Chronology and Sequence of Permanent Tooth Eruption in a Multi-Ethnic Urban Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Sample
3.2. Sequence of Tooth Eruption
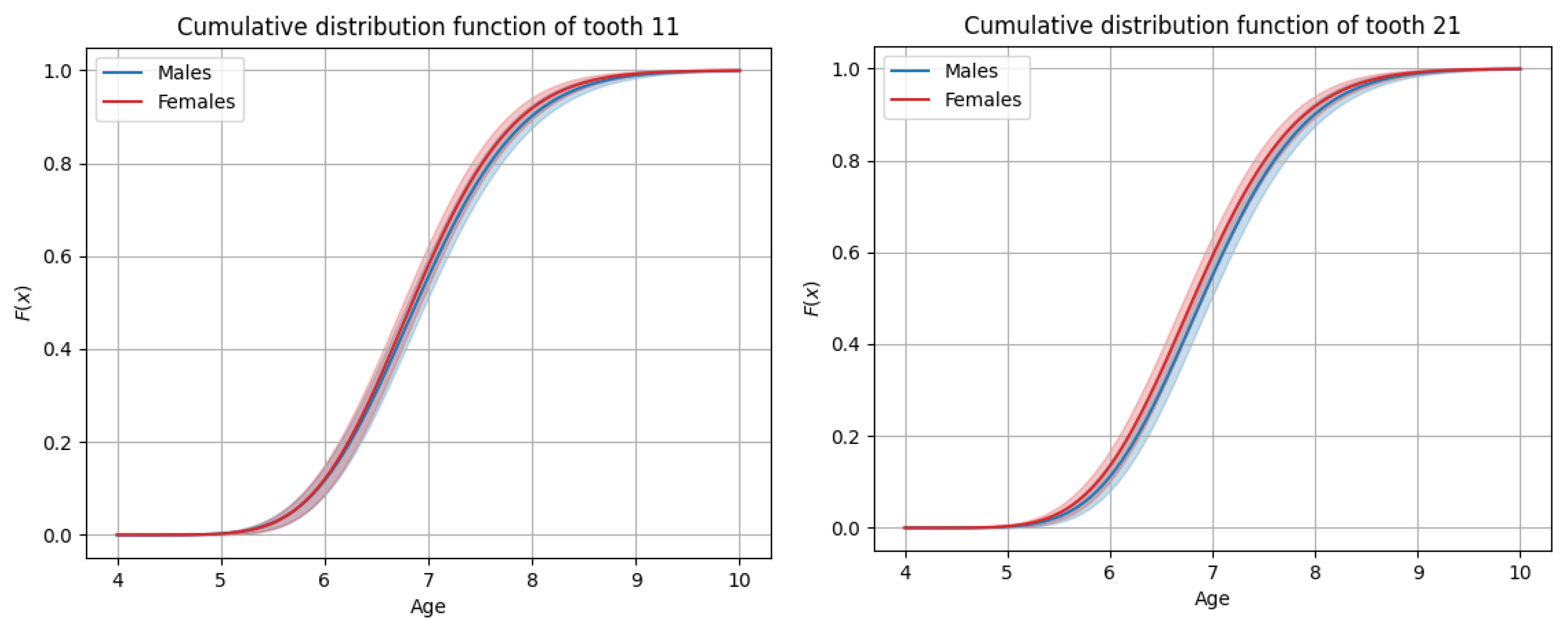
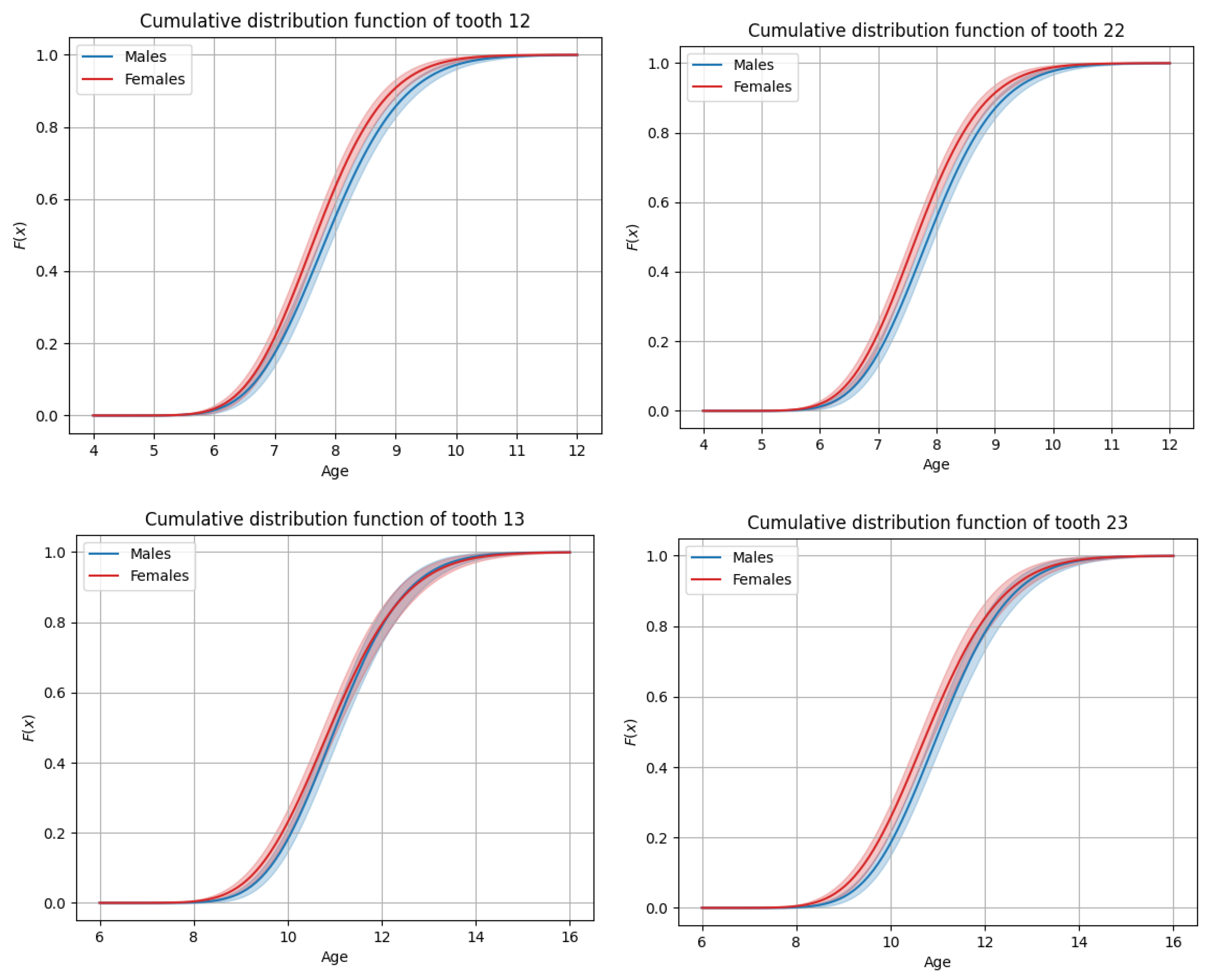
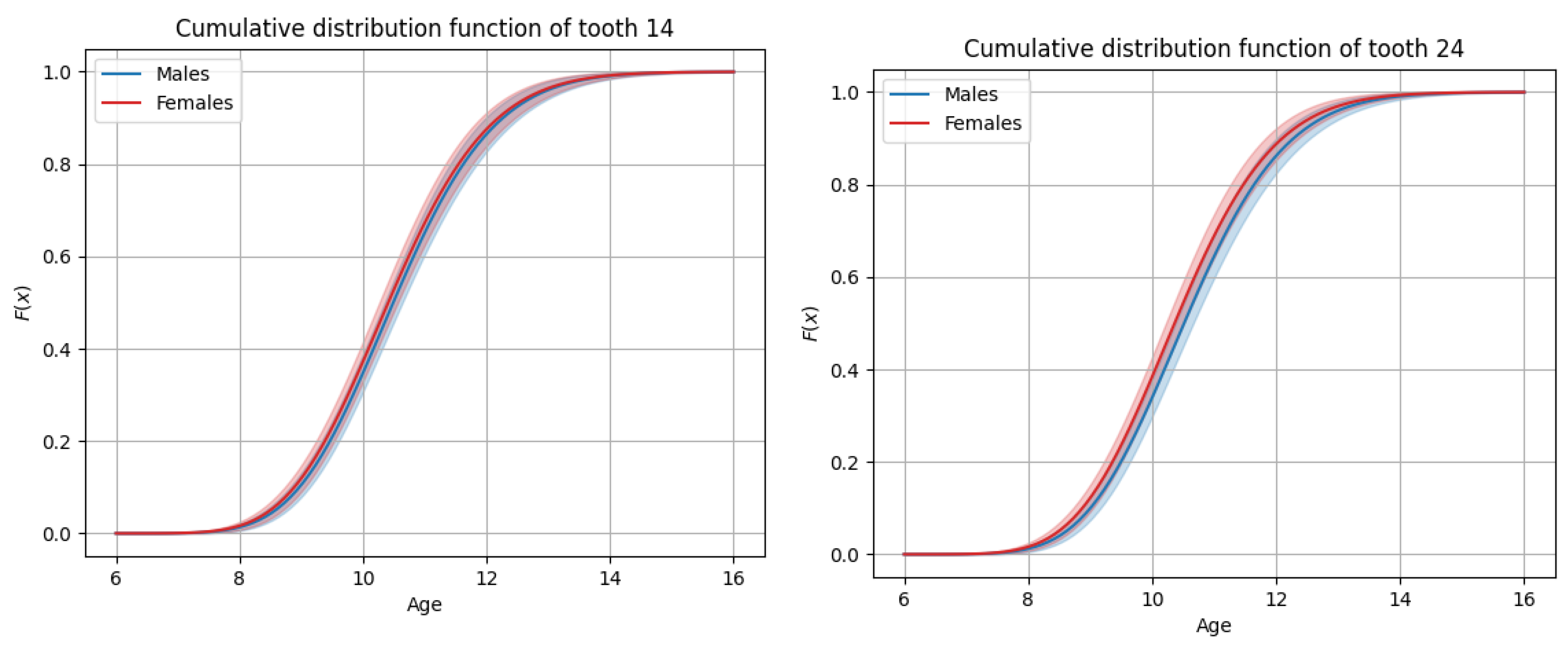
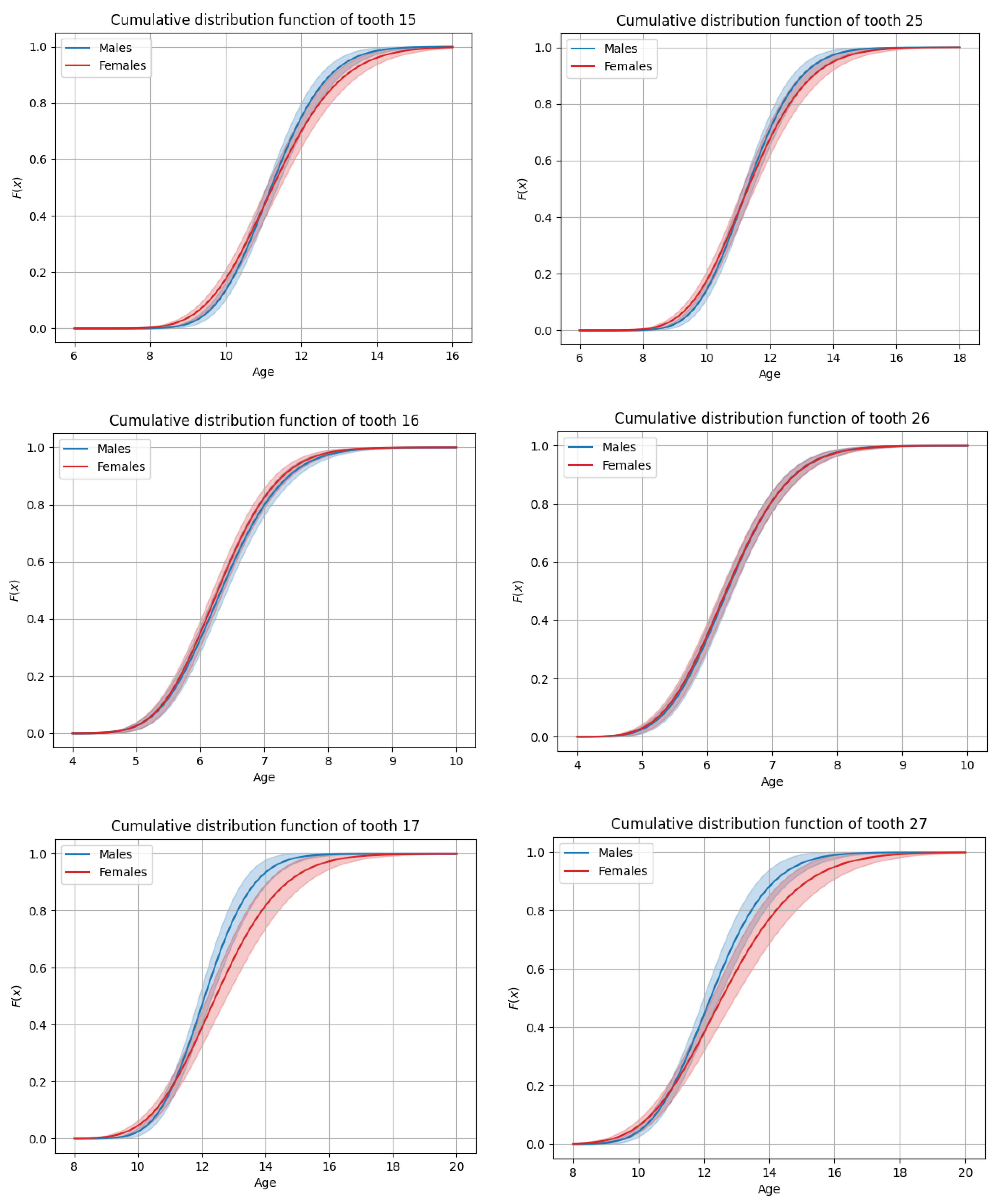
3.3. General Timing of Tooth Eruption
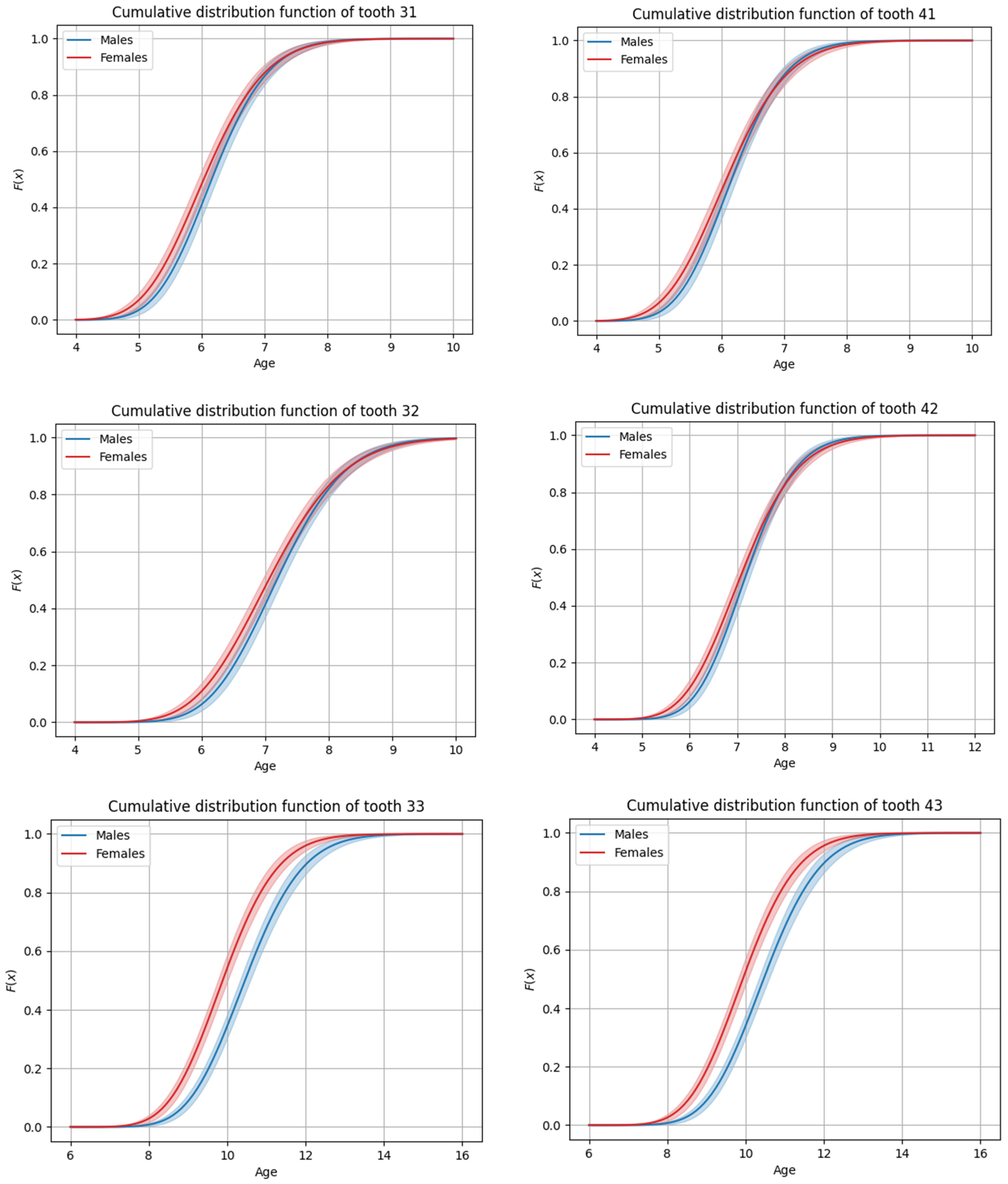
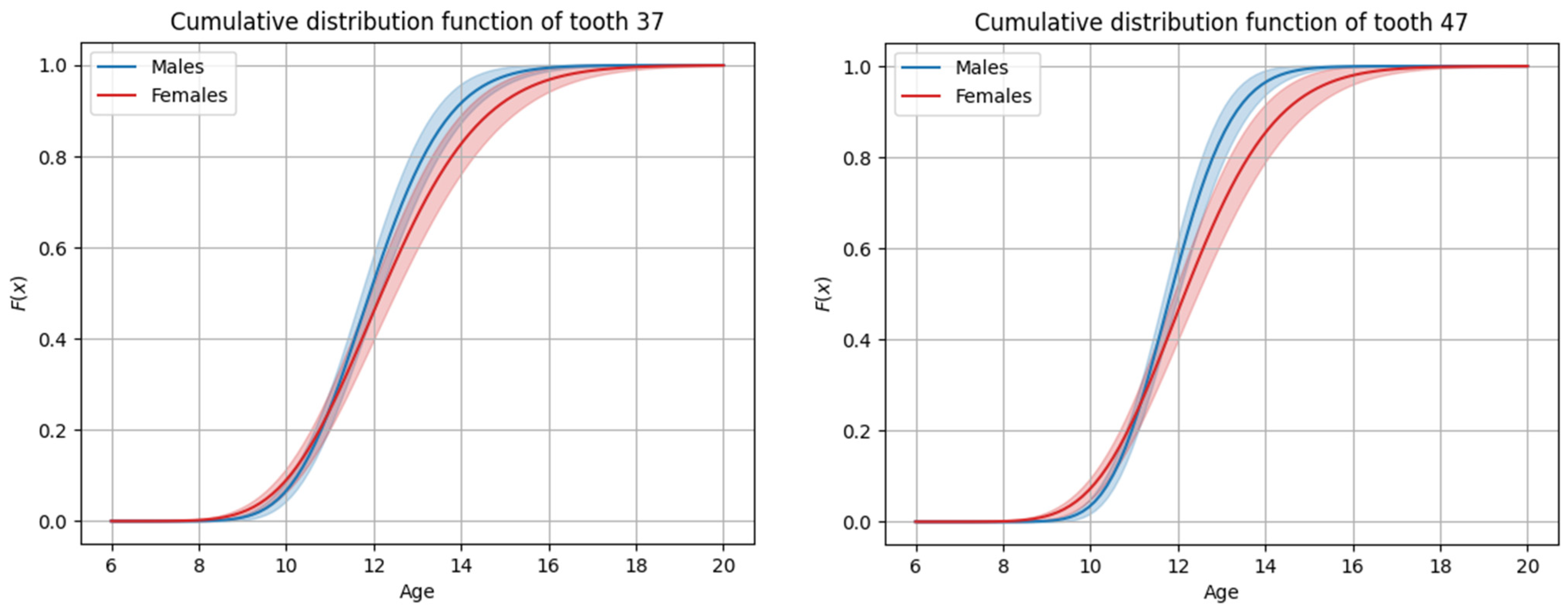
3.4. Sex-Based Differences in Timing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Logan, W.; Kronfeld, R. Development of the Human Jaws and Surrounding Structures from Birth to the Age of Fifteen Years. Am. Dent. Assoc. 1933, 20, 379–427. [Google Scholar]
- Proffit, W.R.; Fields, H.W.; Larson, B.E.; Sarver, D.M. Contemporary Orthodontics, 6th ed.; Elsevier: St. Louis, MO, USA, 2018; pp. 60–83. [Google Scholar]
- Vandana, S.; Muthu, M.S.; Akila, G.; Anusha, M.; Kandaswamy, D.; Narayanan, M.B.A. Global Variations in Eruption Chronology of Permanent Teeth: A Systematic Review and Meta-analysis. Am. J. Hum. Biol. 2024, 36, e24060. [Google Scholar] [CrossRef]
- Eskeli, R.; Lösönen, M.; Ikävalko, T.; Myllykangas, R.; Lakka, T.; Laine-Alava, M.T. Secular Trends Affect Timing of Emergence of Permanent Teeth. Angle Orthod. 2016, 86, 53–58. [Google Scholar] [CrossRef]
- Vucic, S.; Vries, E.d.; Eilers, P.H.C.; Willemsen, S.P.; Kuijpers, M.A.R.; Prahl-Andersen, B.; Jaddoe, V.W.V.; Hofman, A.; Wolvius, E.B.; Ongkosuwito, E.M. Secular Trend of Dental Development in Dutch Children. Am. J. Phys. Anthr. 2014, 155, 91–98. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Al-Dahan, Z.A. Time of Emergence of Permanent Teeth and Impact of Nutritional Status among 4–15 Years Old Children and Teenagers in Basrah City, Iraq. J. Baghdad Coll. Dent. 2016, 28, 134–140. [Google Scholar] [CrossRef]
