Abstract
Background/Objectives: Severe jawbone atrophy, particularly in elderly or medically compromised patients, presents a significant challenge for conventional implant placement. In cases where bone augmentation is not feasible, alternative techniques—such as short, narrow, tilted, and zygomatic implants—may be indicated for the rehabilitation of the atrophic jaw. Subperiosteal implants have re-emerged as a further viable alternative, especially with recent advancements in digital planning and custom fabrication. This study aims to evaluate the clinical outcomes and complications associated with subperiosteal implants through a literature review and a supporting case series, and to propose clinical preliminary guidelines for their use. Methods: Fourteen studies—including case reports, case series, retrospective studies, and systematic reviews—were analyzed to assess the effectiveness and risk profile of subperiosteal implants. Additionally, we present a case series of nine patients with advanced vertical and horizontal alveolar bone atrophy treated using custom-made, digitally-designed subperiosteal implants. Surgical techniques, prosthetic workflows, and complications were recorded and assessed. Results: Subperiosteal implants were found to be particularly suitable for patients with narrow alveolar crests and severe atrophy where traditional implants are contraindicated. Literature and case series data indicated favorable outcomes, with early complications such as soft tissue inflammation and prosthetic misfit being manageable. A low complication rate was recorded in our series, with digital workflows contributing to improved implant fit and reduced technical errors. Conclusions: Subperiosteal implants could offer an effective solution for complex atrophic cases, provided that patient selection, surgical precision, and prosthetic design are meticulously managed. Based on our findings, clinical recommendations are proposed to guide their application in contemporary practice.
1. Introduction
Severe bone atrophy in the alveolar ridge has long posed a significant challenge in dental implantology. This condition often arises following tooth extraction due to periodontal disease, trauma, or congenital defects, leading to progressive bone loss in the maxilla or mandible [1]. As bone volume decreases, achieving fixed rehabilitation with dental implants becomes increasingly difficult, as successful osseointegration requires a stable and sufficiently dense foundation [2]. Patients with significant bone loss frequently experience impaired speech, difficulty chewing, and reduced self-esteem due to the aesthetic and functional consequences of tooth loss, such as sunken cheeks and a decreased vertical dimension of the lower face [3,4].
To address these challenges, various surgical techniques have been developed. These include sinus lifting and zygomatic implant placement in the maxilla, as well as inlay and onlay bone grafting, guided bone regeneration (GBR) followed by standard implant placement, and the use of tilted, narrow, and short implants in both arches [5,6,7,8,9,10,11,12,13,14,15,16]. While these surgical interventions can improve function, aesthetics, and overall quality of life, they present certain limitations. They often require multiple surgical stages, involve extended healing periods, increase treatment costs, and do not always allow for immediate prosthetic rehabilitation. Additionally, failure of osseointegration can result in implant or prosthetic failure, further complicating the clinical outcome [17,18,19,20,21,22,23].
Recent technological advancements—including digital imaging, 3D printing, and customized implant design—have significantly improved treatment options for patients with severe bone deficiencies. One notable development is the renewed interest in subperiosteal implants, which offer a viable alternative for patients who are not suitable candidates for traditional endosseous implants [24,25,26,27]. These implants, placed on the bone surface rather than within it, present a promising solution when bone grafting or osseointegrated intraosseous implants are not feasible. Recent studies have focused on enhancing the precision, survival rates, and patient satisfaction associated with subperiosteal implants, marking a shift in treatment strategies for complex cases of bone atrophy [14,15,16]. Unfortunately, despite these advancements, there are still no clearly defined clinical guidelines for the use of subperiosteal implants, and their indications, surgical protocols, and long-term outcomes remain insufficiently standardized [28,29,30,31,32,33].
As research and technology continue to evolve, implant dentistry is expanding the range of treatment possibilities for patients with severe bone loss [34]. This study aims to provide a comprehensive literature review on subperiosteal implants, examining current applications, clinical outcomes, and recent advancements in technology and design for managing severe bone atrophy in implant dentistry. In addition, a case series based on the authors’ clinical experience is presented to contribute practical insights and propose preliminary guidelines for the effective application of subperiosteal implants in modern clinical practice.
2. Materials and Methods
2.1. Literature Review
This systematic literature review follows PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure transparency and reproducibility. The study protocol was designed to minimize selection bias and ensure a comprehensive evaluation of the available literature [35].
2.2. Inclusion Criteria
- Human clinical studies, including clinical trials, cohort studies, case-control studies, case series, case reports, review articles, letters, editorials, expert opinions, and systematic reviews.
- Studies evaluating survival rates, osseointegration, or complications of subperiosteal implants.
- Studies comparing subperiosteal implants with conventional implants or alternative treatment modalities.
- English-language publications to ensure accessibility and consistency in interpretation.
- No time restrictions to include both historical and modern studies, allowing for a comprehensive perspective on subperiosteal implants’ evolution and clinical outcomes.
2.3. Exclusion Criteria
- Animal or in vitro studies that lack direct clinical implications.
- Studies focusing solely on technical or laboratory procedures without reporting clinical outcomes.
2.4. Search Strategies and Information Sources
A comprehensive literature search was conducted across the following electronic databases: PubMed, Scopus. The search strategy incorporated controlled vocabulary (MeSH terms) and free-text keywords to maximize sensitivity. The search was performed using the following Boolean operators:
((“subperiosteal implants” OR “subperiosteal dental implants” OR “bone-implant interface” OR “custom implants” OR “periosteal implants”) AND (“implant survival” OR “implant failure” OR “long-term success” OR “complications” OR “implant durability” OR “implant prognosis” OR “implant complications” OR “prosthetic failure” OR “implant failure prognosis”)) AND “humans”
Manual screening of reference lists from selected studies was also conducted to capture additional relevant publications.
2.5. Article Selection
The article selection process was conducted in multiple stages:
- Primary Search Results:
Two independent reviewers (M.K. and S.T.) screened search results from PubMed (264 articles), Scopus (452 articles).
- 2.
- Duplicate Removal:
Duplicate articles were identified and removed using Rayyan System (Rayyan Systems Inc.), resulting in the exclusion of 476 duplicate records.
- 3.
- Title and Abstract Screening:
Titles and abstracts were screened for relevance using Rayyan software. Each reviewer categorized articles as “include,” “exclude,” or “undecided.” A third reviewer (C.B.) acted as an arbitrator to resolve discrepancies and reviewed articles marked as “undecided.” After this screening process, 23 articles were selected for full-text evaluation.
- 4.
- Full-Text Evaluation:
Full texts of the selected articles were assessed for adherence to eligibility criteria and methodological quality. Studies that lacked sufficient clinical data or failed to report survival rates, complications, or patient follow-up information were excluded. The final selection included studies that provided relevant and high-quality clinical evidence on subperiosteal implants. Fourteen articles were selected for data extraction (Figure 1) [36,37,38,39,40,41,42,43,44,45,46,47,48,49].
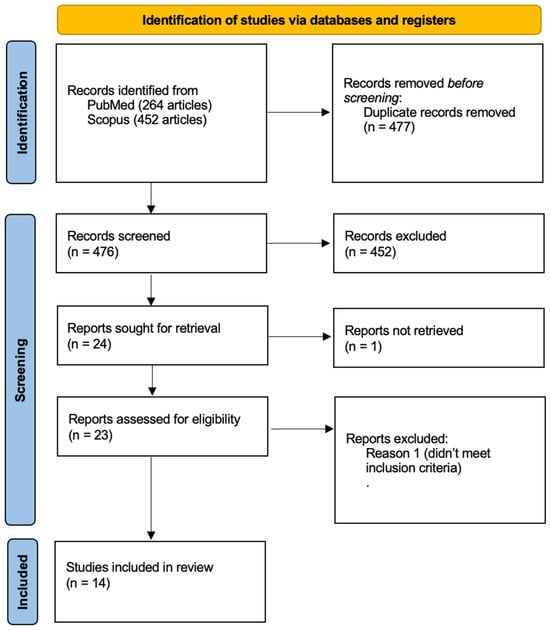
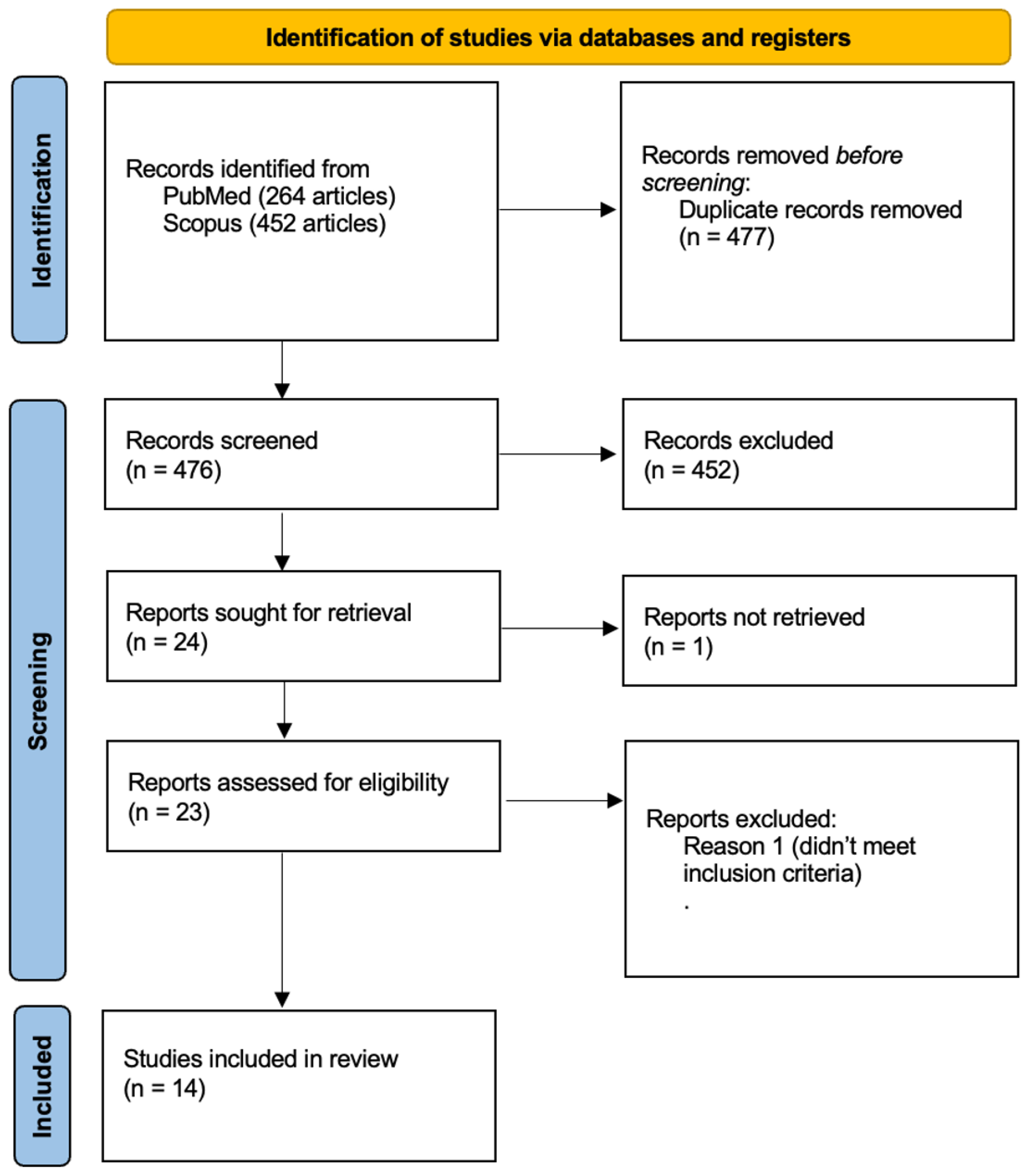
Figure 1.
