The Physiopathological Link Between Bisphenol A Exposure and Molar Incisor Hypomineralization Occurrence: A Systematic Review
Abstract
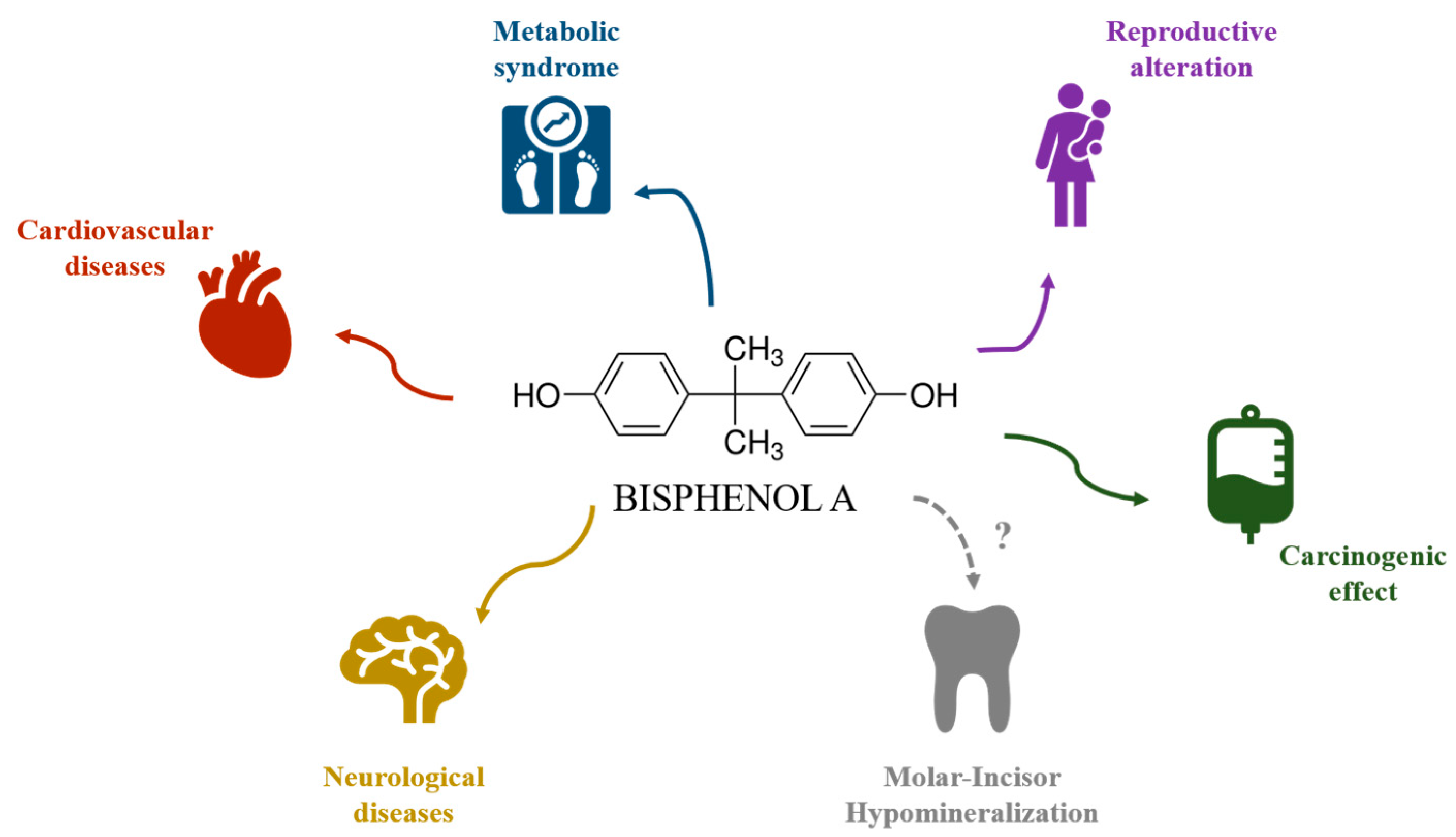
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process and Items
2.6. Summary of Results
2.7. Bias Assessment
3. Results
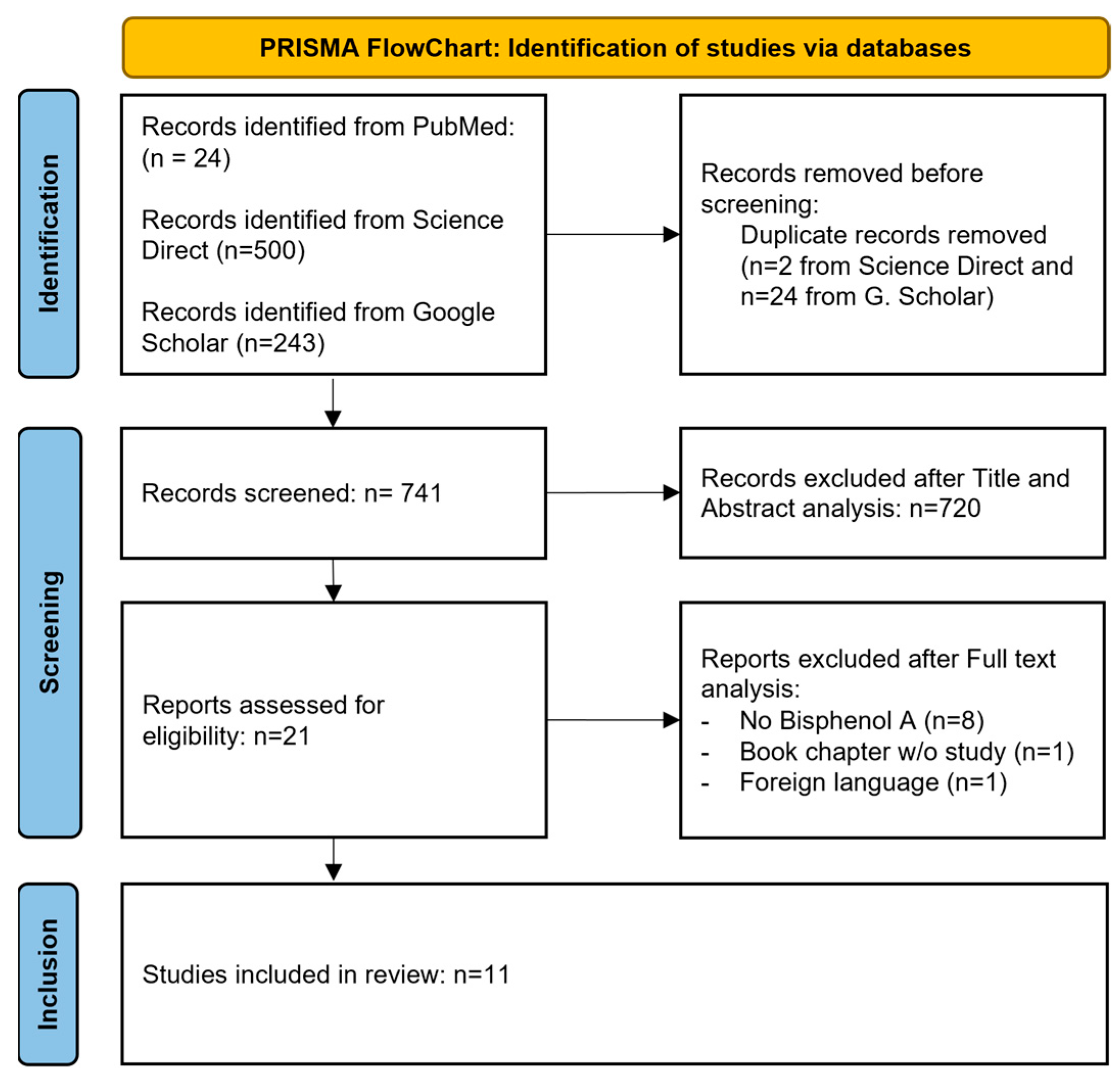
3.1. Study Selection
3.2. Main Characteristics of the Selected Studies
3.3. Results of Individual Studies
3.4. Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPA | Bisphenol A |
| BPS | Bisphenol S |
| ED | Endocrine disruptor |
| KLK4 | Hypomineralized second primary molars |
| HSPM | Kallikrein-related peptidase 4 |
| MIH | Molar incisor hypomineralization |
| REACH | Registration, Evaluation, Authorization and Restriction of Chemicals |
| SVHC | Substance of very high concern |
Appendix A
Appendix A.1
| Query Terms | Number of Results |
|---|---|
| #1: ((((((((((((((((((((((((((((((((MIH [Title/Abstract])) OR (molar incisor [Title/Abstract])) OR (molar-incisor [Title/Abstract])) OR (molar incisor hypomineralisation [Title/Abstract])) OR (molar incisor hypomineralization [Title/Abstract])) OR (molar-incisor hypomineralization [Title/Abstract])) OR (molar incisor-hypomineralization [Title/Abstract])) OR (molar-incisor-hypomineralization [Title/Abstract])) OR (molar-incisor hypomineralization [Title/Abstract])) OR (molar-incisor-hypomineralization [Title/Abstract])) OR (molar incisor-hypomineralization [Title/Abstract])) OR (tooth hypomineralization [Title/Abstract])) OR (tooth hypomineralization [Title/Abstract])) OR (teeth hypomineralization [Title/Abstract])) OR (teeth hypomineralization [Title/Abstract])) OR (hypomineralization defect * [Title/Abstract])) OR (hypomineralization defect * [Title/Abstract])) OR (tooth enamel hypomineralization [Title/Abstract])) OR (teeth enamel hypomineralization [Title/Abstract])) OR (tooth enamel hypomineralization [Title/Abstract])) OR (teeth enamel hypomineralization [Title/Abstract])) OR (molar enamel hypomineralization [Title/Abstract])) OR (incisor enamel hypomineralization [Title/Abstract])) OR (molar incisor enamel hypomineralization [Title/Abstract])) OR (molar-incisor enamel hypomineralization [Title/Abstract])) OR (molar enamel hypomineralization [Title/Abstract])) OR (molar incisor enamel hypomineralization [Title/Abstract])) OR (molar incisor-enamel hypomineralization [Title/Abstract])) OR (incisor enamel hypomineralization [Title/Abstract])) OR (hspm [Title/Abstract])) OR (second primary molar hypomineralization [Title/Abstract])) OR (second primary molar hypomineralization [Title/Abstract]) | 1731 |