- Almonaitiene, R.; Balciuniene, I.; Tutkuviene, J. Factors Influencing Permanent Teeth Eruption. Part One--General Factors. Stomatologija 2010, 12, 67–72. [Google Scholar]
- Seow, W.K. A Study of the Development of the Permanent Dentition in Very Low Birthweight Children. Pediatr. Dent. 1996, 18, 379–384. [Google Scholar]
- Kutesa, A.; Nkamba, E.M.; Muwazi, L.; Buwembo, W.; Rwenyonyi, C.M. Weight, Height and Eruption Times of Permanent Teeth of Children Aged 4–15 Years in Kampala, Uganda. BMC Oral Health 2013, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Mugonzibwa, E.A.; Kuijpers-Jagtman, A.M.; Laine-Alava, M.T.; Hof, M.A.V. Emergence of Permanent Teeth in Tanzanian Children. Community Dent. Oral Epidemiol. 2002, 30, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, K.K.; Akridge, M.; Scheetz, J.P.; Kinane, D.F. Childhood Obesity and Dental Development. Pediatr. Dent. 2006, 28, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Phillips, S.M.; Tybor, D.J.; Lividini, K.; Hayes, C. The Association Between Childhood Obesity and Tooth Eruption. Obesity 2012, 20, 2070–2074. [Google Scholar] [CrossRef]
- Nibali, L. Development of the Gingival Sulcus at the Time of Tooth Eruption and the Influence of Genetic Factors. Periodontol. 2000 2018, 76, 35–42. [Google Scholar] [CrossRef]
- Suri, L.; Gagari, E.; Vastardis, H. Delayed Tooth Eruption: Pathogenesis, Diagnosis, and Treatment. A Literature Review. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 432–445. [Google Scholar] [CrossRef]
