The Correlations Between Diabetes Mellitus and Oro-Maxillofacial Disorders: A Statistical Perspective
Abstract
1. Introduction
2. Materials and Methods
- -
- Oral pathology;
- -
- Availability of complete medical and diagnostic records;
- -
- Confirmed diagnosis regarding the presence or absence of diabetes mellitus.
- -
- Incomplete or inconsistent medical records;
- -
- Unconfirmed diabetic status;
- -
- Patients with maxillofacial trauma or congenital craniofacial anomalies unrelated to systemic disease.
- -
- Malignant tumors (MT);
- -
- Periodontal disease (PD);
- -
- Apical periodontitis (PA);
- -
- Periapical cysts (PC);
- -
- Root remnants (R).
Statistical Analysis
3. Results
- -
- In males (58.7%; RR = 1.06; 95% CI: 0.94–1.20; p = 0.175);
- -
- Slightly more frequently in individuals over 54 years of age (50.6%; RR = 1.01; 95% CI: 0.99–1.02; p = 0.297), with the most affected age group being 60–69 years (20.3%);
- -
- In patients from urban areas (50.7%; RR = 1.15; 95% CI: 1.02–1.29; p = 0.014).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Oral health: Action Plan for Promotion and Integrated Disease Prevention (EB120/10). In Proceedings of the 120th Session of the Executive Board of the World Health Organization (WHO), Geneva, Switzerland, 22–30 January 2007. [Google Scholar]
- Kassebaum, N.J.; Smith, A.G.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, regional, and national prevalence, incidence, and disabilityadjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Wagner, C.; Carmeli, C.; Jackisch, J.; Kivimäki, M.; van der Linden, B.W.; Cullati, S.; Chiolero, A. Lifecourse epidemiology and public health. Lancet Public Health 2024, 9, e261–e269. [Google Scholar] [CrossRef] [PubMed]
- Mayuri, H.M.; Vasantha, S. Knowledge of Dental Caries, Oral Hygiene Practices, and Barriers to Accessing Dental Care among the Patients Attending Endodontic Treatment for Carious Permanent Molar Teeth at Restorative Unit, National Dental Hospital, Sri Lanka. J. Liaquat Natl. Hosp. 2025, 3, 16–22. [Google Scholar]