The flowchart visually represents the systematic selection process for studies included in the review, detailing identification, screening, eligibility, and final inclusion criteria. Fourteen studies met all requirements and were included in the final review.
2.6. Data Extraction Process
Data extraction was performed systematically to ensure consistency and accuracy. All information from the selected articles was extracted into an Excel spreadsheet for structured analysis. The following variables were collected from each included study:
- Study Characteristics: Author(s), year of publication, study title, journal name, and DOI/link to ensure accurate identification and citation of the sources.
- Study Design and Population: Study design, sample size, and study location/country to assess the methodological quality and generalizability of findings.
- Follow-up and Patient Characteristics: Follow-up period, patient demographics, jaw treated (maxilla, mandible, or both), and bone condition to analyze treatment outcomes in different clinical scenarios.
- Implant Characteristics: Type of implant material and fixation method to compare variations in design and placement techniques.
- Outcome Measures: Reported survival rates and success criteria used in each study to evaluate implant longevity and clinical performance.
- Complications and Prosthetic Considerations: Documented complications, prosthetic type, and patient satisfaction levels to assess functional and patient-reported outcomes.
- Key Findings and Study Limitations: Major conclusions, clinical implications, and limitations noted by the authors to contextualize study results.
Extracted data were systematically categorized and analyzed to identify trends, patterns, and potential correlations among study variables. Quantitative outcomes, such as survival rates and complication frequencies, were synthesized descriptively (Table 1).

Table 1.
Main characteristics of the studies included in the review. N/A—not applicable; N/R—not reported; DLMS—direct laser metal sintering.
2.7. Case Series
This retrospective case series includes nine patients with 11 sites who underwent jaw rehabilitation with 11 custom-made subperiosteal implants between 2020 and 2024 at the Department of Oral Surgery, University of Bologna. All patients presented with severe alveolar ridge atrophy and were deemed unsuitable for conventional endosteal implants due to insufficient bone volume or anatomical limitations. Written informed consent was obtained from all patients.
2.8. Patient Selection and Preoperative Assessment
Inclusion criteria consisted of:
- Severe mandibular or maxillary bone atrophy.
- Contraindication to bone grafting or patient refusal of grafting procedures.
- Need for fixed implant-supported rehabilitation.
- Good general health and absence of uncontrolled systemic conditions.
Exclusion criteria consisted of:
- Presence of active oral infections or untreated periodontal disease.
- Uncontrolled systemic conditions (e.g., diabetes, cardiovascular disease, immunosuppressive disorders) or history of head and neck radiation therapy.
- Severe parafunctional habits (e.g., bruxism) that could compromise implant stability.
- Pregnancy or breastfeeding at the time of treatment.
All patients underwent a comprehensive clinical evaluation, including panoramic radiographs and Cone Beam Computed Tomography (CBCT). Digital intraoral scans or conventional impressions were used to assess soft tissue contours and plan implant design. Particular attention was given to the evaluation of mandibular anatomy, mental foramen position, and residual bone morphology.
Custom subperiosteal implants were designed using patient-specific CBCT and intraoral scans. Implant design aimed to achieve passive adaptation to the cortical surface of the mandible and to avoid impingement on critical anatomical structures. Prosthetic abutment positioning was virtually planned based on prosthetic requirements and opposing arch relationships. All implants were manufactured in titanium alloy using selective laser melting technology (BTK, Biotek SRL) and delivered with a custom-made surgical guide.
Surgical procedures were performed under local anesthesia (Articaine 4% with Epinephrine 1:100,000). A mid-crestal incision was made along the edentulous ridge with posterior releasing incisions to allow full-thickness mucoperiosteal flap elevation. The mental nerves in the mandible were carefully identified and protected throughout the procedure. Minimal osteoplasty was performed in areas of bone irregularities to ensure a passive fit of the implant framework. Using the predesigned surgical guide, the implant was positioned and fixated with titanium screws at predetermined anchorage points. The number and position of fixation screws were adapted to each patient’s anatomy to ensure primary mechanical stability.
Soft tissue closure was achieved with resorbable sutures (Vicryl 4-0), and a panoramic radiograph was obtained immediately postoperatively to verify implant positioning. Provisional prostheses were delivered within 24–72 h post-surgery to preserve occlusal function and assist soft tissue contouring. Materials included milled PMMA or reinforced resin frameworks depending on case needs. Definitive prostheses (zirconia or metal-ceramic) were fabricated after a healing period of 4 months, following confirmation of soft tissue stability and implant integrity. All patients received antibiotic prophylaxis (Amoxicillin 2 g preoperatively, followed by 1 g twice daily for 7 days), anti-inflammatory agents (Ibuprofen 600 mg as needed), and Chlorhexidine 0.20% mouthwash twice daily for 1–2 weeks. Sutures were removed at 10–14 days.
Patients were followed up at 1, 3, and 6 months postoperatively, and every 6–12 months thereafter. Follow-up assessments included clinical examinations, panoramic radiographs, CBCT (when indicated), and prosthetic evaluations. Maintenance hygiene protocols included semiannual professional cleaning and individualized oral hygiene instructions.
3. Results
3.1. Literature Review
A total of 14 studies were included in the analysis. The included studies employed diverse methodologies. Retrospective clinical studies were conducted by Cerea & Dolcini (2018), Onică et al. (2024), Ayhan et al. (2024), Vaira et al. (2024), and systematic reviews were performed by Anitua et al. (2024) and El-Sawy & Hegazy (2024). Case series included Mangano et al. (2020), Gellrich et al. (2024), and Santiago et al. (2025), while pilot studies and single-case reports were documented by Nemtoi et al. (2022), Marconcini et al. (2023), Strappa et al. (2022), and Ayhan et al. (2024) [36,37,38,39,40,41,42,43,45,47,49]. A comparative clinical study was performed by Zielinski et al. (2025) [48]. The largest retrospective study by Anitua et al. (2024) analyzed 227 implants, while smaller case series and single-case reports covered samples ranging from 1 to 36 patients [46]. The cumulative sample consisted of 617 patients with severe maxillary or mandibular atrophy, often classified as Cawood and Howell Class V–VI. Most patients were elderly (mean age range: 60.4–69.6 years), although one case involved a young patient with ectodermal dysplasia [50].
3.2. Patient Demographics and Clinical Indications
Patients were predominantly edentulous with severe jaw atrophy, often classified as Cawood and Howell Class V or VI. The average age was typically above 60, with exceptions such as the 18-year-old patient with ectodermal dysplasia reported by Ayhan et al. (2024) [42]. Several studies included medically compromised patients, such as those with osteoporosis (Marconcini et al., 2023) or generalized systemic conditions (El-Sawy & Hegazy, 2024) [40,47].
3.3. Follow-Up Periods and Duration of Evaluation
Follow-up durations ranged from 6 months (Santiago et al., 2025) to up to 6 years (Onică et al., 2024), although the latter reported a low long-term survival rate (~25%). Most studies had follow-up periods of 12–24 months [41,49]. Gellrich et al. (2024) included follow-up up to 68 months, and Zielinski et al. (2025) evaluated outcomes after a minimum of 5 years, highlighting the long-term feasibility of subperiosteal implants [43,48].