- Makino, E.; Tsujino, K.; Ishii, T.; Shintani, S.; Sueishi, K. Difference in Bilateral Timing of Eruption of Permanent Teeth. Bull. Tokyo Dent. Coll. 2018, 59, 277–284. [Google Scholar] [CrossRef]
- Hassanali, J.; Odhiambo, J.W. Ages of Eruption of the Permanent Teeth in Kenyan African and Asian Children. Ann. Hum. Biol. 1981, 8, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Eskeli, R.; Laine-Alava, M.T.; Hausen, H.; Pahkala, R. Standards for Permanent Tooth Emergence in Finnish Children. Angle Orthod. 1999, 69, 529–533. [Google Scholar]
- Diamanti, J.; Townsend, G. New Standards for Permanent Tooth Emergence in Australian Children. Aust. Dent. J. 2003, 48, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; ul Ahmid, W.; Jabbar, A.; Farooq, A. Ages and Sequence of Eruption of Permanent Teeth in a Sample of Pakistani School Children. Pak. Orthod. J. 2010, 2, 52–59. [Google Scholar]
- Clements, E.M.B.; Davies-Thomas, E.; Pickett, K.G. Time of Eruption of Permanent Teeth in British Children at Independent, Rural, and Urban Schools. Br. Méd. J. 1957, 1, 1511. [Google Scholar] [CrossRef]
- Shaweesh, A.I.; Al-Omiri, M.K.; Alsoleihat, F.D. Variation in Time of Emergence of Permanent Teeth among Urban and Rural Jordanian School Children. Saudi Méd. J. 2011, 32, 1066–1072. [Google Scholar] [PubMed]
- Palanisamy, V.; Rao, A.; Shenoy, R.; Baranya, S. Correlation of Dental Age, Skeletal Age, and Chronological Age among Children Aged 9–14 Years: A Retrospective Study. J. Indian Soc. Pedod. Prev. Dent. 2016, 34, 310. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Saha, S.; Yadav, G.; Tripathi, A.M.; Grover, K. Dental and Skeletal Maturity- A Biological Indicator of Chronologic Age. J. Clin. Diagn. Res. 2014, 8, ZC60-4. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Dahlberg, G.; Maunsbach, A.B. The eruption of the permanent teeth in the normal population of Sweden. Hum. Hered. 1950, 1, 77–91. [Google Scholar] [CrossRef]