| #2: (((((((((endocrine disrupt * [Title/Abstract]) OR (endocrine disrupting chemical * [Title/Abstract])) OR (EDC * [Title/Abstract])) OR (phenol * [Title/Abstract])) OR (Bisphenol a [Title/Abstract])) OR (BPA [Title/Abstract])) OR (Bisphenol-a [Title/Abstract])) OR (Bisphenol s [Title/Abstract])) OR (BPS [Title/Abstract])) OR (Bisphenol-s [Title/Abstract]) | 193,924 |
| #3: (((review [Title]) OR review [Publication Type]) OR (meta-analysis [Publication Type]) | 3,936,087 |
| #4: #1 AND #2 | 26 |
| #5: #4 NOT #3 | 24 |
Appendix A.2
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Page 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Page 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Pages 1 to 5 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Page 3 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Page 3 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Page 3 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Page 3 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Page 4 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Page 4 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Page 4 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | ||
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Page 4 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | n/a |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Page 4 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | Page 4 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | ||
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | ||
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | ||
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | ||
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Page 4 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | ||
| Study characteristics | 17 | Cite each included study and present its characteristics. | Page 5 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Page 11 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Pages 6 to 11 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | ||
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | ||
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | ||
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Pages 11 to 13 |
| 23b | Discuss any limitations of the evidence included in the review. | ||
| 23c | Discuss any limitations of the review processes used. | ||
| 23d | Discuss implications of the results for practice, policy, and future research. | ||
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | ||
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | ||
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | Page 13 |
| Competing interests | 26 | Declare any competing interests of review authors. | Page 13 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Page 13 |
References