3.4. Survival Rates and Success Criteria
Implant survival rates were generally high, ranging from 86.7% (Ayhan et al., 2024) to 100% in several studies (Mangano et al., 2020; Strappa et al., 2022; Gellrich et al., 2024) [37,38,42,43]. The lowest survival (around 25%) was reported by Onică et al. (2024) over a long-term 6-year period [41]. Success was commonly defined by implant stability, absence of complications, and functional prosthetic integration.
3.5. Complications and Clinical Outcomes
Complications emerged as a central concern in the evaluation of subperiosteal implants, despite their generally high survival rates. While most studies reported favorable outcomes, the risk of both early and late complications was consistently present.
3.6. Postoperative and Early Complications
Mild postoperative issues, such as pain, swelling, and soft tissue inflammation, were commonly observed. Mangano et al. (2020), Vaira et al. (2024), and Santiago et al. (2025) noted transient edema and discomfort, which typically resolved within days [37,45,49]. However, more severe early complications were reported. Nemtoi et al. (2022) described early implant failure due to postoperative infection, while Ayhan et al. (2024) reported a 13.3% implant failure rate, primarily due to infection and inadequate osseointegration. Onică et al. (2024) provided a particularly critical perspective, with 17 out of 67 implants showing mobility over a 6-year follow-up, suggesting potential design flaws or long-term biological complications [39,41,42].
3.7. Prosthetic Complications
Prosthetic challenges were also noted in several studies. Mangano et al. (2020) reported fractures of provisional restorations in two cases, although these did not compromise the final outcome [37]. Ayhan et al. (2024) encountered significant prosthetic misfit requiring intraoperative adjustments, indicating that manufacturing precision remains a clinical concern even with digital workflows [42]. Additionally, mucosal dehiscence requiring surgical intervention was documented in Strappa et al. (2022) [38].
3.8. Long-Term and Late Complications
Long-term complications, though less frequently reported, raised important questions regarding the stability and integration of subperiosteal implants over time. Zielinski et al. (2025), in a comparative study with 5 years of follow-up, observed mucosal inflammation and prosthesis-related complications but reported no implant failures. On the other hand, the high rate of late implant mobility described by Onică et al. (2024) (25% survival at 6 years) reflects the potential for mechanical or biological degradation over time [41,48].
3.9. Infection and Implant Loss
Infection was one of the most commonly cited serious complications. In addition to the early infections seen in Nemtoi et al. (2022) and Ayhan et al. (2024), Marconcini et al. (2023) noted marginal bone exposure and inflammation, though without implant loss. These findings emphasize the importance of patient selection, surgical technique, and meticulous postoperative care in minimizing infectious complications [39,40,42].
3.10. Summary of Risk Profile
Studies with longer observation periods—such as Onică et al. (2024) and Zielinski et al. (2025)—tended to report more complications, highlighting the importance of long-term monitoring [41,48].
3.11. Case Series
3.11.1. Patient Demographics and Clinical Characteristics
The case series included nine patients (five females and four males), aged between 27 and 69 years, all of whom presented with vertical bone atrophy of the maxilla or mandible that precluded the placement of conventional endosteal implants. One patient had a syndromic condition—EEC syndrome (ectrodactyly–ectodermal dysplasia–cleft syndrome)—while no patients presented systemic contraindications to surgery.
3.11.2. Surgical and Prosthetic Protocol
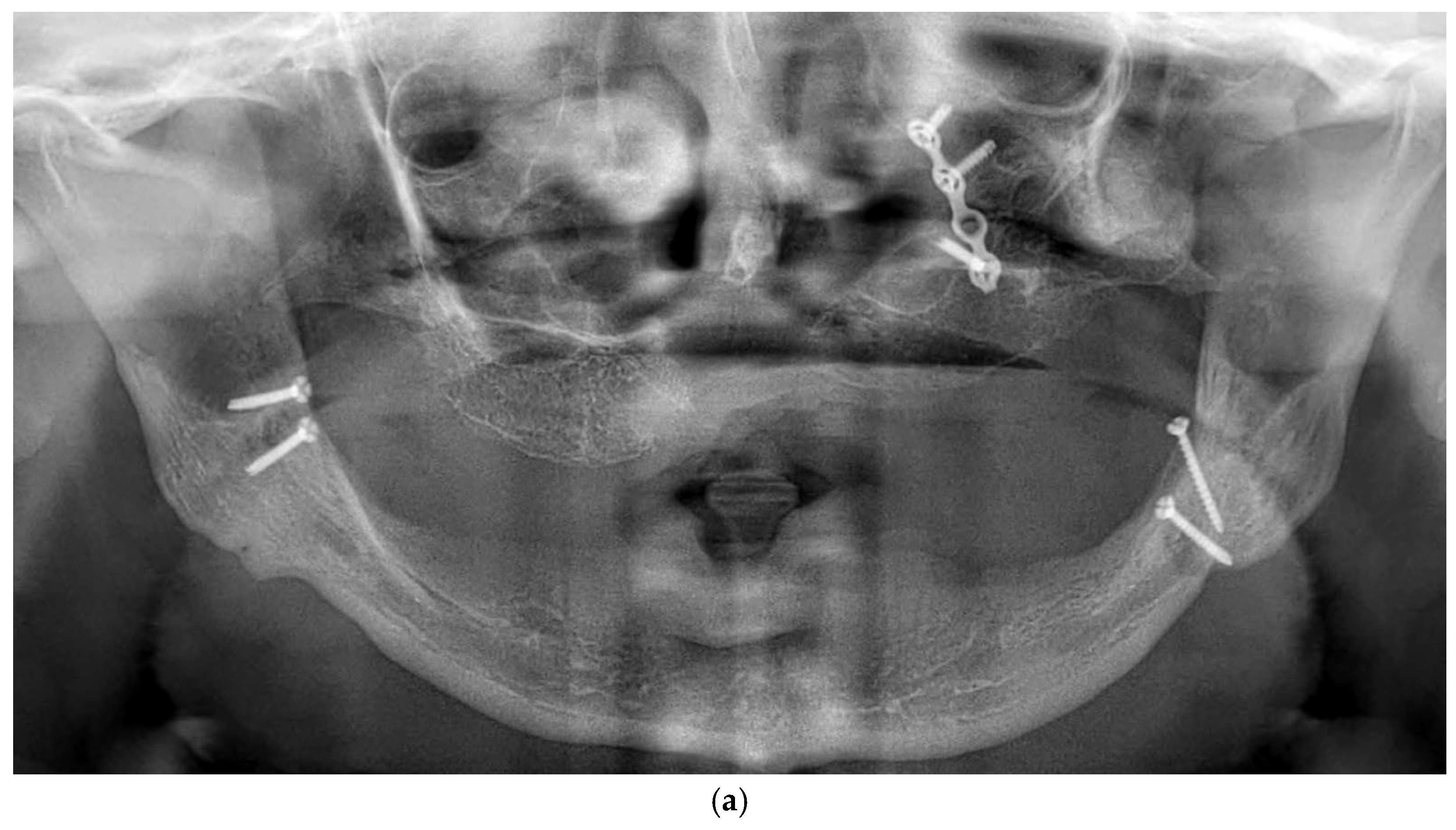
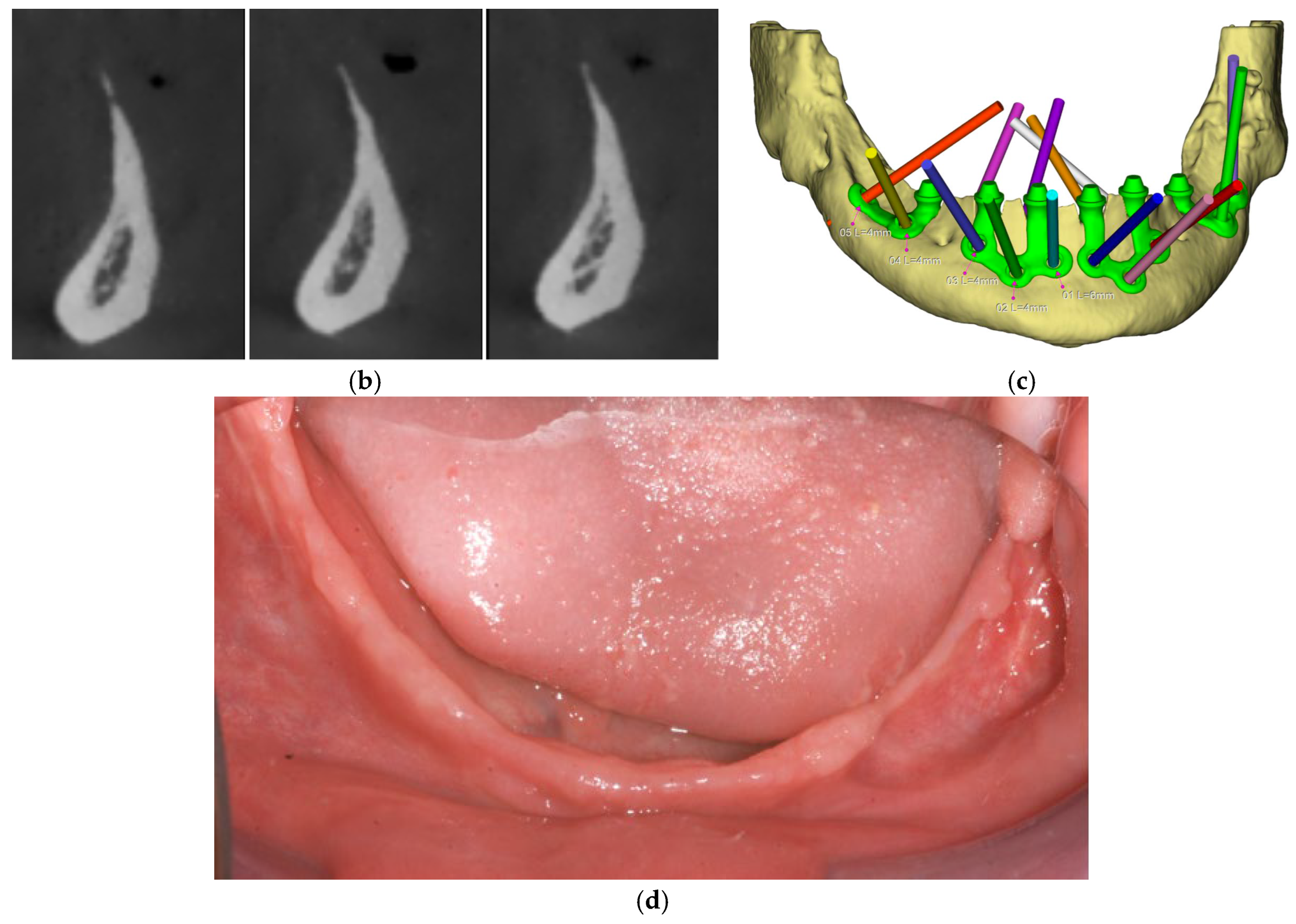
All patients were rehabilitated using custom-made subperiosteal implants, designed from the patients’ CBCT DICOM files to precisely match the anatomical features of each jaw. A fully digital workflow was used in all cases, including the RealGuide® software 5.4 for planning. The implants were designed as segmented frameworks, with two to four divisions per arch and 2–3 abutments per segment (Figure 2a–d).
