Thermal Effects of Pulsed Infrared Lasers on Zirconia Implants at Different Temperatures In Vitro
Abstract
1. Introduction
2. Materials and Methods
- CO2 laser (Denta 2, Lutronic, GPT, Fairfield, NE, USA) with 2 W mean power in a pulsed mode, 700 ms pulse width, and 70 Hz frequency.
- Er,Cr:YSGG laser (Waterlase MD, Foothill Ranch, CA, USA; Biolase, USA, Irvine, CA, USA) with 1.5 W pulsed mode, 45 mJ, and 30 Hz utilizing MZ5 cylindrical glass tips.
3. Results
3.1. Temperature Changes for CO2 Laser
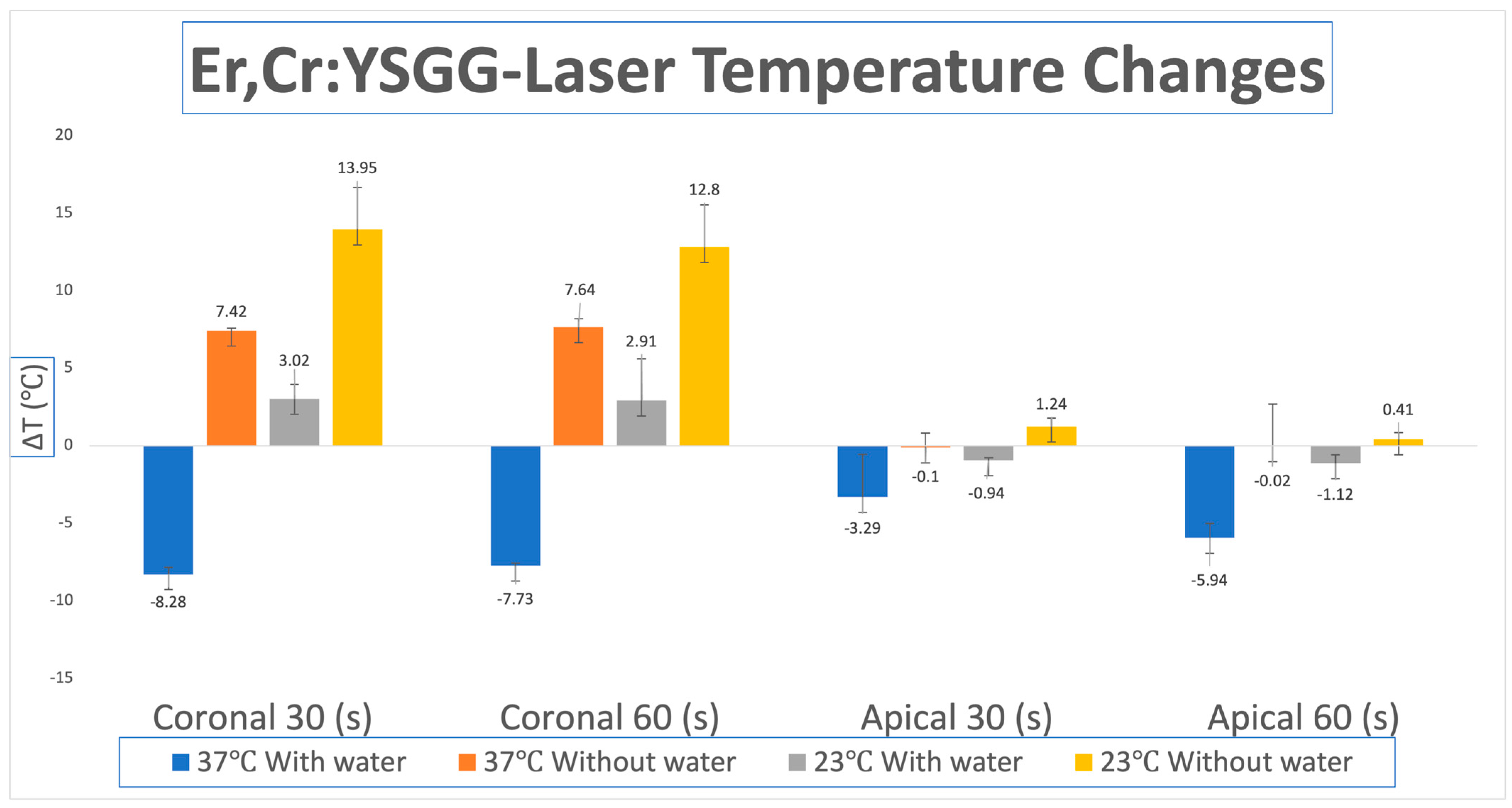
3.2. Temperature Changes for Er,Cr:YSGG Laser
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prathapachandran, J.; Suresh, N. Management of peri-implantitis. Dent. Res. J. 2012, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis Update: Risk Indicators, Diagnosis, and Treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Aoki, A.; Takasaki, A.A. Potential applications of Erbium:YAG laser in periodontics. J. Periodontal. Res. 2004, 39, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Yukna, R.A.; Scott, J.B.; Aichelmann-Reidy, M.E.; LeBlanc, D.M.; Mayer, E.T. Clinical Evaluation of the Speed and Effectiveness of Subgingival Calculus Removal on Single-Rooted Teeth With Diamond-Coated Ultrasonic Tips. J. Periodontol. 1997, 68, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, N.; Yano, K.; He, T.; Umeda, M.; Ishikawa, I. Effect of Initial Periodontal Therapy on the Frequency of Detecting Bacteroides forsythus, Porphyromonas gingivalis, and Actinobacillus actinomycetemcomitans. J. Periodontol. 1999, 70, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Wikström, M.; Dahlén, G.; Slots, J.; Egelberg, J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J. Clin. Periodontol. 1990, 17, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.K.; O’Leary, T.J.; Kafrawy, A.H. The Effectiveness of Hand Versus Ultrasonic Instrumentation in Open Flap Root Planing. J. Periodontol. 