- Gaeta, C.; Malvicini, G.; Di Lascio, D.; Martignoni, M.; Ragucci, G.; Grandini, S.; Marruganti, C. Lifestyle, caries, and apical periodontitis: Results from a university-based cross-sectional study. Int. Endod. J. 2025, 58, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, P.; Madanian, S.; Marshall, S. Investigating the link between oral health conditions and systemic diseases: A cross-sectional analysis. Sci. Rep. 2025, 15, 10476. [Google Scholar] [CrossRef]
- Kim, M.Y.; Pang, E.K. Relationship between periodontitis and systemic health conditions: A narrative review. Ewha Med. J. 2025, 48, e27. [Google Scholar] [CrossRef]
- Souza, P.R.M.; Dupont, L.; Mosena, G.; Dantas, M.L.; Bulcão, L.A. Variations of oral anatomy and common oral lesions. An. Bras. De Dermatol. 2024, 99, 3–18. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021, 236, 72–86. [Google Scholar] [CrossRef]
- Alqadi, S.F. Diabetes mellitus and its influence on oral health. Diabetes Metab. Syndr. Obes. 2024, 17, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Reasner, C.; DeFronzo, R.A.; Cersosimo, E. Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, L.; Martu, M.A.; Ciurcanu, O.E.; Luca, E.O.; Salceanu, M.; Anton, D.; Martu, C.; Martu, S.; Esanu, I.M. Influence of diabetes mellitus and smoking on pro and antiinflammatory cytokine profiles in gingival crevicular fluid. Diagnostics 2023, 13, 3051. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.R.; Huck, O.; Hummers, E.; et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the joint workshop by the European Federation of Periodontology and WONCA Europe. J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef]
- Stöhr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and metaanalysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Taylor, J.J. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J. Clin. Periodontol. 2011, 38, 60–84. [Google Scholar] [CrossRef]
- Maris, M.; Martu, M.A.; Maris, M.; Martu, C.; Anton, D.M.; Pacurar, M.; Earar, K. Clinical and microbiological periodontal biofilm evaluation of patients with type 1 diabetes. J. Clin. Med. 2024, 13, 6724. [Google Scholar] [CrossRef]