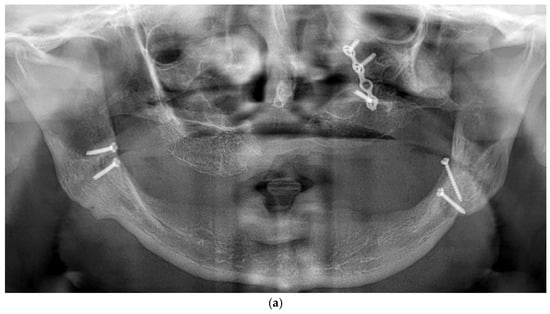
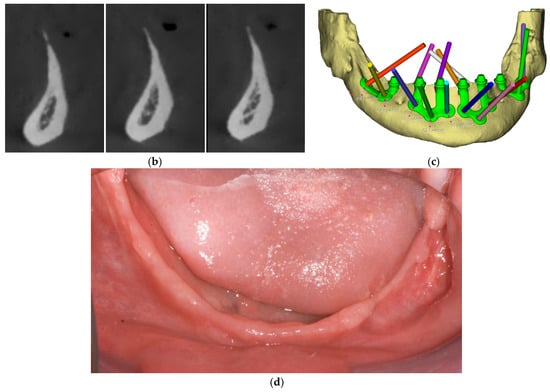
Figure 2.
(a) A panoramic radiograph of the patient at the initial stage was obtained to evaluate the overall dentition, bone structure, and any developmental anomalies. (b) Cross-sectional images show a severely resorbed, knife-edged alveolar bone crest. (c) Digital planning of the subperiosteal implant design and screw positioning. (d) Intraoral clinical image showing a completely edentulous knife-edged alveolar ridge.
Surgical procedures followed a standardized protocol. A mid-crestal incision was made along the edentulous ridge with releasing incisions as needed, followed by full-thickness mucoperiosteal flap elevation to expose the underlying bone. All the nervous structures were carefully identified and isolated to avoid iatrogenic damage. Reference points for implant adaptation were confirmed using a pre-designed surgical guide. In some cases, minor osteoplasty using a surgical guide was performed to regularize bone contours and enhance implant adaptation (Figure 3).
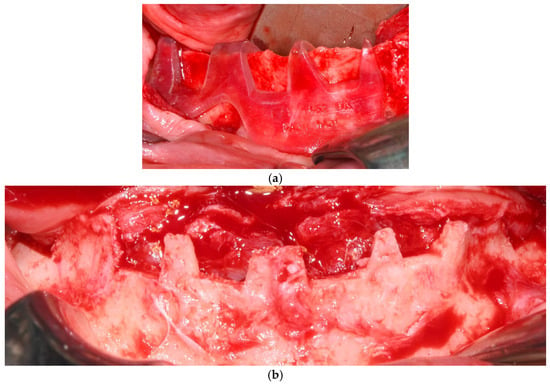
Figure 3.
(a) Surgical guide indicating the osteoplasty to be performed on the left side of the mandible. (b) Post-osteoplasty view showing the entire mandibular crest.
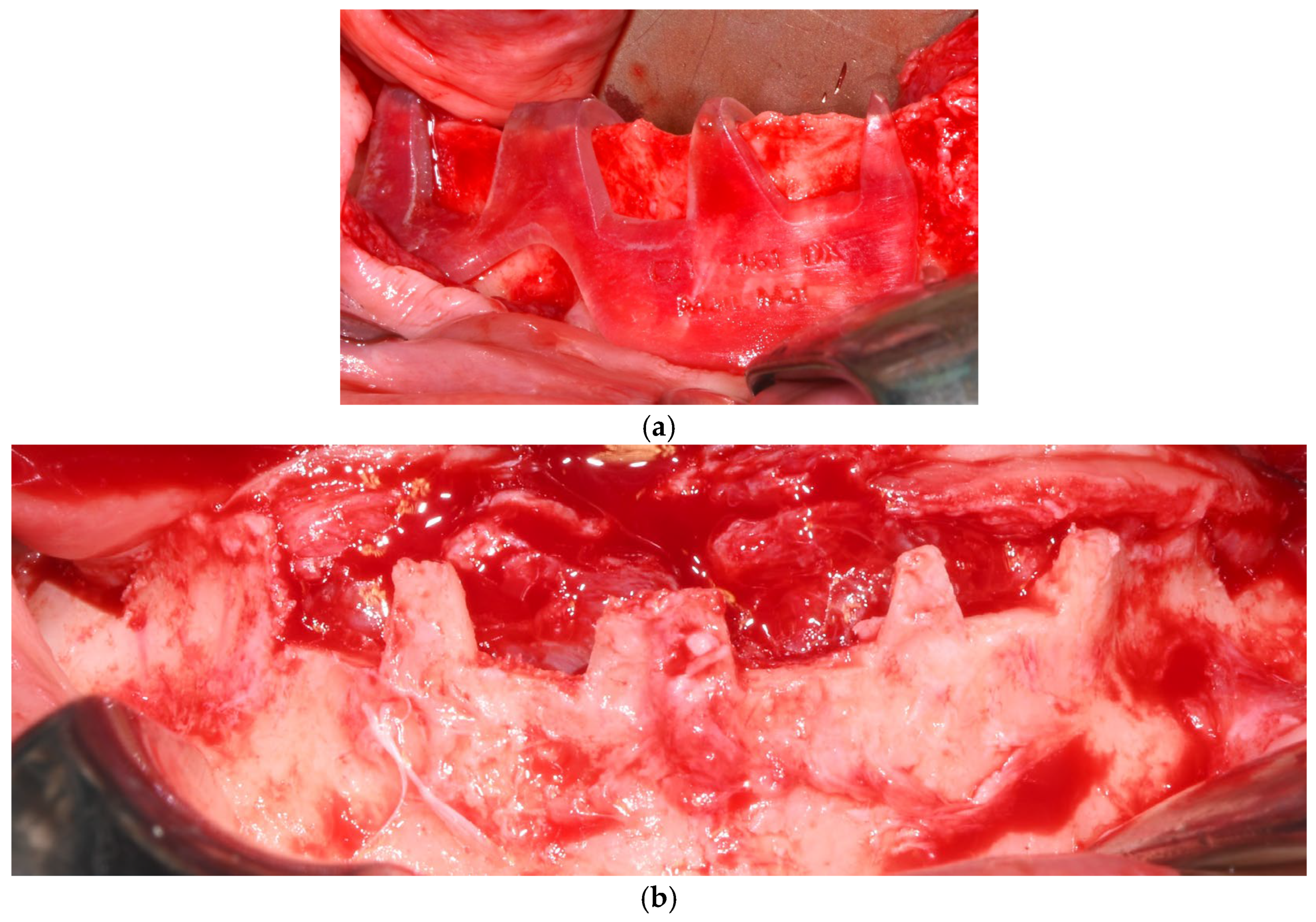
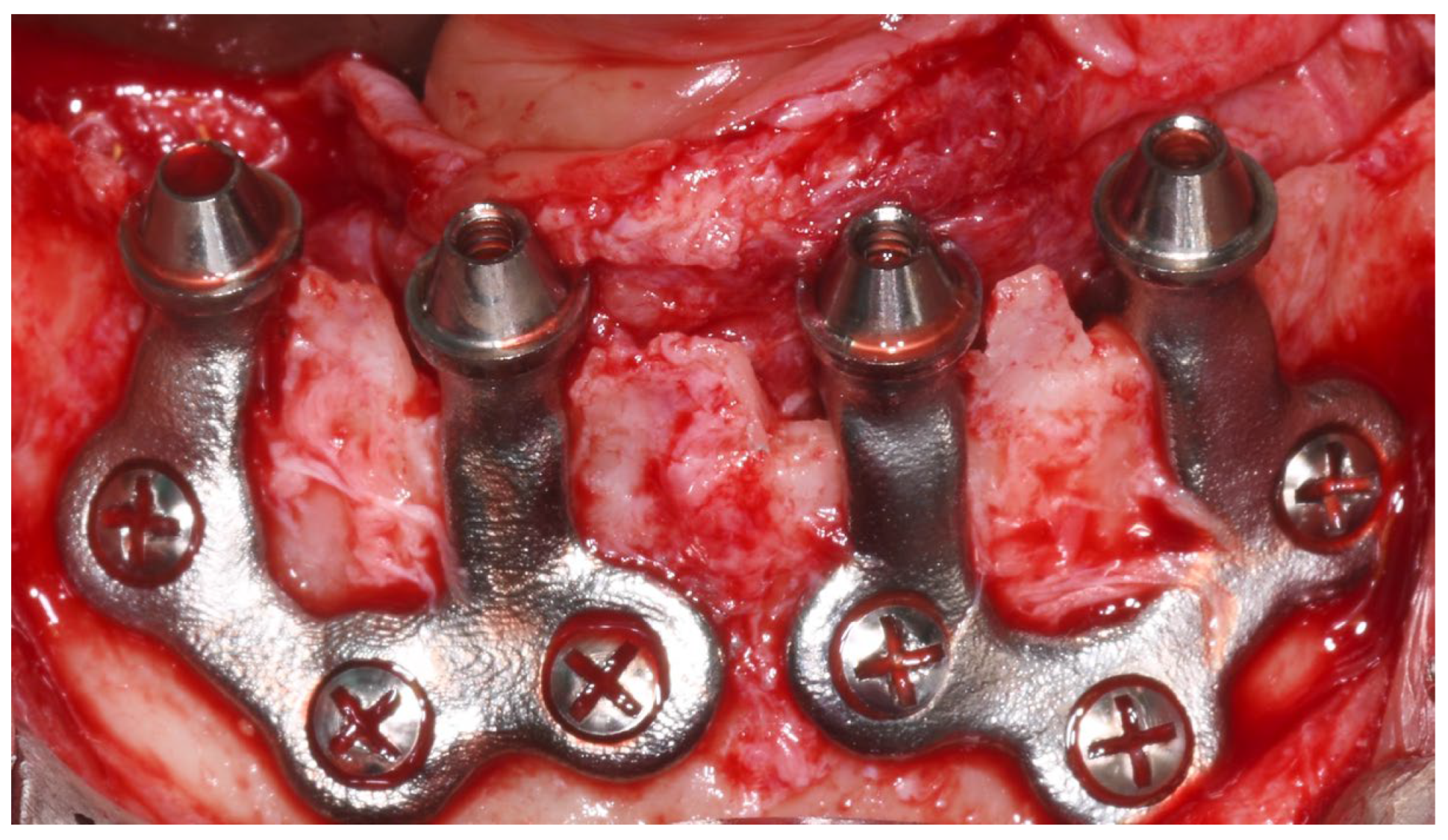
Each subperiosteal implant was passively adapted to the bone and secured with titanium osteosynthesis screws to achieve primary stability (Figure 4).
