Orthod. 1971, 59, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kuo, E.; Duong, T. Invisalign attachments: Materials. In The Invisalign System; Quintessence: Philadelphia, PA, USA, 2006; p. 92. [Google Scholar]
- Dasy, H.; Dasy, A.; Asatrian, G.; Rózsa, N.; Lee, H.F.; Kwak, J.H. Effects of variable attachment shapes and aligner material on aligner retention. Angle Orthod. 2015, 85, 934–940. [Google Scholar] [CrossRef]
- Miller, K.B.; McGorray, S.P.; Womack, R.; Quintero, J.C.; Perelmuter, M.; Gibson, J.; Dolan, T.A.; Wheeler, T.T. A comparison of treatment impacts between Invisalign aligner and fixed appliance therapy during the first week of treatment. Am. J. Orthod. Dentofacial Orthop. 2007, 131, 302.e1–302.e9. [Google Scholar] [CrossRef]
- Karkhanechi, M.; Chow, D.; Sipkin, J.; Sherman, D.; Boylan, R.J.; Norman, R.G.; Craig, R.G.; Cisneros, G.J. Periodontal status of adult patients treated with fixed buccal appliances and removable aligners over one year of active orthodontic therapy. Angle Orthod. 2013, 83, 146–151. [Google Scholar] [CrossRef]
- Kuo, E.; Miller, R.J. Automated custom-manufacturing technology in orthodontics. Am. J. Orthod. Dentofacial Orthop. 2003, 123, 578–581. [Google Scholar] [CrossRef]
- Boyd, R.L.; Miller, R.J.; Vlaskalic, V. The Invisalign system in adult orthodontics: Mild crowding and space closure cases. J. Clin. Orthod. 2000, 34, 203–212. [Google Scholar]
- Tomášik, J.; Zsoldos, M.; Majdakova, K.; Fleischmann, A.; Oravcová, Ľ.; Sónak Ballová, D.; Thurzo, A. The potential of AI-powered face enhancement technologies in face-driven orthodontic treatment planning. Appl. Sci. 2024, 14, 7837. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Alwafi, A.; Liu, X.; Andrews, J.; Ludwig, B.; Bichu, A.Y.; Zou, B. Advances in orthodontic clear aligner materials. Bioact. Mater. 2023, 22, 384–403. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.M.; Mapelli, A.; Maspero, C.; Santaniello, T.; Serafin, M.; Farronato, M.; Caprioglio, A. Direct 3D printing of clear orthodontic aligners: Current state and future possibilities. Materials 2021, 14, 1799. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, J.; Al-Awadhi, E.A. Clear aligners generations and orthodontic tooth movement. J. Orthod. 2016, 43, 68–76. [Google Scholar] [CrossRef]
- Gomez, J.P.; Peña, F.M.; Martínez, V.; Giraldo, D.C.; Cardona, C.I. Initial force systems during bodily tooth movement with plastic aligners and composite attachments: A three-dimensional finite element analysis. Angle Orthod. 2015, 85, 454–460. [Google Scholar] [CrossRef]
- Elkholy, F.; Mikhaiel, B.; Repky, S.; Schmidt, F.; Lapatki, B.G. Effect of different attachment geometries on the mechanical load exerted by PET-G aligners during derotation of mandibular canines: An in vitro study. J. Orofac. Orthop. 2019, 80, 315–326. [Google Scholar] [CrossRef]
- Rossini, G.; Parrini, S.; Castroflorio, T.; Deregibus, A.; Debernardi, C.L. Efficacy of clear aligners in controlling orthodontic tooth movement: A systematic review. Angle Orthod. 2015, 85, 881–889. [Google Scholar] [CrossRef]