- Nibali, L.; Gkranias, N.; Mainas, G.; Di Pino, A. Periodontitis and implant complications in diabetes. Periodontol 2000 2022, 90, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Bolchis, V.; Jumanca, D.; Dumitrescu, R.; Balean, O.; Toderas, N.A.; Popescu, S.; Marcu, A.; Marian, C.; Galuscan, A. Glycaemic control, inflammatory mediators, and periodontal health: A crosssectional study in patients with diabetes. J. Clin. Med. 2025, 14, 2847. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, E.S. Impact of nonsurgical periodontal treatment on glycosylated haemoglobin level in nondiabetic people. Ann. Dent. Spec. 2024, 12, 16. [Google Scholar] [CrossRef]
- Ranbhise, J.S.; Ju, S.; Singh, M.K.; Han, S.; Akter, S.; Ha, J.; Choe, W.; Kim, S.S.; Kang, I. Chronic inflammation and glycaemic control: Exploring the bidirectional link between periodontitis and diabetes. Dent. J. 2025, 13, 100. [Google Scholar] [CrossRef]
- Inoue, M.; Sakanaka, A.; Katakami, N.; Furuno, M.; Nishizawa, H.; Omori, K.; Taya, N.; Ishikawa, A.; Mayumi, S.; Tanaka Isomura, E.; et al. Periodontal tissue susceptibility to glycaemic control in type 2 diabetes. Diabetes Obes. Metab. 2024, 26, 4684–4693. [Google Scholar] [CrossRef]
- RamosGarcia, P.; RocaRodriguez, M.D.M.; AguilarDiosdado, M.; GonzalezMoles, M.A. Diabetes mellitus and oral cancer/oral potentially malignant disorders: A systematic review and metaanalysis. Oral Dis. 2021, 27, 404–421. [Google Scholar] [CrossRef]
- Nicolae, V.; Neamtu, B.; Picu, O.; Stefanache, M.A.M.; Cioranu, V.S.I. Comparative evaluation of salivary biomarkers (calcium, phosphate, salivary pH) in carioresistance versus carioactivity. Rev. Chim. 2016, 67, 821–824. [Google Scholar]
- Cristea, I.; AgopForna, D.; Martu, M.A.; Dascălu, C.; Topoliceanu, C.; Török, R.; Török, B.; Bardis, D.; Bardi, P.M.; Forna, N. Oral and periodontal risk factors of prosthetic success for threeunit toothsupported bridges versus implantsupported fixed dental prostheses. Diagnostics 2023, 13, 852. [Google Scholar] [CrossRef]
- Solomon, S.M.; Timpu, D.; Forna, D.A.; Stefanache, M.A.; Martu, S.; Stoleriu, S. AFM comparative study of root surface morphology after three methods of scaling. Mater. Plast. 2016, 53, 546–549. [Google Scholar]
- Solomon, S.M.; Stoleriu, S.; AgopForna, D.; Timpu, D.; Martu Stefanache, M.A.; Ursarescu, I.G.; Martu, S. Quantitative and qualitative assessment of dental substance loss as consequence of root planing by three techniques. Mater. Plast. 2016, 53, 305–307. [Google Scholar]