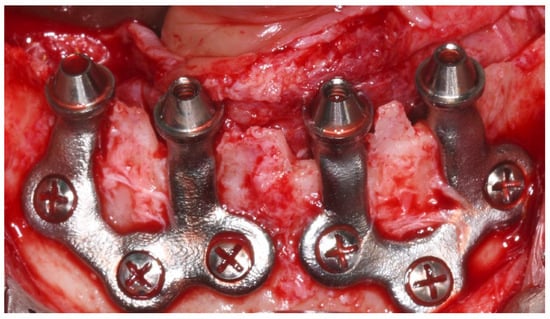
Figure 4.
Intraoperative view of the mandibular crest showing the placement of subperiosteal implants during surgery.
Flaps were repositioned and sutured with resorbable Vicryl 4-0 sutures. Panoramic radiography and clinical pictures were obtained postoperatively (Figure 5a,b). Within 48 h, digital impressions were taken, and a provisional screwed PMMA prosthesis was delivered. In all patients, immediate loading was achieved without intraoperative complications. Postoperative care included Amoxicillin 1 g twice daily for 7 days, Ibuprofen 600 mg as needed, and Chlorhexidine 0.20% mouthwash twice daily for 14 days. Sutures were removed after 14 days.
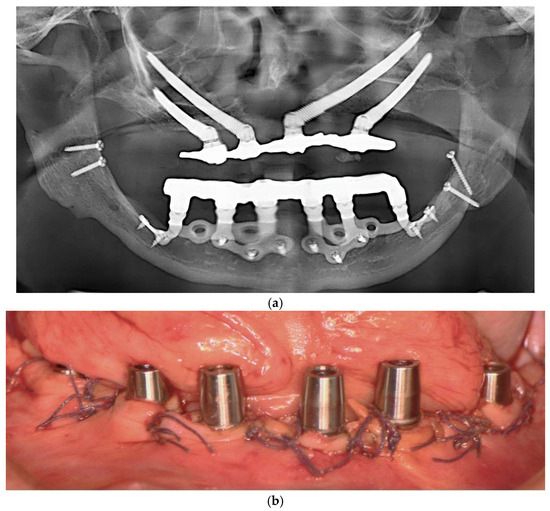
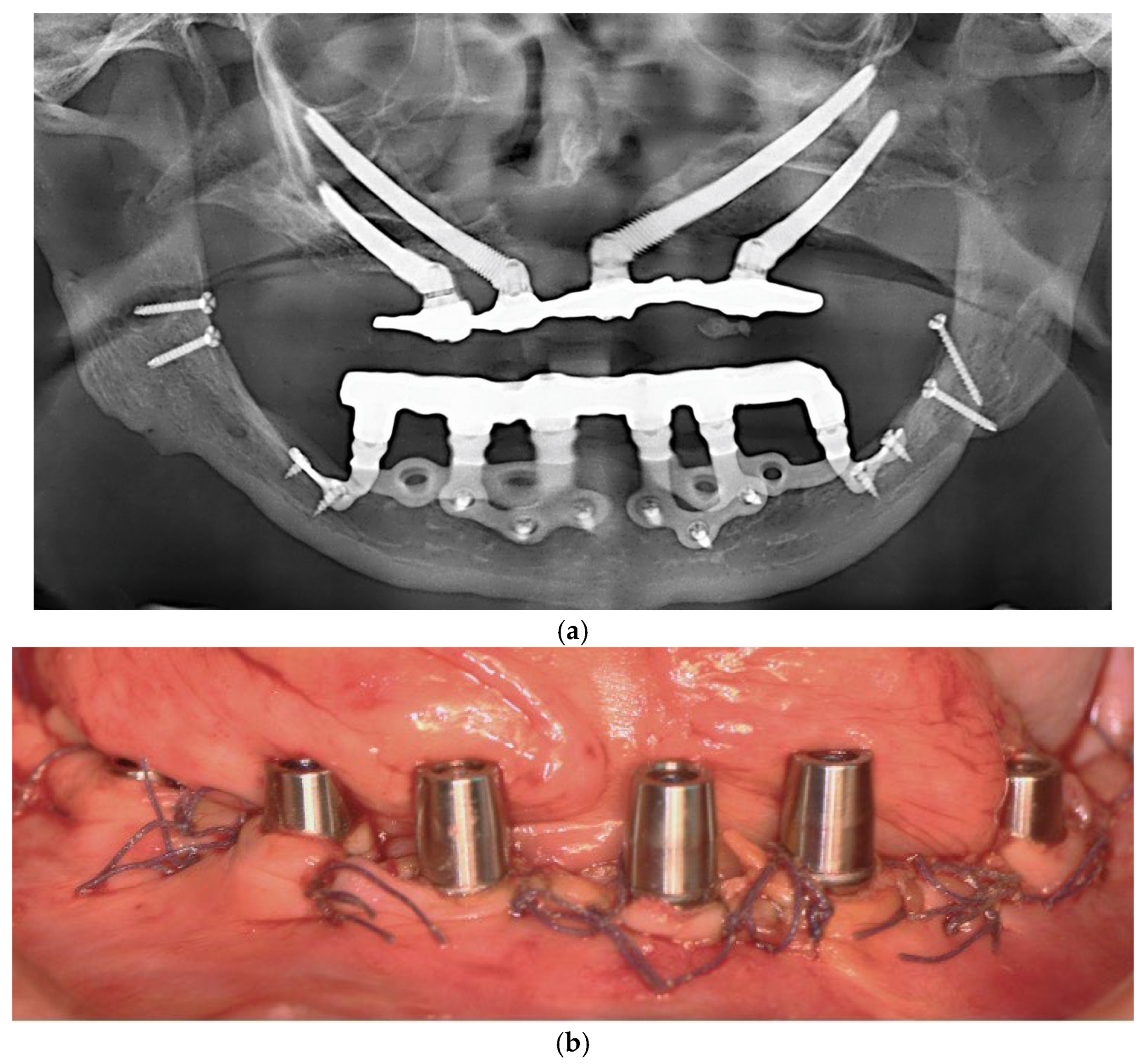
Figure 5.
(a) A panoramic radiograph of the patient after subperiosteal implants placement. (b) Intraoral picture of the mandible following subperiosteal implant placement.
Final prosthetic delivery occurred approximately 3 months post-surgery, following confirmation of soft tissue healing and implant stability. Prosthetic materials included PMMA on a metal bar or zirconia frameworks.
Patients were followed up at 3 and 6 months postoperatively, and then annually. Follow-up assessments included clinical evaluation and panoramic imaging; CBCT scans were obtained when necessary to monitor implant fit, peri-implant tissue response, and bone contour stability. All implants remained functionally stable throughout the observation period. The follow-up period ranged from 12 to 60 months, with a mean duration of 36.2 months (Figure 6a,b). Each patient underwent routine hygiene sessions every six months and an annual special session with prosthesis removal for thorough cleaning.