- Weerheijm, K.L.; Jälevik, B.; Alaluusua, S. Molar-Incisor Hypomineralisation. Caries Res. 2001, 35, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Arheiam, A.; Abbas, S.; Ballo, L.; Borowis, E.; Rashwan, S.; El Tantawi, M. Prevalence, Distribution, Characteristics and Associated Factors of Molar-Incisor Hypo-Mineralisation among Libyan Schoolchildren: A Cross-Sectional Survey. Eur. Arch. Paediatr. Dent. 2021, 22, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Dong, B.; Yu, D.; Ren, Q.; Sun, Y. The Prevalence of Molar Incisor Hypomineralization: Evidence from 70 Studies. Int. J. Paediatr. Dent. 2018, 28, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Weerheijm, K.L. Molar Incisor Hypomineralization (MIH): Clinical Presentation, Aetiology and Management. Dent. Update 2004, 31, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Garot, E.; Denis, A.; Delbos, Y.; Manton, D.; Silva, M.; Rouas, P. Are Hypomineralised Lesions on Second Primary Molars (HSPM) a Predictive Sign of Molar Incisor Hypomineralisation (MIH)? A Systematic Review and a Meta-Analysis. J. Dent. 2018, 72, 8–13. [Google Scholar] [CrossRef] [PubMed]
- da Silva Figueiredo Sé, M.J.; Ribeiro, A.P.D.; Dos Santos-Pinto, L.A.M.; de Cassia Loiola Cordeiro, R.; Cabral, R.N.; Leal, S.C. Are Hypomineralized Primary Molars and Canines Associated with Molar-Incisor Hypomineralization? Pediatr. Dent. 2017, 39, 445–449. [Google Scholar] [PubMed]
- Maserejian, N.N.; Trachtenberg, F.L.; Hauser, R.; McKinlay, S.; Shrader, P.; Tavares, M.; Bellinger, D.C. Dental Composite Restorations and Psychosocial Function in Children. Pediatrics 2012, 130, e328–e338. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Scurrah, K.J.; Craig, J.M.; Manton, D.J.; Kilpatrick, N. Etiology of Molar Incisor Hypomineralization—A Systematic Review. Community Dent. Oral. Epidemiol. 2016, 44, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Molina-López, A.M.; Bujalance-Reyes, F.; Ayala-Soldado, N.; Mora-Medina, R.; Lora-Benítez, A.; Moyano-Salvago, R. An Overview of the Health Effects of Bisphenol A from a One Health Perspective. Animals 2023, 13, 2439. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Lee, C.-Y.; Chuang, Y.-S.; Shih, C.-L. Exposure to Bisphenol A Associated with Multiple Health-Related Outcomes in Humans: An Umbrella Review of Systematic Reviews with Meta-Analyses. Environ. Res. 2023, 237, 116900. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.E.; Cairrao, E. Effect of Bisphenol A on the Neurological System: A Review Update. Arch. Toxicol. 2024, 98, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Kaur, M.; Tyagi, D.; Singh, T.B.; Kaur, G.; Afzal, S.M.; Jauhar, M. Exploring Novel Insights into the Molecular Mechanisms Underlying Bisphenol A-Induced Toxicity: A Persistent Threat to Human Health. Environ. Toxicol. Pharmacol. 2024, 108, 104467. [Google Scholar] [CrossRef] [PubMed]