- Liu, X.; He, G.; Qiu, Z.; Chen, F.; Wang, J.; Huang, Z.; Zhang, P.; Zhang, J.; Zhong, L.; Ding, C.; et al. Diabetes mellitus increases the risk of apical periodontitis in endodonticallytreated teeth: A metaanalysis. J. Endod. 2023, 49, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Oral cancer in Taiwan: Is diabetes a risk factor? Clin. Oral Investig. 2013, 17, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Zaharescu, A.; Solomon, S.M.; Luca, M.G.; Toma, V.; Luchian, I.; Sufaru, I.G.; Martu, S. Quantification of proinflammatory molecules in crevicular fluid and serum in patients with endoperiodontal lesions. Rev. Chim. 2019, 70, 2252–2255. [Google Scholar] [CrossRef]
- Zaharescu, A.; Mârțu, I.; Luchian, A.I.; Mârțu, M.A.; Șufaru, I.G.; Mârțu, C.; Solomon, S.M. Adjunctive subantimicrobial doxycycline therapy improves HbA1c in patients with diabetes and endoperiodontal lesions. Exp. Ther. Med. 2021, 21, 277. [Google Scholar] [CrossRef]
- Smadi, L. Apical periodontitis and endodontic treatment in patients with type 2 diabetes mellitus: A comparative crosssectional survey. J. Contemp. Dent. Pract. 2017, 18, 358–362. [Google Scholar]
- Marica, A.; Chirla, R.; Porumb, M.; Sipos, L.R.; Iurcov, R.O.C.; Cavalu, S. Impact of type 2 diabetes mellitus on prevalence of apical periodontitis in endodontically treated and untreated teeth. J. Med. Life 2024, 17, 918. [Google Scholar] [CrossRef]
- de Barros, M.C.; VelasquezEspedilla, E.G.; de Oliveira, L.D.; Gomes, B.P.F.; Duarte, M.A.H.; Negrato, C.A.; de Andrade, F.B. Impact of type 2 diabetes mellitus on apical periodontitis size, endotoxin levels and bacterial load. J. Endod. 2025, 51, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, R.; Tewari, S.; Gill, P.S.; Monga, N.; Mittal, S.; Duhan, J. Association of apical periodontitis with glycated haemoglobin and systemic inflammatory markers in type 2 diabetes. J. Endod. 2025, 51, 124–131. [Google Scholar] [CrossRef]
- Mortazavi, H.; Baharvand, M.; Movahhedian, A.; Mohammadi, M.; Khodadoustan, A. Xerostomia due to systemic disease: A review of 20 conditions and mechanisms. Ann. Med. Health Sci. Res. 2014, 4, 503–510. [Google Scholar]