1984, 55, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Aoki, A.; Coluzzi, D.; Yukna, R.; Wang, C.; Pavlic, V.; Izumi, Y. Lasers in minimally invasive periodontal and peri-implant therapy. Periodontology 2000 2016, 71, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Al-Falaki, R.; Hughes, F.; Wadia, R.; Eastman, C.; Kontogiorgos, E.; Low, S. The Effect of an Er,Cr:YSGG Laser in the Management of Intrabony Defects Associated with Chronic Periodontitis Using Minimally Invasive Closed Flap Surgery. A Case Series. Laser Ther. 2016, 25, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pavone, C.; Perussi, L.R.; de Oliveira, G.J.P.L.; Scardueli, C.R.; Cirelli, J.A.; Cerri, P.S.; Junior, E.M.; Spolidorio, L.C.; Marcantonio, R.A.C. Effect of Er,Cr:YSGG laser application in the treatment of experimental periodontitis. Lasers Med. Sci. 2014, 30, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.; Ko, H.-H.; Froum, S.; Tarnow, D. The Use of CO2 Laser in the Treatment of Peri-implantitis. Photomed. Laser Surg. 2009, 27, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, R.A.; Albrektsson, T. The effect of heat on bone regeneration: An experimental study in the rabbit using the bone growth chamber. J. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Mouhyi, J.; Sennerby, L.; Nammour, S.; Guillaume, P.; Van Reck, J. Temperature increases during surface decontamination of titanium implants using CO2 laser. Clin. Oral Implant. Res. 1999, 10, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.Q.; Jenny, J.E.; Hargreaves, K.M. Heat generation in hydroxyapatite-coated implants as a result of CO2 laser application. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1995, 79, 410–415. [Google Scholar] [CrossRef]
- Kreisler, M.; Al Haj, H.; Götz, H.; Duschner, H.; D’Hoedt, B. Effect of simulated CO2 and GaAlAs laser surface decontamination on temperature changes in Ti-plasma sprayed dental implants. Lasers Surg. Med. 2002, 30, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Santos, L.; Arnabat-Dominguez, J.; Sierra-Rebolledo, A.; Gay-Escoda, C. Thermal increment due to ErCr:YSGG and CO2 laser irradiation of different implant surfaces. A pilot study. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e782–e787. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Calce, L.; Hong, H.; Hou, W.; Romanos, G.E. Thermal Effects of Diode Laser-Irradiation on Titanium Implants in Different Room Temperatures In Vitro. Photobiomodulation Photomed. Laser Surg. 2022, 40, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Calce, L.; Hafeez, M.; Hou, W.; Romanos, G.E. Thermal Effects of 445-nm Diode Laser Irradiation on Titanium and Ceramic Implants. J. Oral Implant. 