- Kochhar, R.; Richardson, A. The Chronology and Sequence of Eruption of Human Permanent Teeth in Northern Ireland. Int. J. Paediatr. Dent. 1998, 8, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Leroy, R.; Bogaerts, K.; Lesaffre, E.; Declerck, D. The Emergence of Permanent Teeth in Flemish Children. Community Dent. Oral Epidemiol. 2003, 31, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Leroy, R.; Cecere, S.; Lesaffre, E.; Declerck, D. Variability in Permanent Tooth Emergence Sequences in Flemish Children. Eur. J. Oral Sci. 2008, 116, 11–17. [Google Scholar] [CrossRef]
- Almonaitiene, R.; Balciuniene, I.; Tutkuviene, J. Standards for Permanent Teeth Emergence Time and Sequence in Lithuanian Children, Residents of Vilnius City. Stomatologija 2012, 14, 93–100. [Google Scholar]
- Cecere, S.; Leroy, R.; Groenen, P.J.F.; Lesaffre, E.; Declerck, D. Estimating Emergence Sequences of Permanent Teeth in Flemish Schoolchildren Using Interval-censored Biplots: A Graphical Display of Tooth Emergence Sequences. Community Dent. Oral Epidemiol. 2012, 40, 49–55. [Google Scholar] [CrossRef]
- Fekonja, A. Evaluation of the Eruption of Permanent Teeth and Their Association with Malocclusion. Clin. Exp. Dent. Res. 2022, 8, 836–842. [Google Scholar] [CrossRef]
- Šindelářová, R.; Žáková, L.; Broukal, Z. Standards for Permanent Tooth Emergence in Czech Children. BMC Oral Health 2017, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, P.; Reddy, J.S.; Suhasini, K.; Chandrika, I.H.; Praveen, D. Time and Eruption Sequence of Permanent Teeth in Hyderabad Children: A Descriptive Cross-Sectional Study. Int. J. Clin. Pediatr. Dent. 2018, 11, 330–337. [Google Scholar] [CrossRef]