- Khan, F.R.; Iftikhar, K.; Hashmi, A.; Ismail, M.; Siddiqui, S.H.; Siddiqui, H.K. Extraction socket complications among diabetic, hypertensive and smoker patients versus controls. Adv. Oral Maxillofac. Surg. 2021, 2, 100032. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.J.; OtomoCorgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading periodontitis: Framework and proposal. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Darby, I. Risk factors for periodontitis and periimplantitis. Periodontol 2000 2022, 90, 9–12. [Google Scholar] [CrossRef]
- Ceriello, A.; Prattichizzo, F. Variability of risk factors and diabetes complications. Cardiovasc. Diabetol. 2021, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Templer, S.; Abdo, S.; Wong, T. Preventing diabetes complications. Intern. Med. J. 2024, 54, 1264–1274. [Google Scholar] [CrossRef]
- Jiao, Y.R.; Chen, K.X.; Tang, X.; Tang, Y.L.; Yang, H.L.; Yin, Y.L.; Li, C.J. Exosomes derived from mesenchymal stem cells in diabetes and diabetic complications. Cell Death Dis. 2024, 15, 271. [Google Scholar] [CrossRef]
- Jia, D.; Liu, L.; Liu, W.; Li, J.; Jiang, X.; Xin, Y. Copper metabolism and its role in diabetic complications: A review. Pharmacol. Res. 2024, 206, 107264. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative stress in diabetes mellitus and its complications: Pathogenetic role and therapeutic approaches. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Yang, T.; Qi, F.; Guo, F.; Shao, M.; Song, Y.; Ren, G.; Linlin, Z.; Qin, G.; Zhao, Y. Chronic complications of diabetes mellitus: Mechanisms to therapeutic strategies with focus on metabolic memory. Mol. Med. 2024, 30, 71. [Google Scholar] [CrossRef]
- Wondm, S.A.; Zeleke, T.K.; Dagnew, S.B.; Moges, T.A.; Tarekegn, G.Y.; Belachew, E.A.; Tamene, F.B. Association between selfcare and glycaemic control in type 2 diabetes: A multicentre study. Sci. Rep. 2024, 14, 23198. [Google Scholar] [CrossRef]
- Oluwatoyin, A.E.; Arinola, E.; Olufemi, O.E.; Jokotade, A. Selfreported oral health and quality of life in patients with diabetes mellitus. BMC Oral Health 2024, 24, 181. [Google Scholar] [CrossRef]
- Slowik, J.; Kaczynski, L.; Kaczor, M.; Wnuk, M. Oral healthrelated quality of life in patients with type II diabetes mellitus: Systematic review and metaanalysis. BMC Oral Health 2025, 25, 485. [Google Scholar] [CrossRef] [PubMed]