- Kravitz, N.D.; Kusnoto, B.; BeGole, E.; Obrez, A.; Agran, B. How well does Invisalign work? A prospective clinical study evaluating the efficacy of tooth movement with Invisalign. Am. J. Orthod. Dentofacial Orthop. 2009, 135, 27–35. [Google Scholar] [CrossRef]
- Grünheid, T.; Loh, C.; Larson, B.E. How accurate is Invisalign in nonextraction cases? Are predicted tooth positions achieved? Angle Orthod. 2017, 87, 809–815. [Google Scholar] [CrossRef]
- Djeu, G.; Shelton, C.; Maganzini, A. Outcome assessment of Invisalign and traditional orthodontic treatment compared with the American Board of Orthodontics objective grading system. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 292–298. [Google Scholar] [CrossRef]
- Savignano, R.; Valentino, R.; Razionale, A.V.; Michelotti, A.; Barone, S.; D’Antò, V. Biomechanical effects of different auxiliary-aligner designs for the extrusion of an upper central incisor: A finite element analysis. J. Healthc. Eng. 2019, 2019, 687127. [Google Scholar] [CrossRef]
- Cai, Y.; He, B.; Yang, X.; Yao, J. Optimization of configuration of attachment in tooth translation with transparent tooth correction by appropriate moment-to-force ratios: Biomechanical analysis. Biomed. Mater. Eng. 2015, 26, S507–S517. [Google Scholar] [CrossRef]
- Jones, M.L.; Mah, J.; O’Toole, B.J. Retention of thermoformed aligners with attachments of various shapes and positions. J. Clin. Orthod. 2009, 43, 113–117. [Google Scholar] [PubMed]
- Al Noor, H.S.S.; Al-Joubori, S.K. Retention of different orthodontic aligners according to their thickness and the presence of attachments. Int. J. Med. Res. Health Sci. 2018, 7, 115–121. [Google Scholar]
- Jindal, P.; Worcester, F.; Siena, F.L.; Forbes, C.; Juneja, M.; Breedon, P. Mechanical behaviour of 3D printed vs thermoformed clear dental aligner materials under non-linear compressive loading using FEM. J. Mech. Behav. Biomed. Mater. 2020, 112, 104045. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, E.; Castroflorio, E.; Rossini, G.; Garino, F.; Cugliari, G.; Deregibus, A.; Castroflorio, T. Scanning electron microscopy evaluation of aligner fit on teeth. Angle Orthod. 2018, 88, 596–601. [Google Scholar] [CrossRef]
- Mantovani, E.; Castroflorio, E.; Rossini, G.; Garino, F.; Cugliari, G.; Deregibus, A.; Castroflorio, T. Scanning electron microscopy analysis of aligner fitting on anchorage attachments. J. Orofac. Orthop. 2019, 80, 79–87. [Google Scholar] [CrossRef]
- Lombardo, L.; Palone, M.; Longo, M.; Arveda, N.; Nacucchi, M.; De Pascalis, F.; Spedicato, G.; Siciliani, G. MicroCT X-ray comparison of aligner gap and thickness of six brands of aligners: An in- vitro study. Prog. Orthod. 2020, 21, 12. [Google Scholar] [CrossRef]
- Cortona, A.; Rossini, G.; Parrini, S.; Deregibus, A.; Castroflorio, T. Clear aligner orthodontic therapy of rotated mandibular round-shaped teeth: A finite element study. Angle Orthod. 2020, 90, 247–254. [Google Scholar] [CrossRef]
- Elkholy, F.; Schmidt, F.; Jäger, R.; Lapatki, B.G. Forces and moments delivered by novel, thinner PET-G aligners during labiopalatal bodily movement of a maxillary central incisor: An in vitro study. Angle Orthod. 2016, 86, 883–890. [Google Scholar] [CrossRef]