- Vithanaarachchi, N.; Nawarathna, L.; Wijeyeweera, L. Standards for Permanent Tooth Emergence in Sri Lankan Children. Ceylon Méd. J. 2021, 66, 44. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Nagar, P.; Singh, P.; Bharti, M. Changes in the Sequence of Eruption of Permanent Teeth; Correlation between Chronological and Dental Age and Effects of Body Mass Index of 5–15-Year-Old Schoolchildren. Int. J. Clin. Pediatr. Dent. 2020, 13, 368–380. [Google Scholar] [CrossRef]
- Ghose, L.J.; Baghdady, V.S. Eruption Time of Permanent Teeth in Iraqi School Children. Arch. Oral Biol. 1981, 26, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Nizam, A.; Naing, L.; Mokhtar, N. Age and Sequence of Eruption of Permanent Teeth in Kelantan, North-Eastern Malaysia. Clin. Oral Investig. 2003, 7, 222–225. [Google Scholar] [CrossRef]
- Nassif, N.; Sfeir, E. Age and Sequence of Permanent Teeth Eruption in Lebanese Children. Sci. World J. 2020, 2020, 9238679. [Google Scholar] [CrossRef]
- Höuffding, J.; Maeda, M.; Yamaguchi, K.; Tsuji, H.; Kuwabara, S.; Nohara, Y.; Yoshida, S. Emergence of Permanent Teeth and Onset of Dental Stages in Japanese Children. Community Dent. Oral Epidemiol. 1984, 12, 55–58. [Google Scholar] [CrossRef]
- Esan, T.A.; Mothupi, K.A.; Schepartz, L.A. Permanent Tooth Emergence: Timing and Sequence in a Sample of Black Southern African Children. Am. J. Phys. Anthr. 2018, 167, 827–839. [Google Scholar] [CrossRef]
- Houpt, M.I.; Adu-Aryee, S.; Grainger, R.M. Eruption Times of Permanent Teeth in the Brong Ahafo Region of Ghana. Am. J. Orthod. 1967, 53, 95–99. [Google Scholar] [CrossRef]