- Olea, N.; Pulgar, R.; Pérez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of Resin-Based Composites and Sealants Used in Dentistry. Environ. Health Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Elzein, R.; Chouery, E.; Abdel-Sater, F.; Bacho, R.; Ayoub, F. Molar-Incisor Hypomineralisation in Lebanon: Association with Prenatal, Natal and Postnatal Factors. Eur. Arch. Paediatr. Dent. 2021, 22, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, N.; Naito, T.; Narimatsu, S. Human UDP-Glucuronosyltransferase Isoforms Involved in Bisphenol A Glucuronidation. Chemosphere 2008, 74, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Ekström, L.; Johansson, M.; Rane, A. Tissue Distribution and Relative Gene Expression of UDP-Glucuronosyltransferases (2B7, 2B15, 2B17) in the Human Fetus. Drug Metab. Dispos. 2013, 41, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a Narrative Biomedical Review: Considerations for Authors, Peer Reviewers, and Editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufunaru, C.; Aromataris, E.; Sears, K.; Sfetc, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Systematic Reviews of Aetiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; JBI: North Adelaide, SA, Australia, 2024; ISBN 978-0-6488488-2-0. [Google Scholar]
- Tufunaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Systematic Reviews of Effectiveness. In JBI Manual for Evidence Synthesis; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; JBI: North Adelaide, SA, Australia, 2024; ISBN 978-0-6488488-2-0. [Google Scholar]
- Tran, L.; Tam, D.N.H.; Elshafay, A.; Dang, T.; Hirayama, K.; Huy, N.T. Quality Assessment Tools Used in Systematic Reviews of in Vitro Studies: A Systematic Review. BMC Med. Res. Methodol. 2021, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.B.; Houari, S.; Loiodice, S.; Jedeon, K.; Berdal, A.; Babajko, S. Steroid Receptors Involvement in Enamel Hypomineralization Resulting from Exposure to Low-Dose DEHP and Bisphenol A. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2017. [Google Scholar] [CrossRef]
- Winkler, J.R.; Dixon, B.L.; Singh, I.; Soto, R.; Qiu, Y.; Zhang, Y.; Porucznik, C.A.; Stanford, J.B. Prenatal Exposure to Environmental Toxins and Comprehensive Dental Findings in a Population Cohort of Children. BMC Oral Health 2024, 24, 326. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Reichl, F.-X.; Milz, S.; Wölfle, U.C.; Kühnisch, J.; Schmitz, C.; Geist, J.; Hickel, R.; Högg, C.; Sternecker, K. Disrupted Biomineralization in Zebra Mussels after Exposure to Bisphenol-A: Potential Implications for Molar-Incisor Hypomineralization. Dent. Mater. 