- Hahn, W.; Zapf, A.; Dathe, H.; Fialka-Fricke, J.; Fricke-Zech, S.; Gruber, R.; Kubein-Meesenburg, D.; Sadat-Khonsari, R. Torquing an upper central incisor with aligners—Acting forces and biomechanical principles. Eur. J. Orthod. 2010, 32, 607–613. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Yassen, G.H.; Platt, J.A.; Hara, A.T. Bovine teeth as substitute for human teeth in dental research: A review of literature. J. Oral Sci. 2011, 53, 273–282. [Google Scholar] [CrossRef] [PubMed]
- de Dios Teruel, J.; Alcolea, A.; Hernández, A.; Ruiz, A.J. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch. Oral Biol. 2015, 60, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Ender, A.; Mehl, A. In-vitro evaluation of the accuracy of conventional and digital methods of obtaining full-arch dental impressions. Quintessence Int. 2015, 46, 9–17. [Google Scholar]
- Ye, N.; Wu, T.; Dong, T.; Yuan, L.; Fang, B.; Xia, L. Precision of 3D-printed splints with different dental model offsets. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 733–738. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Wezgowiec, J.; Mikulewicz, M. Comparison of two chosen 3D printing resins designed for orthodontic use: An in vitro study. Materials 2023, 16, 2237. [Google Scholar] [CrossRef]
- Jindal, P.; Juneja, M.; Siena, F.L.; Bajaj, D.; Breedon, P. Mechanical and geometric properties of thermoformed and 3D printed clear dental aligners. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 694–701. [Google Scholar] [CrossRef]
- Mitić, V.; Janošević, M. The effect of phosphoric acid application time on the bond strength of orthodontic brackets. Stomatol. Glas. Srb. 2008, 55, 23–33. [Google Scholar] [CrossRef]
- Parrish, B.C.; Katona, T.R.; Isikbay, S.C.; Stewart, K.T.; Kula, K.S. The effects of application time of a self-etching primer and debonding methods on bracket bond strength. Angle Orthod. 2012, 82, 131–136. [Google Scholar] [CrossRef]
- Turk, T.; Elekdag-Turk, S.; Isci, D. Effects of self-etching primer on shear bond strength of orthodontic brackets at different debond times. Angle Orthod. 2007, 77, 108–112. [Google Scholar] [CrossRef]
- Elkholy, F.; Lapatki, B.G. Recommendation of a novel film-thickness sequence, 0.4, 0.5 and 0.75 mm, for aligner systems. J. Align. Orthod. 2018, 4, 295–304. [Google Scholar]
- Krey, K.F.; Behyar, M.; Hartmann, M.; Corteville, F.; Ratzmann, A. Behaviour of monolayer and multilayer foils in the aligner thermoforming process. J. Align. Orthod. 2019, 3, 139–145. [Google Scholar]
- Ekşi, O.; Karabeyoğlu, S.S. The effect of process parameters on thickness distribution in thermoforming. Adv. Sci. Technol. Res. J. 2017, 11, 195–203. [Google Scholar] [CrossRef][Green Version]
- Cowley, D.P. Effect of Gingival Margin Design on Retention of Thermoformed Orthodontic Aligners. Master’s Thesis, University of Nevada, Las Vegas, NV, USA, 2012. [Google Scholar]