- Oziegbe, E.O.; Esan, T.A.; Oyedele, T.A. Brief Communication: Emergence Chronology of Permanent Teeth in Nigerian Children. Am. J. Phys. Anthr. 2014, 153, 506–511. [Google Scholar] [CrossRef]
- Savara, B.S.; Steen, J.C. Timing and Sequence of Eruption of Permanent Teeth in a Longitudinal Sample of Children from Oregon. J. Am. Dent. Assoc. 1978, 97, 209–214. [Google Scholar] [CrossRef]
- Esan, T.A.; Schepartz, L.A. The WITS Atlas: A Black Southern African Dental Atlas for Permanent Tooth Formation and Emergence. Am. J. Phys. Anthr. 2018, 166, 208–218. [Google Scholar] [CrossRef]
- Esan, T.A.; Schepartz, L.A. The Timing of Permanent Tooth Development in a Black Southern African Population Using the Demirjian Method. Int. J. Leg. Med. 2019, 133, 257–268. [Google Scholar] [CrossRef]
- Šindelářová, R.; Broukal, Z. Polymorphism in Sequence of Permanent Tooth Emergence in Czech Children. Cent. Eur. J. Public Health 2019, 27, 165–169. [Google Scholar] [CrossRef] [PubMed]
- García-Gil, M.; Alarcón, J.A.; Cacho, A.; Yañez-Vico, R.; Palma-Fernández, J.C.; Martin, C. Association between Eruption Sequence of Posterior Teeth, Dental Crowding, Arch Dimensions, Incisor Inclination, and Skeletal Growth Pattern. Children 2023, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Vandana, S.; Kandaswamy, S.; Muthu, M.S.; Aswath Narayanan, M.B. Sequence and Chronology of Permanent Teeth Eruption in 5–18-Year-Old School Children of Chennai and the Influence of Sex, BMI, and Socio-Economic Status. Am. J. Hum. Biol. 2025, 37, e24211. [Google Scholar] [CrossRef] [PubMed]