2023, 49, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Deppe, H.; Greim, H.; Brill, T.; Wagenpfeil, S. Titanium Deposition After Peri-Implant Care with The Carbon Dioxide Laser. Int. J. Oral Maxillofac. Implant. 2002, 17, 707–714. [Google Scholar]
- Schou, S.; Odont, D.; Berglundh, T.; Dr, O.; Lang, N.P. Surgical Treatment of Peri-implantitis n.d. J. Clin. Periodontol. 2008, 35, 316–332. [Google Scholar]
- Ting, C.; Fukuda, M.; Watanabe, T.; Aoki, T.; Sanaoka, A.; Noguchi, T. Effects of Er,Cr:YSGG Laser Irradiation on the Root Surface: Morphologic Analysis and Efficiency of Calculus Removal. J. Periodontol. 2007, 78, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Hakki, S.S.; Korkusuz, P.; Berk, G.; Dundar, N.; Saglam, M.; Bozkurt, B.; Purali, N. Comparison of Er,Cr:YSGG Laser and Hand Instrumentation on the Attachment of Periodontal Ligament Fibroblasts to Periodontally Diseased Root Surfaces: An In Vitro Study. J. Periodontol. 2010, 81, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Al-Falaki, R.; Cronshaw, M.; Hughes, F.J. Treatment outcome following use of the erbium, chromium:yttrium, scandium, gallium, garnet laser in the non-surgical management of peri-implantitis: A case series. Br. Dent. J. 2014, 217, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Shimada, K.; Iwasaki, H.; Ito, K. Inhibitory effects of a super pulsed carbon dioxide laser at low energy density on periodontopathic bacteria and lipopolysaccharide in vitro. J. Periodontal. Res. 2005, 40, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Hellak, A.; Schauseil, M.; Korbmacher-Steiner, H.; Braun, A. Effects of 445-nm Diode Laser-Assisted Debonding of Self-Ligating Ceramic Brackets on Shear Bond Strength. Photomed. Laser Surg. 2018, 36, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Frentzen, M.; Kraus, D.; Reichelt, J.; Engelbach, C.; Dehn, C.; Meister, J. A novel blue light diode laser (445 nm) for dental application Biomedical testing and clinical aspects. Laser Int. Mag. Laser Dent. 2016, 8, 6–13. [Google Scholar]