- Mubayrik, A.F.B.; Alhoqail, R.I.; Dous, R.A. Oral healthrelated quality of life among diabetic patients: A crosssectional controlled study. J. Fam. Med. Prim. Care 2024, 13, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Zafar, S.; Sharma, A.; Quaranta, A. Awareness of diabetic patients regarding the bidirectional association between periodontal disease and diabetes mellitus. J. Contemp. Dent. Pract. 2020, 21, 1270–1274. [Google Scholar]
- Chung, Y.L.; Lee, J.J.; Chien, H.H.; Chang, M.C.; Jeng, J.H. Interplay between diabetes mellitus and periodontal/pulpalperiapical diseases. J. Dent. Sci. 2024, 19, 1338–1347. [Google Scholar] [CrossRef]
- Memon, N.H.; Tanvir, S.; Munawar, A.; Abbasi, M.A.; Khan, A.; Ashraf, M. Diabetes in the pathogenesis of periapical lesions. J. Health Rehabil. Res. 2024, 4, 15. [Google Scholar]
- Sowmya, S.; Sangavi, R. Effectiveness of oral health education and interventions on oral health outcomes in type II diabetes mellitus patients: Prospective study. Cureus 2024, 16, e58227. [Google Scholar]
- Borgnakke, W.S.; Poudel, P. Diabetes and oral health: Current evidence for transdisciplinary collaboration. Front. Dent. Med. 2021, 2, 709–831. [Google Scholar] [CrossRef]
- Siddiqi, A.; Zafar, S.; Sharma, A.; Quaranta, A. Diabetic patients’ knowledge of the bidirectional link: Are dental health care professionals effectively conveying the message? Aust. Dent. J. 2019, 64, 312–326. [Google Scholar] [CrossRef]
- Simpson, T.C.; Weldon, J.C.; Worthington, H.V.; Needleman, I.; Wild, S.H.; Moles, D.R.; Stevenson, B.; Furness, S.; Iheozor-Ejiofor, Z. Periodontal treatment for glycaemic control in diabetes mellitus. Cochrane Database Syst. Rev. 2015, 11, CD004714. [Google Scholar]
- Budala, D.G.; Martu, M.A.; Maftei, G.A.; DiaconuPopa, D.A.; Danila, V.; Luchian, I. Natural compounds in contemporary dental treatment—Current status and future trends. J. Funct. Biomater. 2023, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Sufaru, I.G.; Martu, M.A.; Luchian, I.; Stoleriu, S.; DiaconuPopa, D.; Martu, C.; Teslaru, S.; Pasarin, L.; Solomon, S.M. Effect of 810 nm diode laser and indocyanine green on periodontal parameters and HbA1c in periodontitis with type 2 diabetes. Diagnostics 2022, 12, 1614. [Google Scholar] [CrossRef]