- Diéguez-Pérez, M.; Paz-Cortés, M.M.; Muñoz-Cano, L. Evaluation of the Relationship between the Weight and Height Percentiles and the Sequence and Chronology of Eruption in Permanent Dentition. Healthcare 2022, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Paz-Cortés, M.M.; Muñoz-Cano, L.; Diéguez-Pérez, M. Evaluation of the Relationship between the BMI and the Sequence and Chronology of Eruption in Permanent Dentition in Spanish Population. Healthcare 2022, 10, 1046. [Google Scholar] [CrossRef]

| Maxilla | Mandible | ||||||
|---|---|---|---|---|---|---|---|
| Tooth | Median age | Average age | Standard deviation | Tooth | Median age | Average age | Standard deviation |
| 11 | 6.89 | 6.93 | 0.81 | 31 | 6.16 | 6.20 | 0.72 |
| 12 | 7.88 | 7.94 | 1.00 | 32 | 7.18 | 7.23 | 0.85 |
| 13 | 11.02 | 11.08 | 1.18 | 33 | 10.45 | 10.52 | 1.16 |
| 14 | 10.49 | 10.57 | 1.30 | 34 | 10.54 | 10.61 | 1.19 |
| 15 | 11.20 | 11.26 | 1.16 | 35 | 11.30 | 11.37 | 1.27 |
| 16 | 6.33 | 6.37 | 0.77 | 36 | 6.21 | 6.25 | 0.74 |
| 17 | 12.10 | 12.16 | 1.18 | 37 | 11.91 | 12.00 | 1.40 |
| 21 | 6.90 | 6.95 | 0.80 | 41 | 6.15 | 6.19 | 0.69 |
| 22 | 7.87 | 7.92 | 0.95 | 42 | 7.17 | 7.22 | 0.84 |
| 23 | 11.03 | 11.09 | 1.21 | 43 | 10.46 | 10.52 | 1.15 |
| 24 | 10.52 | 10.59 | 1.29 | 44 | 10.51 | 10.58 | 1.20 |
| 25 | 11.28 | 11.35 | 1.28 | 45 | 11.27 | 11.33 | 1.18 |
| 26 | 6.31 | 6.35 | 0.76 | 46 | 6.21 | 6.25 | 0.70 |
| 27 | 12.20 | 12.29 | 1.43 | 47 | 11.84 | 11.89 | 1.11 |
| Maxillary eruption sequence: M1-CI-LI-PM1-C-PM2-M2 | Mandibular eruption sequence: CI-M1-LI-C-PM1-PM2-M2 | ||||||
| Maxilla | Mandible | ||||||
|---|---|---|---|---|---|---|---|
| Tooth | Median age | Average age | Standard deviation | Tooth | Median age | Average age | Standard deviation |
| 11 | 6.84 | 6.89 | 0.77 | 31 | 6.03 | 6.08 | 0.78 |
| 12 | 7.68 | 7.74 | 0.92 | 32 | 7.05 | 7.11 | 0.98 |