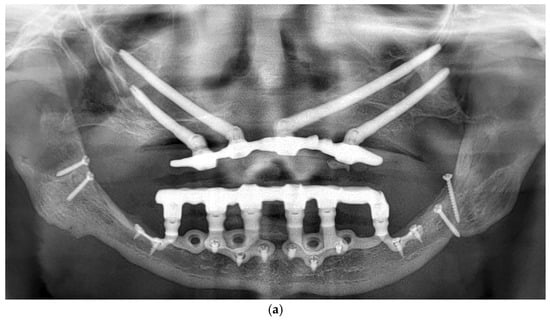
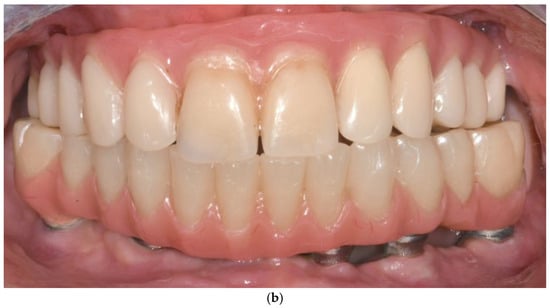
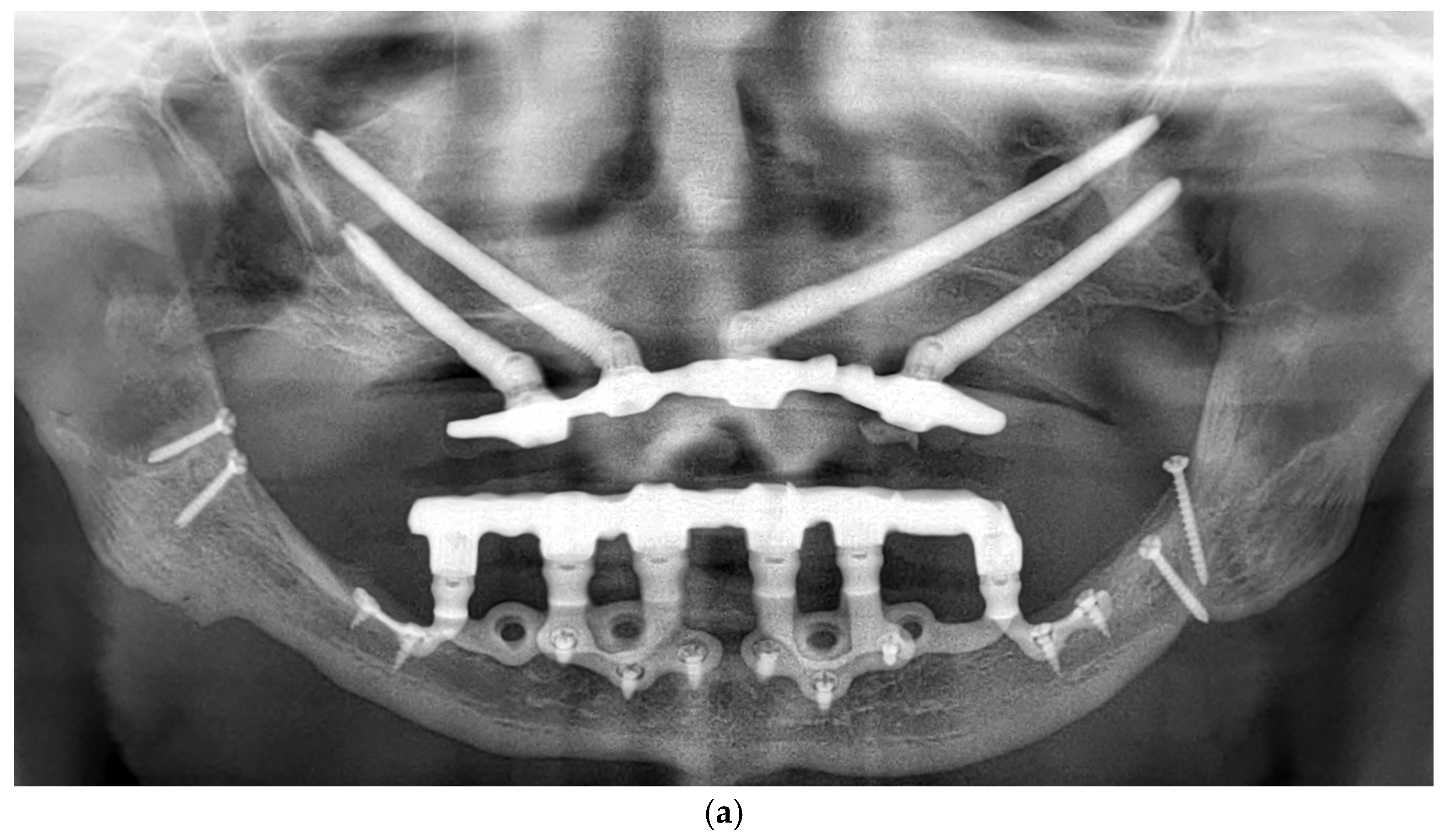
Figure 6.
(a) A panoramic radiograph of the patient at 5-year follow-up. (b) Intraoral clinical image of the patient at 5-year follow-up.
3.11.3. Complications and Management
A total of three minor complications were observed, corresponding to a complication rate of 37.5%. All complications were managed conservatively and did not require surgical re-intervention (Table 2).

Table 2.
Main characteristics of the complications associated with subperiosteal implants.
- Patient 1: Developed a localized abscess in the region of tooth 13 three months postoperatively, accompanied by soft tissue dehiscence at sites 13, 15, and 17. These were managed with local irrigation and hygiene reinforcement, with full recovery.
- Patient 2: Presented with tissue dehiscence on the lingual side.
- Patient 3: Presented with tissue dehiscence around the second abutment of the upper left implant. The condition was resolved through improved oral hygiene.
There were no reports of early implant failure, screw loosening, prosthetic fracture, or exposure of the implant framework in any case.
4. Discussion
The review of 14 studies investigating the use of subperiosteal implants in patients with severe jawbone atrophy reveals a mixture of positive outcomes and notable complications, which are important for assessing the viability of this treatment option. The studies included in this review span a wide range of methodologies, sample sizes, and follow-up periods, contributing to a comprehensive understanding of the effectiveness and challenges associated with these implants.
The patient populations in the reviewed studies generally consisted of elderly individuals, typically over 60 years old, which corresponds to the progressive nature of jawbone atrophy. This finding is in line with the studies by Anitua et al. and El-Sawy et al., where subperiosteal implants were primarily used in older adults for whom conventional implant placement was not feasible due to anatomical limitations [46,47]. However, certain studies, such as Ayhan et al. (2024), included younger individuals with congenital or syndromic conditions like ectodermal dysplasia, demonstrating the versatility of subperiosteal implants in managing both age-related and developmental bone deficiencies [42]. Cerea et al. (2019) further broadened this scope by treating patients who had previously failed conventional implant therapy, underscoring subperiosteal implants as a last-resort solution in complex cases [36].
Follow-up durations varied significantly across studies, ranging from 6 months to 6 years. Longer follow-up durations, such as those reported by Zielinski et al. (2025) and Onică et al. (2024), showed a higher incidence of late complications, suggesting potential challenges in long-term maintenance [41,48]. In contrast, Anitua et al. reported stable outcomes over a 17-month period with no implant failures, and El-Sawy et al. reported similar success over an average of 18 months [46,47]. Cerea et al., with up to 36 months of follow-up, observed a few soft tissue complications, confirming the need for extended monitoring to fully assess durability [36]. These differences indicate that while short- and medium-term results are promising, long-term data are necessary for a conclusive evaluation.
Complications ranged from mild and self-limiting to more serious issues affecting treatment success. Early postoperative complications—pain, swelling, and inflammation—were common but typically manageable, as confirmed by the experiences in Anitua and El-Sawy ‘s studies [47]. More severe issues such as infection and implant failure were less frequent but notable. Ayhan et al. (2024) reported a 13.3% failure rate primarily due to infection, while Nemtoi et al. (2022) also linked failure to infection [39,46]. Cerea et al. (2019) documented one case of implant loss due to infection in a smoker, highlighting the importance of patient-related risk factors [36]. These findings suggest that success is highly dependent on surgical technique, systemic health, and postoperative care.
Prosthetic challenges such as misfit and provisional restoration fractures were reported in several studies. The adoption of fully digital workflows by Anitua, El-Sawy, and Ayhan was associated with reduced prosthetic complications [42,46,47]. These workflows enable high-precision implant manufacturing and better adaptation to patient anatomy, which contributes to improved outcomes and patient satisfaction. However, even with digital advancements, Cerea et al. noted occasional prosthetic issues, especially in early designs, emphasizing that digital tools, while beneficial, are not foolproof [36]. The most frequent complication remains implant exposure. Its impact on the long-term survival rate of these implants, as well as its association with the onset of mucositis, remains unclear.
Infection remains one of the most concerning complications due to its impact on implant survival. Nemtoi et al. (2022), Ayhan et al. (2024), and Marconcini et al. (2023) all reported infection as a leading cause of early implant loss [39,40,44]. These contrasting results highlight the critical role of surgical protocols and the potential benefits of minimally invasive techniques in reducing infection risk.
Several implant designs and manufacturing techniques were used by different authors, along with varying surgical approaches. These factors further increase the uneven nature of the available literature. However, more clinical data and longer follow-up periods are needed to better evaluate clinical outcomes and establish definitive guidelines [34]. In vitro data based on finite element analysis may be useful for understanding the biomechanical properties of subperiosteal implants, particularly when conducted using realistic virtual models.