- Hurjui, I.; Carmen, D.; Liliana, H.L.; Raluca, J.; Mihaela, M.; Carina, B.; Oana, A.A.; Alexandra, M.M.; Irina, G. Platelet derivatives with dental applications. Rom. J. Oral Rehabil. 2020, 12, 142–152. [Google Scholar]
- Tse, S.Y. Diabetes mellitus and periodontal disease: Awareness and practice among physicians in Hong Kong. BMC Fam. Pract. 2018, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.; Griffiths, R.; Wong, V.W.; Arora, A.; Flack, J.R.; Khoo, C.L.; George, A. General practitioners’ perceptions on providing oral health care to people with diabetes. BMC Fam. Pract. 2020, 21, 34. [Google Scholar] [CrossRef]
- Poudel, P.; Griffiths, R.; Wong, V.W.; Arora, A.; Flack, J.R.; Khoo, C.L.; George, A. Diabetes educators’ practices in providing oral health care. Diabetes Educ. 2018, 44, 454–464. [Google Scholar] [CrossRef]
- AlShwaimi, E.; Idrees, M.; Berri, Z.; ElSakka, H.; Kujan, O. Association between diabetes mellitus and periodontal diseases: Opinions of dental professionals. Med. Princ. Pract. 2019, 28, 141–149. [Google Scholar] [CrossRef]
- Fu, D.; Shu, X.; Zhou, G.; Ji, M.; Liao, G.; Zou, L. Connection between oral health and chronic diseases. MedComm 2025, 6, e70052. [Google Scholar] [CrossRef]
- Ziebolz, D.; Bauwe, I.; Schmidt, J.; Kottmann, T.; Rinke, S.; Schmalz, G. Diabetes screening in dental practice using the FINDRISK questionnaire: Pilot study. Oral Health Prev. Dent. 2019, 17, 147–156. [Google Scholar] [PubMed]
- Harada, Y.; Giri, P.; Prajapati, D.; Sakamoto, H.; Sugishita, T.; Rawal, L. Engaging community health workers to promote oral health for people with type 2 diabetes in Nepal: Qualitative study. BMC Prim. Care 2025, 26, 89. [Google Scholar] [CrossRef]
- SáenzRavello, G.; CastilloRiquelme, M.; Cuadrado, C.; Gamonal, J.; Baeza, M. Efficiency of periodontal treatment to improve type 2 diabetes outcomes: Systematic review and economic evaluation. Community Dent. Oral Epidemiol. 2025, 53, 135–151. [Google Scholar] [CrossRef] [PubMed]
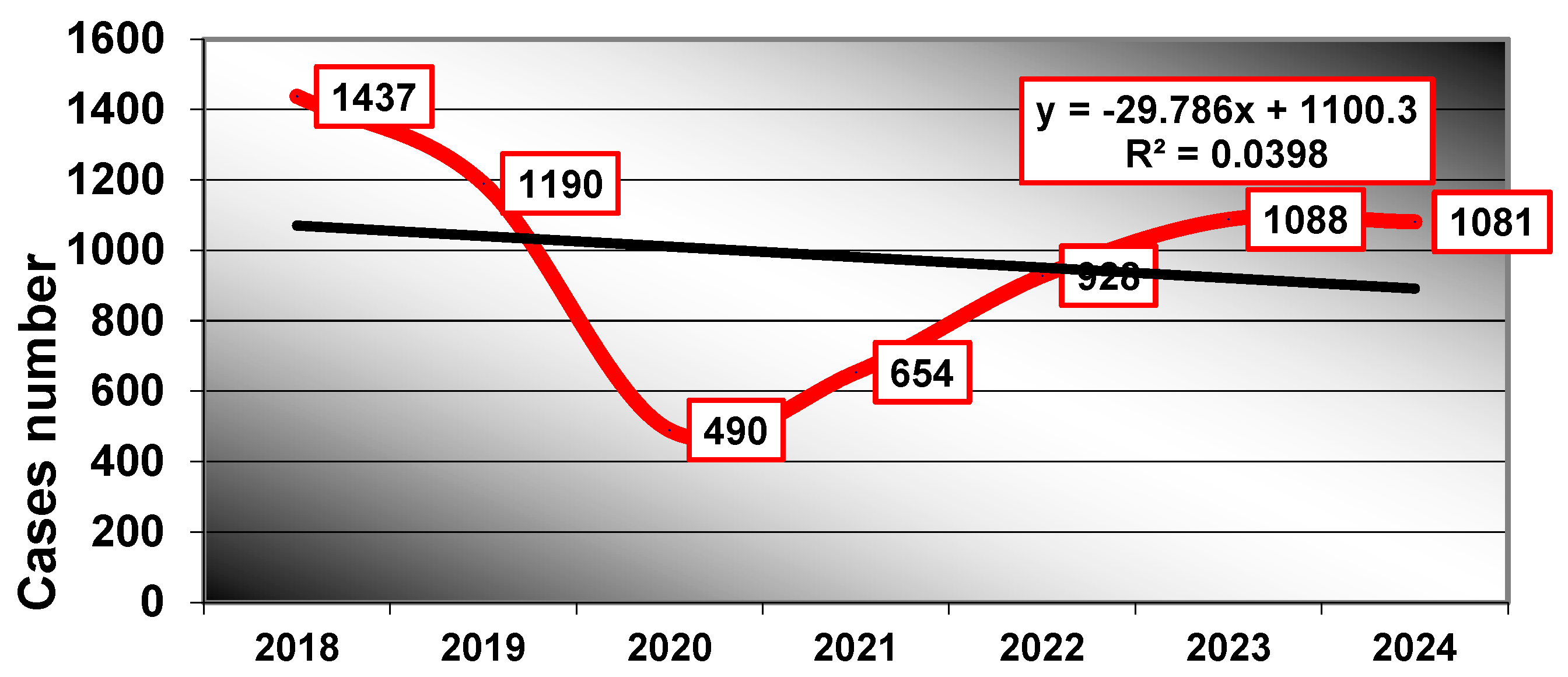


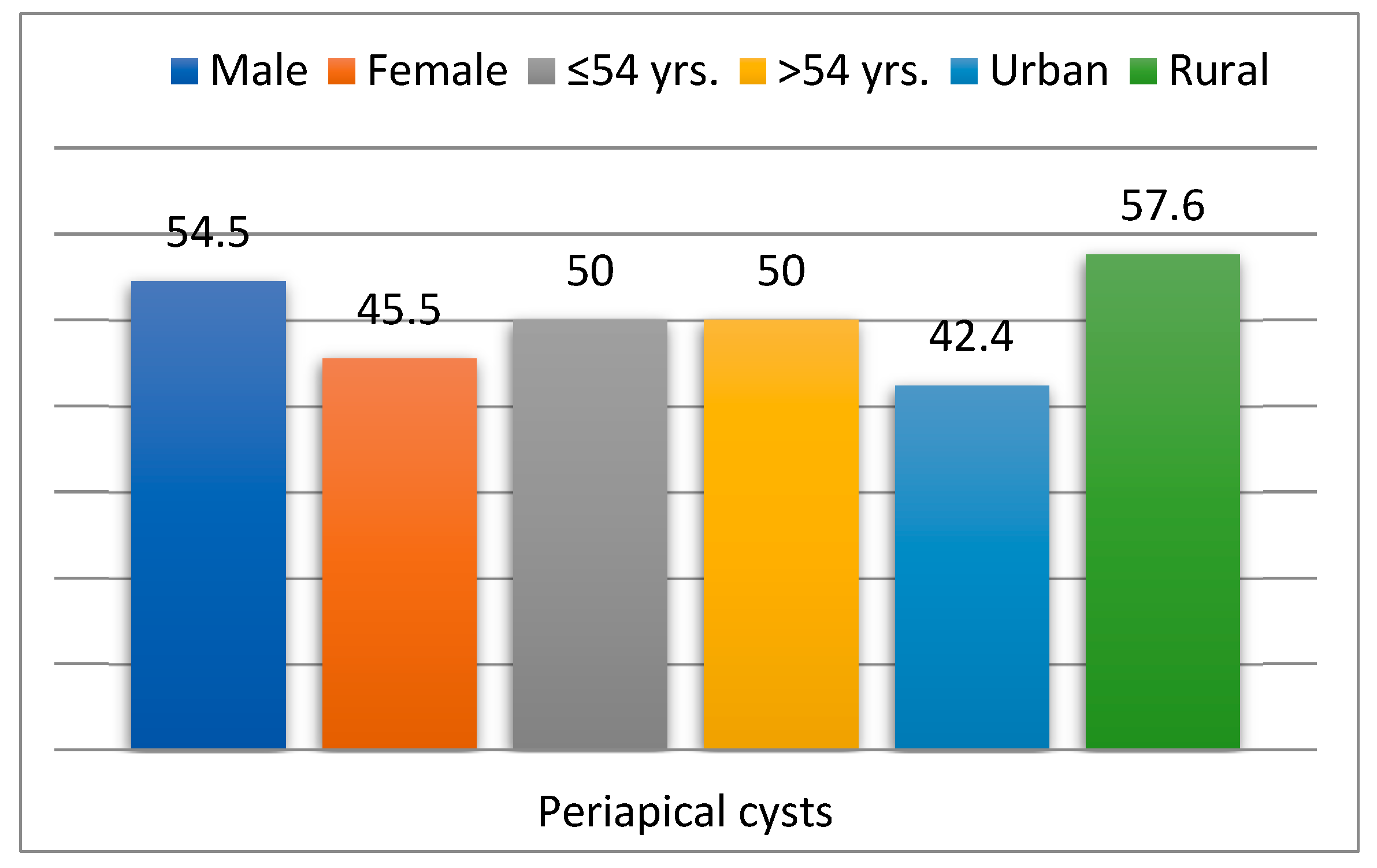

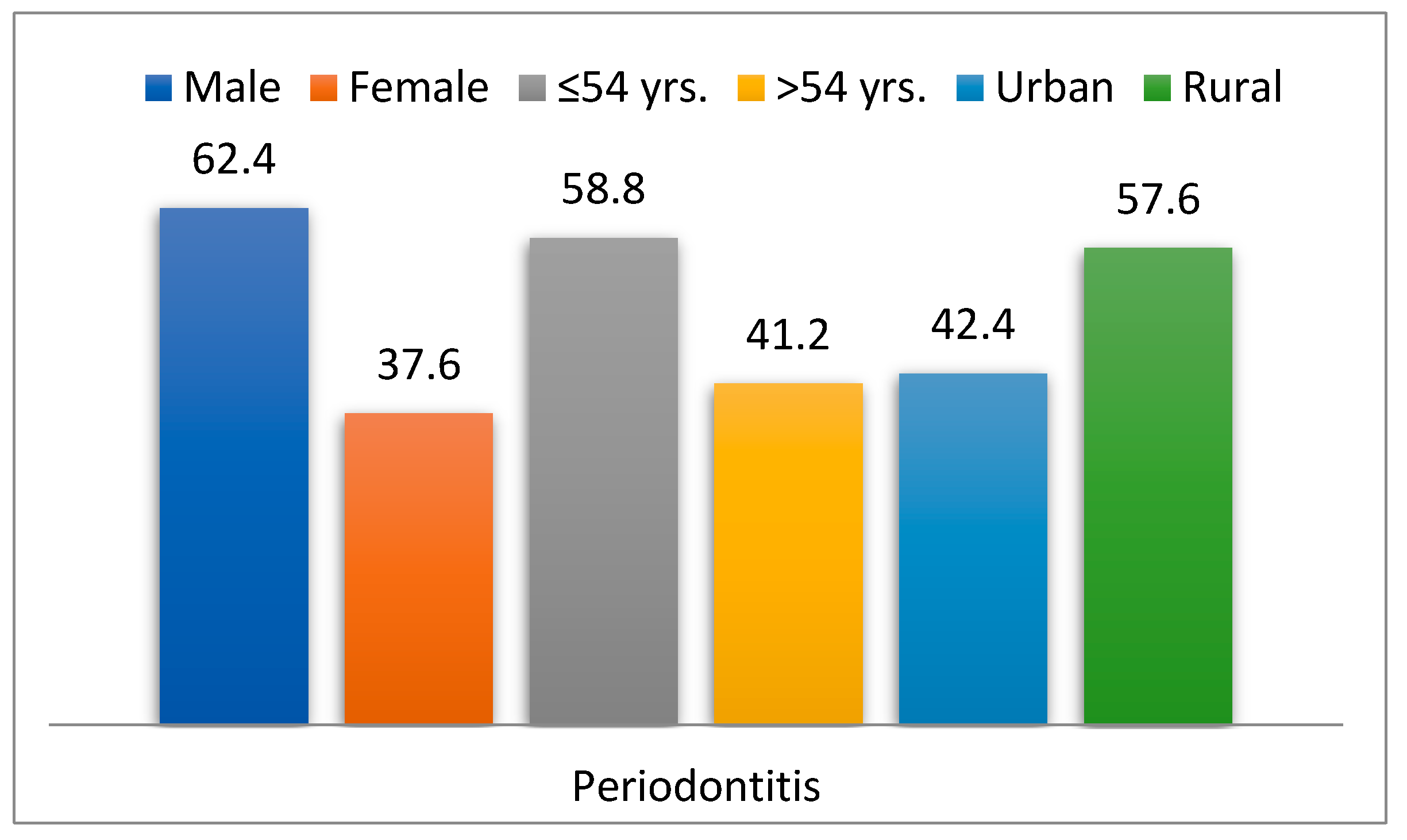
| Demographic Features | Diabetes Mellitus Group (n = 925) | Non-Diabetes Group (n = 5943) | Chi2 Test p | RR | 95% IC | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Gender | 0.175 | ||||||