| 13 | 10.90 | 10.98 | 1.29 | 33 | 9.89 | 9.95 | 1.11 |
| 14 | 10.41 | 10.49 | 1.30 | 34 | 10.37 | 10.45 | 1.25 |
| 15 | 11.24 | 11.33 | 1.42 | 35 | 11.23 | 11.32 | 1.42 |
| 16 | 6.28 | 6.32 | 0.74 | 36 | 6.17 | 6.21 | 0.76 |
| 17 | 12.45 | 12.56 | 1.63 | 37 | 12.18 | 12.32 | 1.82 |
| 21 | 6.81 | 6.86 | 0.79 | 41 | 6.07 | 6.12 | 0.78 |
| 22 | 7.66 | 7.71 | 0.91 | 42 | 7.06 | 7.13 | 0.95 |
| 23 | 10.78 | 10.86 | 1.26 | 43 | 9.93 | 9.99 | 1.11 |
| 24 | 10.36 | 10.44 | 1.27 | 44 | 10.31 | 10.39 | 1.23 |
| 25 | 11.31 | 11.40 | 1.50 | 45 | 11.23 | 11.33 | 1.48 |
| 26 | 6.29 | 6.34 | 0.77 | 46 | 6.17 | 6.22 | 0.75 |
| 27 | 12.55 | 12.69 | 1.88 | 47 | 12.16 | 12.27 | 1.65 |
| Maxillary eruption sequence: M1-CI-LI-PM1-C-PM2-M2 | Mandibular eruption sequence: CI-M1-LI-C-PM1-PM2-M2 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheli, O.; Athanasiou, M.; Kristof, V.; Antonarakis, G.S. Chronology and Sequence of Permanent Tooth Eruption in a Multi-Ethnic Urban Population. Dent. J. 2025, 13, 356. https://doi.org/10.3390/dj13080356
Micheli O, Athanasiou M, Kristof V, Antonarakis GS. Chronology and Sequence of Permanent Tooth Eruption in a Multi-Ethnic Urban Population. Dentistry Journal. 2025; 13(8):356. https://doi.org/10.3390/dj13080356
Chicago/Turabian StyleMicheli, Olivia, Maria Athanasiou, Victor Kristof, and Gregory S. Antonarakis. 2025. "Chronology and Sequence of Permanent Tooth Eruption in a Multi-Ethnic Urban Population" Dentistry Journal 13, no. 8: 356. https://doi.org/10.3390/dj13080356
APA StyleMicheli, O., Athanasiou, M., Kristof, V., & Antonarakis, G. S. (2025). Chronology and Sequence of Permanent Tooth Eruption in a Multi-Ethnic Urban Population. Dentistry Journal, 13(8), 356. https://doi.org/10.3390/dj13080356