2022, 38, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Duman, C.; Özkan Yenal, N.; Menteş, A. How Prenat. Environ. Factors Affect. Rat. Molar Enamel. Form? Odontology 2022, 110, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, K.; Houari, S.; Loiodice, S.; Thuy, T.T.; Normand, M.L.; Berdal, A.; Babajko, S. Chronic Exposure to Bisphenol A Exacerbates Dental Fluorosis in Growing Rats. J. Bone Miner. Res. 2016, 31, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, K.; Loiodice, S.; Salhi, K.; Le Normand, M.; Houari, S.; Chaloyard, J.; Berdal, A.; Babajko, S. Androgen Receptor Involvement in Rat Amelogenesis: An Additional Way for Endocrine-Disrupting Chemicals to Affect Enamel Synthesis. Endocrinology 2016, 157, 4287–4296. [Google Scholar] [CrossRef] [PubMed]
- Houari, S.; Loiodice, S.; Jedeon, K.; Berdal, A.; Babajko, S. Expression of Steroid Receptors in Ameloblasts during Amelogenesis in Rat Incisors. Front. Physiol. 2016, 7, 503. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, K.; Berdal, A.; Babajko, A. Impact of Three Endocrine Disruptors, Bisphenol A, Genistein and Vinclozolin on Female Rat Enamel. Bull. Group. Int. Rech. Sci. Stomatol. Odontol. 2016, 53, e28–e32. [Google Scholar] [PubMed]
- Jedeon, K.; Loiodice, S.; Marciano, C.; Vinel, A.; Canivenc Lavier, M.-C.; Berdal, A.; Babajko, S. Estrogen and Bisphenol A Affect Male Rat Enamel Formation and Promote Ameloblast Proliferation. Endocrinology 2014, 155, 3365–3375. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, K.; Marciano, C.; Loiodice, S.; Boudalia, S.; Canivenc Lavier, M.-C.; Berdal, A.; Babajko, S. Enamel Hypomineralization Due to Endocrine Disruptors. Connect. Tissue Res. 2014, 55 (Suppl. 1), 43–47. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, K.; De la Dure-Molla, M.; Brookes, S.J.; Loiodice, S.; Marciano, C.; Kirkham, J.; Canivenc-Lavier, M.-C.; Boudalia, S.; Bergès, R.; Harada, H.; et al. Enamel Defects Reflect Perinatal Exposure to Bisphenol A. Am. J. Pathol. 2013, 183, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.; Monfort, C.; Lainé, F.; Gaudreau, É.; Tillaut, H.; Bonnaure-Mallet, M.; Cordier, S.; Meuric, V.; Chevrier, C. Prenatal Exposure to Persistent Organic Pollutants and Molar-Incisor Hypomineralization among 12-Year-Old Children in the French Mother-Child Cohort PELAGIE. Environ. Res. 2023, 231, 116230. [Google Scholar] [CrossRef] [PubMed]
- Berenstein Ajzman, G.; Dagon, N.; Iraqi, R.; Blumer, S.; Fadela, S. The Prevalence of Developmental Enamel Defects in Israeli Children and Its Association with Perinatal Conditions: A Cross-Sectional Study. Children 2023, 10, 903. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.T.; Houari, S.; Loiodice, S.; Bazin, D.; Sadoine, J.; Roubier, N.; Vennat, E.; Tran, T.T.; Berdal, A.; Ricort, J.-M.; et al. Use of Dental Defects Associated with Low-Dose Di(2-Ethylhexyl)Phthalate as an Early Marker of Exposure to Environmental Toxicants. Environ. Health Perspect. 2022, 130, 67003. [Google Scholar] [CrossRef] [PubMed]