| Males | 543 | 58.7 | 3388 | 57.0 | 1.06 | 0.94–1.20 | |
| Females | 382 | 41.3 | 2555 | 43.0 | 0.99 | 0.97–1.01 | |
| Age groups | 0.297 | ||||||
| ≤54 years. | 457 | 49.4 | 2996 | 50.4 | 0.97 | 0.86–1.09 | |
| >54 years. | 468 | 50.6 | 2947 | 49.6 | 1.01 | 0.99–1.02 | |
| Environment of origin | 0.014 | ||||||
| Urban | 469 | 50.7 | 2780 | 46.8 | 1.15 | 1.02–1.29 | |
| Rural | 456 | 49.3 | 3163 | 53.2 | 0.98 | 0.96–1.00 | |
| Comorbidities | DM Group (n = 925) | Non-DM Group (n = 5943) | Chi2 Test p | RR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Malignant tumors | 139 | 15.0 | 83 | 1.4 | 0.001 | 5.29 | 4.69–5.98 |
| Root remnants | 83 | 9.0 | 361 | 6.1 | 0.001 | 1.43 | 1.16–1.75 |
| Periapical cysts | 22 | 2.4 | 43 | 0.7 | 0.001 | 2.55 | 1.81–3.60 |
| Apical periodontitis | 49 | 5.3 | 58 | 1.0 | 0.001 | 3.53 | 2.85–4.38 |
| Periodontal disease | 51 | 5.5 | 34 | 0.6 | 0.001 | 4.66 | 3.87–5.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botezatu, I.C.; Salceanu, M.; Botez, A.E.; Dimitriu, C.D.; Ciurcanu, O.E.; Topoliceanu, C.; Cotrutz, E.-C.; Martu, M.-A. The Correlations Between Diabetes Mellitus and Oro-Maxillofacial Disorders: A Statistical Perspective. Dent. J. 2025, 13, 373. https://doi.org/10.3390/dj13080373
Botezatu IC, Salceanu M, Botez AE, Dimitriu CD, Ciurcanu OE, Topoliceanu C, Cotrutz E-C, Martu M-A. The Correlations Between Diabetes Mellitus and Oro-Maxillofacial Disorders: A Statistical Perspective. Dentistry Journal. 2025; 13(8):373. https://doi.org/10.3390/dj13080373
Chicago/Turabian StyleBotezatu, Ionut Catalin, Mihaela Salceanu, Ana Emanuela Botez, Cristina Daniela Dimitriu, Oana Elena Ciurcanu, Claudiu Topoliceanu, Elena-Carmen Cotrutz, and Maria-Alexandra Martu. 2025. "The Correlations Between Diabetes Mellitus and Oro-Maxillofacial Disorders: A Statistical Perspective" Dentistry Journal 13, no. 8: 373. https://doi.org/10.3390/dj13080373
APA StyleBotezatu, I. C., Salceanu, M., Botez, A. E., Dimitriu, C. D., Ciurcanu, O. E., Topoliceanu, C., Cotrutz, E.-C., & Martu, M.-A. (2025). The Correlations Between Diabetes Mellitus and Oro-Maxillofacial Disorders: A Statistical Perspective. Dentistry Journal, 13(8), 373. https://doi.org/10.3390/dj13080373