In our case series of nine patients treated with custom-made subperiosteal implants using a fully digital workflow, clinical outcomes were favorable. The overall complication rate was low: only three minor cases of localized soft tissue dehiscence occurred, all of which were resolved with conservative measures. No instances of early implant failure, screw loosening, or prosthetic fractures were observed. These findings align with existing literature and support the potential effectiveness of subperiosteal implants in the rehabilitation of severely atrophic ridges, particularly when enhanced by modern digital planning and manufacturing protocols. Notably, the surgical procedure is less invasive and can typically be performed under local anesthesia, with postoperative complications manageable in an outpatient setting. This contrasts with alternative solutions like zygomatic implants, which often require general anesthesia and carry greater surgical risks.
Across the reviewed studies, fixed full-arch prosthetic solutions were the preferred choice. Cerea and Dolcini (2019), Mangano et al. (2022), Marconcini et al. (2023), Nemtoi et al. (2022), and Onică et al. (2024) all reported using fixed restorations—either screw-retained or cemented—with definitive materials such as ceramic or zirconia [36,37,39,41]. Strappa et al. (2022) used a cement-retained Toronto Bridge [38]. Ayhan and colleagues, in multiple studies (2023; 2024), also employed full-arch fixed prostheses, including cases of immediate loading [42,46]. In this case series, the authors exclusively adopted screw-retained full-arch prostheses supported by custom subperiosteal frameworks. This decision was based on their retrievability, structural stability, and ease of hygiene maintenance. No mechanical or prosthetic complications occurred during the observation period, and patient satisfaction was high.
When compared with other implant strategies, subperiosteal implants present a distinct set of advantages and limitations. Zygomatic implants provide stable anchorage in cases of advanced maxillary atrophy but are associated with higher morbidity and potential complications such as sinusitis or oroantral communication. Short and tilted implants offer a less invasive alternative but may lack long-term biomechanical stability in severely resorbed jaws. Subperiosteal implants, by contrast, are custom-designed to conform to the bone surface, allowing for stable prosthetic support even in cases of extreme bone loss—especially when vertical height must be preserved. However, their success depends on adequate soft tissue coverage, and complications such as exposure or prosthetic misfit can still occur, despite the precision offered by digital workflows. Based on the results of our case series and supported by the reviewed literature, we propose the following clinical preliminary guidelines for the successful application of subperiosteal implants.
4.1. Patient Selection
- Patients with severe alveolar bone atrophy (Cawood and Howell Class V–VI). (Ideal for those with advanced horizontal and vertical bone loss where standard implants are contraindicated).
- Narrow alveolar crest cases unsuitable for endosseous implants. (Allows rehabilitation without vertical bone reduction. Subperiosteal implant is the only technique that allows for the rehabilitation of narrow bone crests without the loss of vertical height).
- Elderly or systemically stable patients unwilling or unable to undergo bone grafting. (Minimally invasive alternative to augmentative procedures) [50].
4.2. Digital Planning
- Fully digital workflows.
- High-resolution CBCT scan without artifacts. (Provides accurate anatomical data for implant customization).
- Use of prosthetically driven planning software. (Ensures correct abutment angulation and emergence profile).
- Collaborative workflow between surgeon, prosthodontist, and technician. (Aligns surgical feasibility with prosthetic functionality) [26].
4.3. Surgical Technique
- Full-thickness flap elevation with identification of vital structures. (Prevents nerve injury and ensures proper implant bed access).
- Minimal osteoplasty when needed for passive fit. (Improves implant adaptation to the bony surface).
- Fixation with titanium screws at preplanned anchorage points. (Provides mechanical stability without intraosseous engagement).
- Strict aseptic handling of the implant. (Reduces risk of postoperative contamination).
4.4. Implant Manufacturing and Fitting
- High-precision manufacturing and appropriate surface treatments are essential to allow a successful rehabilitation.
- The implant must achieve a passive sitting and fixation, avoiding biomechanical stress on both the structure and fixation screws, as well as the aseptic implant handling [51].
4.5. Prosthetic Design
- Immediate loading with provisional prosthesis (24–72 h post-op). (Supports soft tissue healing and functional rehabilitation).
- Final prosthesis placement after 4 months. (Ensures tissue maturation and long-term durability).
- Passive fit and absence of considerable cantilevers. (Prevents biomechanical overload and prosthetic complications) [52].
4.6. Postoperative Protocols and Long-Term Monitoring
- Scheduled evaluations at 1, 3, and 6 months post-surgery, then annually. (Allows early detection of complications and implant integration monitoring).
- Annual prosthesis removal and peri-implant hygiene assessment. (Enables thorough decontamination and inspection of framework integrity).
- Customized hygiene instructions and use of chlorhexidine rinses. (Minimizes plaque accumulation and soft tissue inflammation) [53,54].
This study has several limitations that should be acknowledged. First, the retrospective nature of our case series introduces inherent selection bias. The small sample size (n = 9) limits statistical power and generalizability. Follow-up durations varied across cases and were limited in some instances, which may not capture long-term outcomes such as framework degradation or chronic soft tissue changes. Additionally, the absence of a control group treated with alternative methods (e.g., zygomatic or short implants) precludes direct comparative analysis. Despite these limitations, our findings provide valuable preliminary insights and support further prospective, controlled studies with larger patient cohorts.
5. Conclusions
Subperiosteal implants represent a promising alternative for the rehabilitation of patients with advanced jawbone atrophy. While short to medium-term outcomes are encouraging—particularly with the integration of digital technologies. The long-term clinical data and standardized protocols are still essential to optimize success and minimize complications. Currently, a narrow alveolar crest is considered the most suitable clinical indication for subperiosteal implants, as no other technique offers a comparable ability to preserve vertical bone height. Implant exposure remains one of the most unwanted complications; however, its impact on long-term success rates is still unclear.
Author Contributions
Conceptualization, G.P. and M.K.; methodology, A.G.; formal analysis, A.A.; data curation, R.P.; writing—original draft preparation, M.K. and C.B.; writing—review and editing, M.K. and P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as it qualifies as a case report/series and, according to the local ethical committee regulations, does not require formal evaluation.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank BTK Biotek SRL for their technical support and collaboration throughout the study. Their contribution was instrumental in the planning and development of the customized subperiosteal implant systems.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 195–223, Erratum in J. Clin. Periodontol. 2020, 47, 129. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F.F.; Rocchietta, I.; Buti, J.; D’Aiuto, F. Comparative evidence of different surgical techniques for the management of vertical alveolar ridge defects in terms of complications and efficacy: A systematic review and network meta-analysis. J. Clin. Periodontol. 2023, 50, 1487–1519. [Google Scholar] [CrossRef] [PubMed]
- Korn, P.; Gellrich, N.C.; Spalthoff, S.; Jehn, P.; Eckstein, F.; Lentge, F.; Zeller, A.N.; Rahlf, B. Managing the severely atrophic maxilla: Farewell to zygomatic implants and extensive augmentations? J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P. Horizontal bone-augmentation procedures in implant dentistry: Prosthetically guided regeneration. Periodontology 2000 2018, 77, 213–240. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009, 24, 237. [Google Scholar]
- Corbella, S.; Taschieri, S.; Del Fabbro, M. Long-term outcomes for the treatment of atrophic posterior maxilla: A systematic review of literature. Clin. Implant. Dent. Relat. Res. 2015, 17, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Barausse, C.; Balercia, A.; Pistilli, R.; Ippolito, D.R.; Felice, P. Conventional drills vs piezoelectric surgery preparation for placement of four immediately loaded zygomatic oncology implants in edentulous maxillae: Results from 1-year split-mouth randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 147–158. [Google Scholar] [PubMed]
- Felice, P.; Pistilli, R.; Piattelli, M.; Soardi, E.; Pellegrino, G.; Corvino, V.; Esposito, M. 1-stage versus 2-stage lateral maxillary sinus lift procedures: 4-month post-loading results of a multicenter randomised controlled trial. Eur. J. Oral Implantol. 2013, 6, 153–165. [Google Scholar] [PubMed]
- Esposito, M.; Barausse, C.; Pistilli, R.; Checchi, V.; Diazzi, M.; Gatto, M.R.; Felice, P. Posterior jaws rehabilitated with partial prostheses supported by 4.0 x 4.0 mm or by longer implants: Four-month post-loading data from a randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 221–230. [Google Scholar] [PubMed]