- Babajko, S.; Jedeon, K.; Houari, S.; Loiodice, S.; Berdal, A. Disruption of Steroid Axis, a New Paradigm for Molar Incisor Hypomineralization (MIH). Front. Physiol. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Grandin, F.C.; Lacroix, M.Z.; Gayrard, V.; Viguié, C.; Mila, H.; de Place, A.; Vayssière, C.; Morin, M.; Corbett, J.; Gayrard, C.; et al. Is Bisphenol S a Safer Alternative to Bisphenol A in Terms of Potential Fetal Exposure? Placental Transfer across the Perfused Human Placenta. Chemosphere 2019, 221, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Grandin, F.C.; Lacroix, M.Z.; Gayrard, V.; Gauderat, G.; Mila, H.; Toutain, P.-L.; Picard-Hagen, N. Bisphenol S Instead of Bisphenol A: Toxicokinetic Investigations in the Ovine Materno-Feto-Placental Unit. Environ. Int. 2018, 120, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.-J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A New Chapter in the Bisphenol A Story: Bisphenol S and Bisphenol F Are Not Safe Alternatives to This Compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Balicco, A.; Bidondo, M.-L.; Filol, C.; Gane, J.; Oleko, A.; Saoudi, A.; Zeghnoun, A. Impregnation of the French Population by Bisphenols A, S and F. National Bio-Monitoring Program: Esteban 2014–2016; Santé Publique France: Saint-Maurice, France, 2019; p. 57. [Google Scholar]
- Dursun, E.; Fron-Chabouis, H.; Attal, J.-P.; Raskin, A. Bisphenol A Release: Survey of the Composition of Dental Composite Resins. Open Dent. J. 2016, 10, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, A.F.; Sheffield, P.E.; Chinn, C.; Edelstein, B.L.; Landrigan, P.J. Bisphenol A and Related Compounds in Dental Materials. Pediatrics 2010, 126, 760–768. [Google Scholar] [CrossRef] [PubMed]
| Year | Authors | Country | Objective | Study Type | Male (Yes/No) | Female (Yes/No) | Number of Subjects | Cellular Analyses (Yes/No) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| 2024 | Winkler et al. [25] | USA | To investigate the relationship between intrauterine exposure to ED and enamel developmental defects in children. | Clinical retrospective study | Yes (53%) | Yes (47%) | 356 | No | Weak positive correlations (but not statistically significant) were found between an increasing concentration of BPA and an increased number or proportion of teeth with hypomineralization. |
| 2022 | Liu et al. [26] | Germany | To investigate the feasibility of zebra mussel (Dreissena polymorpha) as a novel model to screen potential MIH-related factors. | In vivo | 46 groups of 7 | BPA was toxic to zebra mussels and interfered quantitively with shell biomineralization. Nacre is composed of 95% mineral (aragonite, not hydroxyapatite) with an organic matrix, similarly to tooth enamel. | |||