- Apicella, D.; Veltri, M.; Chieffi, N.; Polimeni, A.; Giovannetti, A.; Ferrari, M. Implant adaptation of stock abutments versus CAD/CAM abutments: A radiographic and scanning electron microscopy study. Ann. Stomatol. 2011, 1, 9–13. [Google Scholar][Green Version]
- Wagner, R.J. Techniques in Biological Electron Microscope; Academic Press: New York, NY, USA, 2000. [Google Scholar]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists; Jones and Bartlett Publishers: Sudbury, MA, USA, 1999. [Google Scholar]
- Son, K.; Lee, S.; Kang, S.H.; Park, J.; Lee, K.B.; Jeon, M.; Yun, B.J. A comparison study of marginal and internal fit assessment methods for fixed dental prostheses. J. Clin. Med. 2019, 8, 785. [Google Scholar] [CrossRef]
- Ryokawa, H.; Miyazaki, Y.; Fujishima, A.; Miyazaki, T.; Maki, K. The mechanical properties of dental thermoplastic materials in a simulated intraoral environment. Orthod. Waves 2006, 65, 64–72. [Google Scholar] [CrossRef]
- Bucci, R.; Rongo, R.; Levatè, C.; Michelotti, A.; Barone, S.; Razionale, A.V.; D’Antò, V. Thickness of orthodontic clear aligners after thermoforming and after 10 days of intraoral exposure: A prospective clinical study. Prog. Orthod. 2019, 20, 36. [Google Scholar] [CrossRef]
- Burashed, H. Quantifying the efficacy of overbite reduction in patients treated with clear aligners using optimized versus conventional attachments. J. World Fed. Orthod. 2023, 12, 172–179. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Xu, X.; Chen, J. Impacts of surface wear of attachments on maxillary canine distalization with clear aligners: A three-dimensional finite element study. Front. Bioeng. Biotechnol. 2025, 13, 1234567. [Google Scholar] [CrossRef]
- Hassanaly, T.; Smith, R.; Johnson, M. A comparison of the upper anterior teeth movements with optimized and conventional attachment. J. Clin. Exp. Dent. 2024, 16, e234–e241. [Google Scholar] [CrossRef]
- Groody, J.T.; Miller, K.; Williams, P. Effect of clear aligner attachment design on extrusion of maxillary lateral incisors: A multicenter, single-blind randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2023, 164, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Keilig, L.; Schwarze, J.; Jung, B.A.; Bourauel, C. Forces and moments generated by removable thermoplastic aligners: Incisor torque, premolar derotation, and molar distalization. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Paoli, A.; Neri, P.; Razionale, A.V.; Giannese, M. Mechanical and geometrical properties assessment of thermoplastic materials for biomedical application. In Advances on Mechanics, Design Engineering and Manufacturing; Eynard, B., Nigrelli, V., Oliveri, S.M., Peris-Fajarnes, G., Rizzuti, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 437–446. [Google Scholar]
- Ryu, J.H.; Kwon, J.S.; Jiang, H.B.; Cha, J.Y.; Kim, K.M. Effects of thermoforming on the physical and mechanical properties of thermoplastic materials for transparent orthodontic aligners. Korean J. Orthod. 2018, 48, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, L.; Martines, E.; Mazzanti, V.; Arreghini, A.; Mollica, F.; Siciliani, G. Stress relaxation properties of four orthodontic aligner materials: A 24-hour in vitro study. Angle Orthod. 2017, 87, 11–18. [Google Scholar] [CrossRef]