- Cucchi, A.; Vignudelli, E.; Fiorino, A.; Pellegrino, G.; Corinaldesi, G. Vertical ridge augmentation (VRA) with Ti-reinforced d-PTFE membranes or Ti meshes and collagen membranes: 1-year results of a randomized clinical trial. Clin. Oral Implant. Res. 2021, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Lizio, G.; Corinaldesi, G.; Marchetti, C. Titanium Mesh Technique in Rehabilitation of Totally Edentulous Atrophic Maxillae: A Retrospective Case Series. J. Periodontol. 2016, 87, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Pedrosa, A.R.; Custódio, A.L.N. Zygomatic implants: Critical review of surgical techniques. Oral Maxillofac. Surg. 2013, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, H.; Raes, S.; Östman, P.O.; Cosyn, J. Immediate loading in edentulous jaws: Literature review with clinical guidelines. Periodontology 2000 2014, 66, 153–187. [Google Scholar] [CrossRef] [PubMed]
- Keleş, H.G.; Karaca, Ç. Stress distribution among standard, zygomatic and subperiosteal implants in the atrophic maxilla: 3-D FEA. Int. J. Oral Maxillofac. Implant. 2023, 38, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H. Clinical performance of short implants supporting single crowns: Cement vs screw retention. Int. J. Oral Maxillofac. Implant. 2019, 34, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Fernández, H.; Gómez-Delgado, A.; Trujillo-Saldarriaga, S.; Varón-Cardona, D.; Castro-Núñez, J. Zygomatic implants for the management of the severely atrophied maxilla: A retrospective analysis of 244 implants. J. Oral Maxillofac. Surg. 2014, 72, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Andolsek, F.; Berton, F.; Perinetti, G.; Navarra, C.O.; Di Lenarda, R. Intraoperative Complications During Sinus Floor Elevation with Lateral Approach: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2017, 32, e107–e118. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Survival and Complications of Zygomatic Implants: An Updated Systematic Review. J. Oral Maxillofac. Surg. 2016, 74, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Classification of complications in guided bone regeneration with non-resorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265–273. [Google Scholar]
- Molina, A.; Sanz-Sánchez, I.; Sanz-Martín, I.; Ortiz-Vigón, A.; Sanz, M. Complications in sinus lifting: Classification and management. Periodontology 2000 2022, 88, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 319–339. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H. 15-year follow-up of short dental implants placed in the partially edentulous patient: Mandible Vs. maxilla. Ann. Anat. 2019, 222, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Vetromilla, B.M.; Mazzetti, T.; Pereira-Cenci, T. Short versus standard implants associated with sinus floor elevation: An umbrella review of meta-analyses of multiple outcomes. J. Prosthet. Dent. 2021, 126, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, L.V.; Franchina, A.; Pranno, A.; Pellegrino, G.; Ferri, A.; Pranno, N.; Di Carlo, S.; De Angelis, F. Use of Intraoral Scanners for Full Dental Arches: Could Different Strategies or Overlapping Software Affect Accuracy? Int. J. Environ. Res. Public Health 2021, 18, 9946. [Google Scholar] [CrossRef] [PubMed]
- De Moor, E.; Huys, S.E.F.; van Lenthe, G.H.; Mommaerts, M.Y.; Vander Sloten, J. Finite-element evaluation of a patient-specific AMSJI. Int. J. Oral Maxillofac. Surg. 2022, 51, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Karaban, M.; Scalchi, V.; Urbani, M.; Giudice, A.; Barausse, C.; Felice, P. Finite Element Analysis of Functionally Loaded Subperiosteal Implants Evaluated on a Realistic Model Reproducing Severe Atrophic Jaws. Methods Protoc. 2025, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Cipollina, A.; Ceddia, M.; Di Pietro, N.; Inchingolo, F.; Tumedei, M.; Romasco, T.; Piattelli, A.; Specchiulli, A.; Trentadue, B. Finite Element Analysis (FEA) of a Premaxillary Device: A New Type of Subperiosteal Implant to Treat Severe Atrophy of the Maxilla. Biomimetics 2023, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Kundakcioglu, A.; Ayhan, M. Subperiosteal implant thickness: Mechanical strength and bone stress by finite element analysis. Int. J. Med. Sci. 2024, 21, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol. 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, M.Y. Evolutionary steps in the design and biofunctionalization of the additively manufactured sub-periosteal jaw implant ‘AMSJI’ for the maxilla. Int. J. Oral Maxillofac. Surg. 2019, 48, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; Hansen, P.A. 18-year retrospective review of subperiosteal implants in severely atrophied edentulous mandibles. J. Prosthet. Dent. 2004, 92, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Vatteroni, E.; Covani, U.; Menchini Fabris, G.B. The New Generation of Subperiosteal Implants for Patient-Specific Treatment of Atrophic Dental Arches: Literature Review and Two Case Reports. Int. J. Periodontics Restor. Dent. 2023, 43, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Van den Borre, C.; Rinaldi, M.; De Neef, B.; Loomans, N.A.J.; Nout, E.; Van Doorne, L.; Naert, I.; Politis, C.; Schouten, H.; Klomp, G.; et al. Outcomes for AMSJI in the maxilla: Multicentre 1-year follow-up. Int. J. Oral Maxillofac. Surg. 2022, 51, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Herce-López, J.; Pingarrón, M.D.C.; Tofé-Povedano, Á.; García-Arana, L.; Espino-Segura-Illa, M.; Sieira-Gil, R.; Rodado-Alonso, C.; Sánchez-Torres, A.; Figueiredo, R. Customized Subperiosteal Implants for the Rehabilitation of Atrophic Jaws: A Consensus Report and Literature Review. Biomimetics 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cerea, M.; Dolcini, G.A. Custom-made direct metal laser sintering titanium sub-periosteal implants: A retrospective clinical study on 70 patients. BioMed Res. Int. 2018, 2018, 5420391. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Bianchi, A.; Mangano, F.G.; Dana, J.; Colombo, M.; Solop, I.; Admakin, O. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: A case series. 3D Print. Med. 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Strappa, E.M.; Memè, L.; Cerea, M.; Roy, M.; Bambini, F. Custom-made additively manufactured subperiosteal implant. Minerva Dent. Oral Sci. 2022, 71, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Nemtoi, A.; Covrig, V.; Nemtoi, A.; Stoica, G.; Vatavu, R.; Haba, D.; Zetu, I. Custom-Made Direct Metal Laser Sintering Titanium Subperiosteal Implants in Oral and Maxillofacial Surgery for Severe Bone-Deficient Patients-A Pilot Study. Diagnostics 2022, 12, 2531. [Google Scholar] [CrossRef] [PubMed]
- Marconcini, S.; Giammarinaro, E.; Covani, U. The rehabilitation of the atrophic posterior maxilla with a custom-made subperiosteal implant in a patient with a history of medications related to osteonecrosis of the jaws: One-year case report. Oral Maxillofac. Surg. Cases 2023, 9, 100292. [Google Scholar] [CrossRef]
- Onică, N.; Budală, D.G.; Baciu, E.R.; Onică, C.A.; Gelețu, G.L.; Murariu, A.; Balan, M.; Pertea, M.; Stelea, C. Long-Term Clinical Outcomes of 3D-Printed Subperiosteal Titanium Implants: A 6-Year Follow-Up. J. Pers. Med. 2024, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, M.; Yey Özkeskin, S.Z. Rehabilitation of Severely Atrophic Maxilla with Le Fort I Advancement Surgery and Subperiosteal Implant in a Patient With Ectodermal Dysplasia. J. Craniofacial Surg. 2024, 35, e484–e486. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, N.C.; Korn, P.; Jehn, P.; Neuhaus, M.; Lentge, F.; Rahlf, B. Exceptional Cases Demand Exceptional Personalized Solutions: The Next Level in Dental Rehabilitation. J. Pers. Med. 2024, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, M.; Ozturk Muhtar, M.; Kundakcioglu, A.; Kucukcakir, O.; Cansiz, E. Evaluation of Clinical Success of the 3D-Printed Custom-Made Subperiosteal Implants. J. Craniofacial Surg. 2024, 35, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Biglio, A.; Favro, A.; Salzano, G.; Abbate, V.; Lechien, J.R.; De Riu, G. Implant-prosthetic rehabilitation of the atrophic posterior mandible with additively manufactured custom-made subperiosteal implants: A cohort study. Int. J. Oral Maxillofac. Surg. 2024, 53, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Eguia, A.; Staudigl, C.; Alkhraisat, M.H. Clinical performance of additively manufactured subperiosteal implants: A systematic review. Int. J. Implant. Dent. 2024, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, M.A.; Hegazy, S.A. Subperiosteal implants constructed with digital technology: A systematic review. Oral Maxillofac. Surg. 2024, 28, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, R.; Okulski, J.; Piechaczek, M.; Łoś, J.; Sowiński, J.; Sadowska-Sowińska, M.; Kołkowska, A.; Simka, W.; Kozakiewicz, M. Five-Year Comparative Study of Zygomatic and Subperiosteal Implants: Clinical Outcomes, Complications, and Treatment Strategies for Severe Maxillary Atrophy. J. Clin. Med. 2025, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Santiago, P.H.; Tavares, M.G.; Grillo, R. Personalized Rehabilitation of Atrophic Customized Subperiosteal Implants. J. Craniofacal Surg. 2025, 36, e20–e22. [Google Scholar] [CrossRef] [PubMed]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Vatteroni, E.; Toti, P.; Covani, U.; Crespi, R.; Cosola, S.; Menchini-Fabris, G.B. A Retrospective Radiological and Clinical Survey of Full-Arch Immediate Fixed Prostheses Supported by Custom-Made Three- Dimensional Printed Subperiosteal Titanium Implants in Patients with Severe Atrophic Jaws: Implant Success Code. Int. J. Oral Maxillofac. Implant. 2025, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Biglio, A.; Salzano, G.; Lechien, J.R.; De Riu, G. Additively manufactured custom-made subperiosteal implant rehabilitation for severely atrophic maxillary molar area: A technical note. J. Stomatol. Oral Maxillofac. Surg. 2024, 125 (Suppl. 1), 101917. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Bertacci, A.; Bonifazi, L.; Karaban, M.; Canullo, L.; Pistilli, R.; Sammartino, P.; Gasparro, R.; Barausse, C. A Proposed Protocol for Ordinary and Extraordinary Hygienic Maintenance in Different Implant Prosthetic Scenarios. Appl. Sci. 2021, 11, 2957. [Google Scholar] [CrossRef]
- Monje, A.; Aranda, L.; Diaz, K.T.; Alarcón, M.A.; Bagramian, R.A.; Wang, H.L.; Catena, A. Impact of maintenance therapy on prevention of peri-implant diseases: Systematic review and meta-analysis. J. Dent. Res. 2016, 95, 372–379. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).