- Deppe, H.; Ahrens, M.; Behr, A.V.; Marr, C.; Sculean, A.; Mela, P.; Ritschl, L.M. Thermal effect of a 445 nm diode laser on five dental implant systems: An in vitro study. Sci. Rep. 2021, 11, 20174. [Google Scholar] [CrossRef] [PubMed]
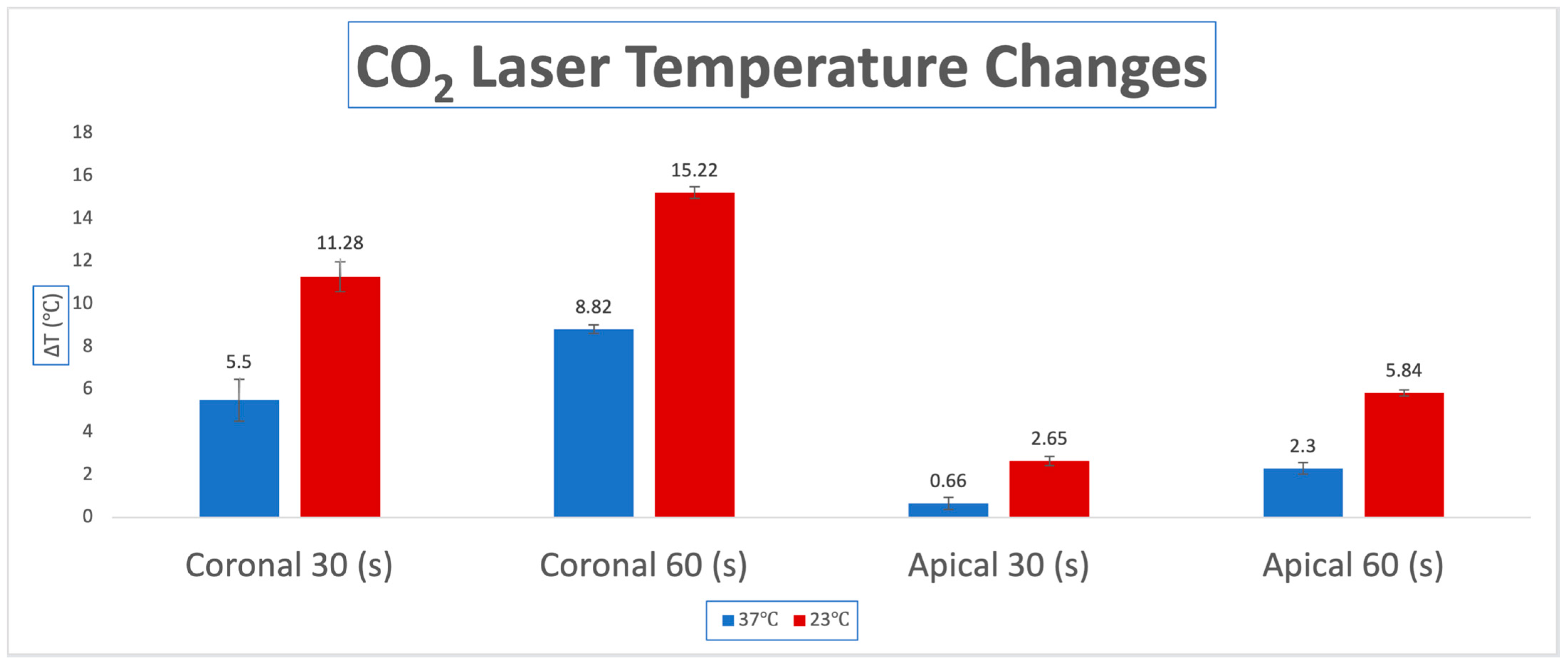
| CO2 Laser Pulsed | Body Temp. 37 °C | Room Temp. 23 °C | p Value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| ΔΤ coronal | |||
| 30 s | 5.50 (0.98) | 11.28 (0.70) | <0.0001 |
| 60 s | 8.82 (0.21) | 15.22 (0.28) | <0.0001 |
| ΔΤ apical | |||
| 30 s | 0.66 (0.28) | 2.65 (0.21) | <0.0001 |
| 60 s | 2.30 (0.28) | 5.84 (0.14) | <0.0001 |
| Er,Cr:YSGG-Laser | Body Temp. 37 °C | Room Temp. 23 °C | ||||
|---|---|---|---|---|---|---|
| With Water | Without Water | p-Value | With Water | Without Water | p-Value | |
| ΔΤ coronal | Mean (SD) | Mean (SD) | ||||
| 30 s | −8.28 (0.44) | 7.42 (0.17) | <0.0001 | 3.02 (2.73) | 13.95 (0.92) | <0.0001 |
| 60 s | −7.73 (0.17) | 7.64 (0.55) | <0.0001 | 2.91 (0.92) | 12.80 (2.70) | <0.0001 |
| ΔΤ apical | Mean (SD) | Mean (SD) | ||||
| 30 s | −3.29 (0.92) | −0.1 (2.70) | <0.0001 | −0.94 (0.17) | 1.24 (0.55) | <0.0001 |
| 60 s | −5.94 (2.70) | −0.02 (2.73) | <0.0001 | −1.12 (0.55) | 0.41 (0.44) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinos, G.; Hafeez, M.; De Leon, J.; Romanos, G.E. Thermal Effects of Pulsed Infrared Lasers on Zirconia Implants at Different Temperatures In Vitro. Dent. J. 2025, 13, 342. https://doi.org/10.3390/dj13080342
Kokkinos G, Hafeez M, De Leon J, Romanos GE. Thermal Effects of Pulsed Infrared Lasers on Zirconia Implants at Different Temperatures In Vitro. Dentistry Journal. 2025; 13(8):342. https://doi.org/10.3390/dj13080342
Chicago/Turabian StyleKokkinos, George, Maryam Hafeez, Joseph De Leon, and Georgios E. Romanos. 2025. "Thermal Effects of Pulsed Infrared Lasers on Zirconia Implants at Different Temperatures In Vitro" Dentistry Journal 13, no. 8: 342. https://doi.org/10.3390/dj13080342
APA StyleKokkinos, G., Hafeez, M., De Leon, J., & Romanos, G. E. (2025). Thermal Effects of Pulsed Infrared Lasers on Zirconia Implants at Different Temperatures In Vitro. Dentistry Journal, 13(8), 342. https://doi.org/10.3390/dj13080342