- Robertson, L.; Kaur, H.; Fagundes, N.C.F.; Romanyk, D.; Major, P.; Flores Mir, C. Effectiveness of clear aligner therapy for orthodontic treatment: A systematic review. Orthod. Craniofac. Res. 2020, 23, 133–142. [Google Scholar] [CrossRef]
- Gao, L.; Tian, Y.; Liu, W.; Song, J. Artificial intelligence in orthodontics: A systematic review and meta-analysis of diagnostic performance and treatment prediction. Am. J. Orthod. Dentofac. Orthop. 2024, 165, 251–262. [Google Scholar]


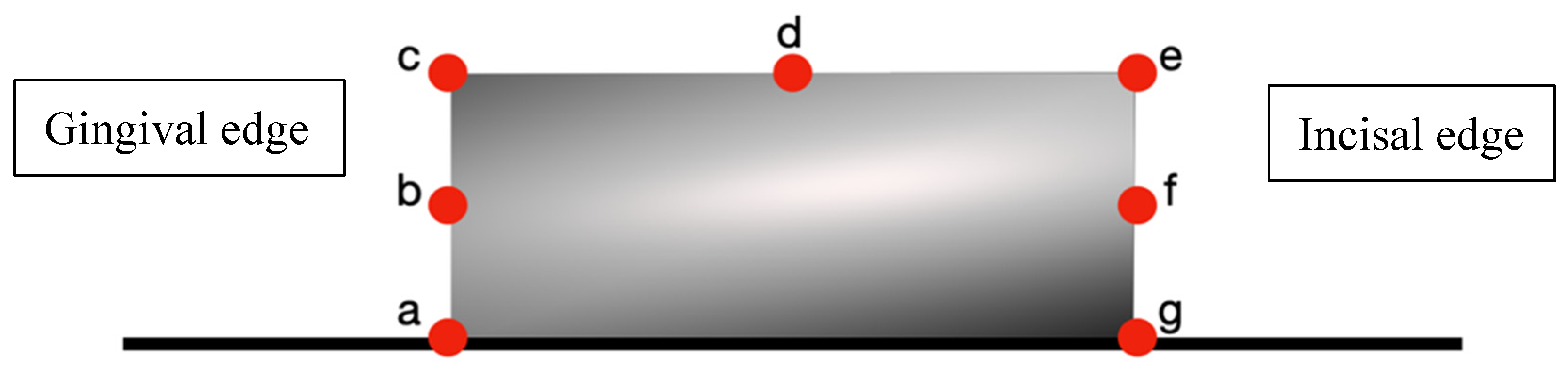
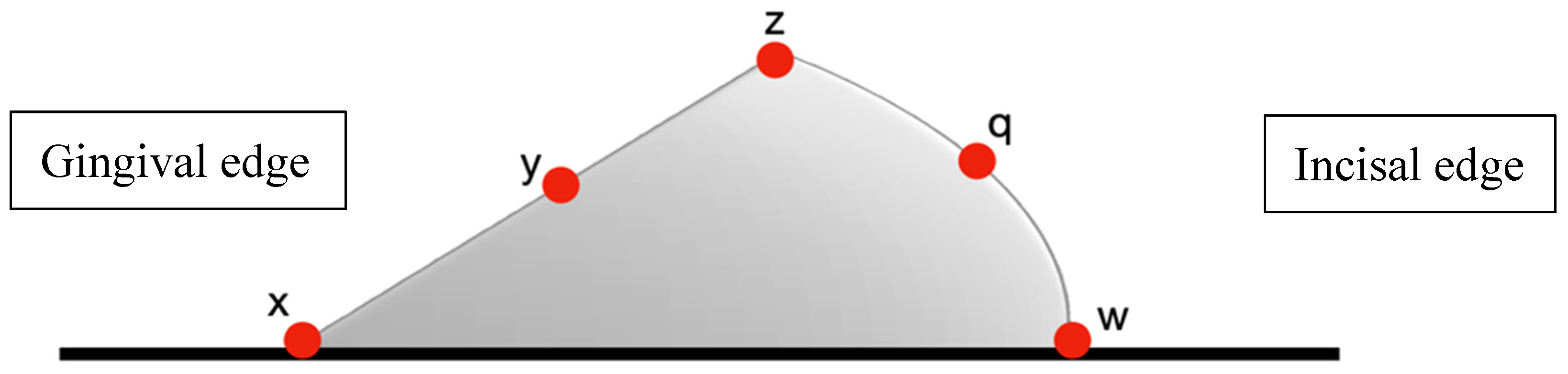

| Measurement Points. | Duran+ (n = 14) | Zendura FLX (n = 14) | p-Value |
|---|---|---|---|
| a (Gingival border) | 91.07 ± 3.11 | 85.77 ± 4.46 | 0.001 ** |
| b (Gingival midpoint) | 70.71 ± 2.42 | 68.51 ± 2.34 | 0.022 * |
| c (Gingival angle) | 50.78 ± 2.26 | 48.75 ± 2.16 | 0.023 * |
| d (Buccal midpoint) | 20.98 ± 2.24 | 19.88 ± 1.74 | 0.159 |
| e (Occlusal angle) | 15.62 ± 1.58 | 14.75 ± 1.41 | 0.138 |
| f (Occlusal midpoint) | 18.87 ± 2.611 | 17.62 ± 2.49 | 0.206 |
| g (Occlusal border) | 43.70 ± 4.123 | 38.41 ± 3.40 | 0.006 * |
| Measurement Points | Duran+ (n = 14) | Zendura FLX (n = 14) | p-Value |
|---|---|---|---|
| x (Gingival border) | 69.91 ± 4.87 | 67.18 ± 4.92 | 0.152 |
| y (Gingival midpoint) | 29.62 ± 3.19 | 27.68 ± 3.27 | 0.127 |
| z (Buccal angle) | 32.38 ± 3.11 | 29.70 ± 3.07 | 0.030 * |
| q (Occlusal midpoint) | 18.25 ± 2.831 | 16.50 ± 2.96 | 0.122 |
| w (Occlusal border) | 47.28 ± 4.07 | 45.50 ± 3.93 | 0.248 |
| Attachment Type | Duran+ | Zendura FLX | p-Value |
|---|---|---|---|
| Rectangular attachment | 44.52 ± 1.51 | 42.10 ± 1.07 | 0.000 ** |
| Optimized attachment | 39.41 ± 3.20 | 37.30 ± 3.09 | 0.089 |
| Measurement Points | Mean ± SD (µm) |
|---|---|
| a (Gingival border) | 88.39 ± 4.62 |
| b (Gingival midpoint) | 69.61 ± 2.59 |
| c (Gingival angle) | 49.77 ± 2.40 |
| d (Buccal midpoint) | 20.43 ± 2.05 |
| e (Occlusal angle) | 15.19 ± 1.54 |
| f (Occlusal midpoint) | 18.24 ± 2.58 |
| g (Occlusal border) | 41.56 ± 4.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sever, C.; Arslan, C. Evaluation of Compatibility of Different Attachment Types Used in Orthodontic Clear Aligners with Electron Microscopy. Dent. J. 2025, 13, 379. https://doi.org/10.3390/dj13080379
Sever C, Arslan C. Evaluation of Compatibility of Different Attachment Types Used in Orthodontic Clear Aligners with Electron Microscopy. Dentistry Journal. 2025; 13(8):379. https://doi.org/10.3390/dj13080379
Chicago/Turabian StyleSever, Can, and Can Arslan. 2025. "Evaluation of Compatibility of Different Attachment Types Used in Orthodontic Clear Aligners with Electron Microscopy" Dentistry Journal 13, no. 8: 379. https://doi.org/10.3390/dj13080379
APA StyleSever, C., & Arslan, C. (2025). Evaluation of Compatibility of Different Attachment Types Used in Orthodontic Clear Aligners with Electron Microscopy. Dentistry Journal, 13(8), 379. https://doi.org/10.3390/dj13080379