| 2022 | Duman et al. [27] | Turkey | To investigate how prenatal environmental factors (e.g., BPA) affect AMELX and AMBN production of ameloblasts. | In vivo | No | Yes (100%) | 15 (5 groups of 3) | No | Abnormal enamel matrix formation was observed in all experimental groups, including those with BPA exposure. AMELX and AMBN staining was significantly lower than that of the control. |
| 2017 | Ai Thu et al. [24] | France | To compare mouse enamel defects resulting from exposure to low-dose BPA and/or phthalates; To elucidate the mechanism of action of both endocrine disruptors during amelogenesis. | In vivo (conference abstract) | Unknown | Unknown | Unknown | Yes | Depending on their nature and the time of exposure, phthalates and BPA could affect enamel quality and/or quantity. |
| 2016 | Jedeon et al. [28] | France | To study the impact of exposure to BPA and sodium fluoride (NaF) on fluorosis and hypomineralization. | In vivo and in vitro | Yes | Yes | 4 groups of 12 rats | Yes | BPA and NaF decrease the expression of proteases and disrupt pH, causing the inhibition of crystal growth and thus the hypomineralization of enamel. |
| 2016 | Jedeon et al. [29] | France | To explore androgen receptors due to the preferential enamel impact of BPA on male rats. | In vivo and in vitro | Yes | No | 8 groups of 8 rats | Yes | The androgen signaling pathway is involved throughout the enamel mineralization process. The highest expression of androgen receptors is in maturation-stage ameloblasts. BPA and V exert an anti-androgenic effect, preferentially in male rats, which can specifically affect enamel. |
| 2016 | Houari et al. [30] | France | To explore the molecular pathways stimulated by BPA during amelogenesis and the different receptors known to regulate the effects of BPA. | In vivo and in vitro | Yes | Yes | 6 groups of 3 rats. | Yes | Many steroid receptors are expressed by ameloblasts, in particular at the stage of maturation, impacting enamel quality rather than quantity. A parallel can be made with MIH pathology, especially involving enamel quality, and, therefore, the final stages of enamel synthesis. |
| 2016 | Jedeon et al. [31] | France | To assess differences in hypomineralization between female and male rats following exposure to 3 EDs (V, G, and BPA). | In vivo and in vitro | No | Yes | 6 groups of 8 rats | Yes | Female rats are less affected than males by the three EDs chosen in this study. The modulation of the gene expression of kallikrein 4 and enamelin was higher in males than females. |
| 2014 | Jedeon et al. [32] | France | To assess the effects of BPA on ameloblasts and the potential involvement of the estrogen signaling pathway. | In vivo and in vitro | Yes | Yes | Male and female groups, each containing 16 control rats and 16 treated rats | Yes | Both BPA and estrogen stimulate the proliferation of ameloblasts via the estrogen receptor Erα (but not only). BPA impacts, preferentially, amelogenesis in male rats with a longer stage of secretion of ameloblasts and a shorter maturation stage. |
| 2014 | Jedeon et al. [33] | France | To assess the effect of the combination of several EDs (Genistein (G), Vinclozolin (V), and BPA) on tooth enamel. | In vivo and in vitro | Yes | No | 6 groups of 8 rats | Yes | In vivo, the different combinations tested had less impact on enamel than BPA alone. In addition, the combination of G and/or V with BPA reduces the effects of BPA on enamel hypomineralization. |
| 2013 | Jedeon et al. [34] | France | To analyze the impact of BPA on amelogenesis. | In vivo and in vitro | Yes | No | 16 treated rats and 16 control rats | Yes | The incisors of rats treated with BPA present, in 75% of cases, asymmetrical white spots at the enamel level with a phenotype similar to those of human MIH. BPA disrupts the normal removal of proteins from the enamel matrix (increased enamelin, decreased KLK4). There is a specific window of sensitivity. |
| Authors, Date | Bias Score |
|---|---|
| Winkler et al., 2024 [25] | 62.5% |
| Liu et al., 2022 [26] | 87.5% |
| Duman et al., 2022 [27] | 87.5% |
| Ai Thu et al., 2017 (not evaluated, abstract only) [24] | - |
| Jedeon et al., 2016 (J Bone & Min Res) [28] | 100% |
| Jedeon et al., 2016 (Endocrinology) [29] | 100% |
| Houari et al., 2016 [30] | 87.5% |
| Jedeon et al., 2016 (Bull Group Int Rech Sto Od) [31] | 87.5% |
| Jedeon et al., 2014 (Endocrinology) [32] | 100% |
| Jedeon et al., 2014 (Connect Tissue Res) [33] | 100% |
| Jedeon et al., 2013 [34] | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathonat, E.; Canceill, T.; Marty, M.; Prosper, A.; Vinel, A.; Noirrit-Esclassan, E. The Physiopathological Link Between Bisphenol A Exposure and Molar Incisor Hypomineralization Occurrence: A Systematic Review. Dent. J. 2025, 13, 332. https://doi.org/10.3390/dj13080332
Mathonat E, Canceill T, Marty M, Prosper A, Vinel A, Noirrit-Esclassan E. The Physiopathological Link Between Bisphenol A Exposure and Molar Incisor Hypomineralization Occurrence: A Systematic Review. Dentistry Journal. 2025; 13(8):332. https://doi.org/10.3390/dj13080332
Chicago/Turabian StyleMathonat, Estelle, Thibault Canceill, Mathieu Marty, Alison Prosper, Alexia Vinel, and Emmanuelle Noirrit-Esclassan. 2025. "The Physiopathological Link Between Bisphenol A Exposure and Molar Incisor Hypomineralization Occurrence: A Systematic Review" Dentistry Journal 13, no. 8: 332. https://doi.org/10.3390/dj13080332
APA StyleMathonat, E., Canceill, T., Marty, M., Prosper, A., Vinel, A., & Noirrit-Esclassan, E. (2025). The Physiopathological Link Between Bisphenol A Exposure and Molar Incisor Hypomineralization Occurrence: A Systematic Review. Dentistry Journal, 13(8), 332. https://doi.org/10.3390/dj13080332









