Detection of Protein Carbonylation in Gingival Biopsies from Periodontitis Patients with or Without Diabetes Mellitus—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Clinical Procedure
- ➢
- Pocket Probing Depth (PPD), 6 sites per tooth, utilizing a UNC-15 probe
- ➢
- Clinical Attachment Loss (CAL), 6 sites per tooth, from CEJ to free gingival margin
- ➢
- Gingival Bleeding Index (GI) and Plaque Index (PI), by Silness and Loe [35]
2.5. Gingival Tissue Biopsies
2.6. Immunohistochemistry {IHC} Procedure
2.7. Histopathologic Evaluation
3. Results
Histopathologic Evaluation
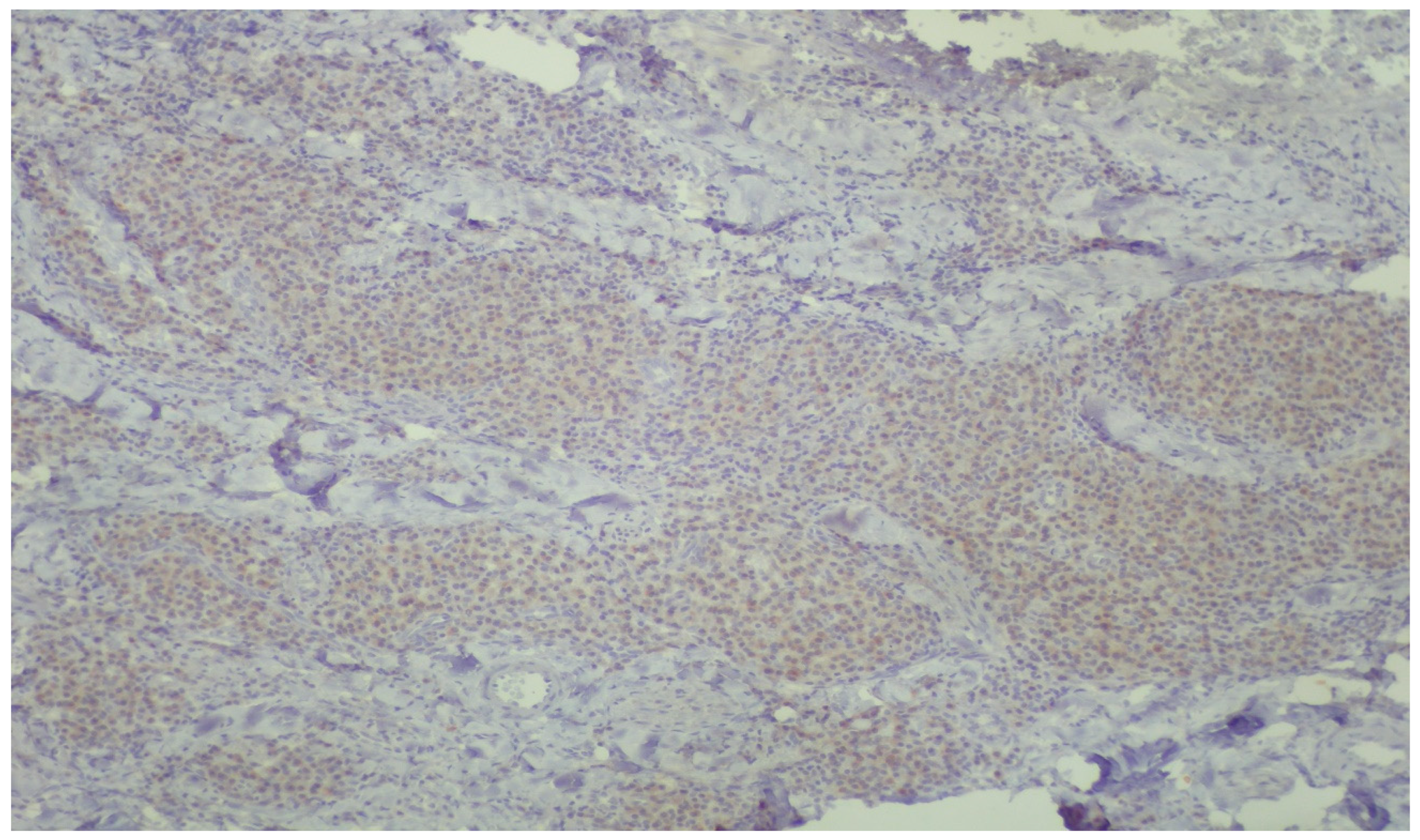

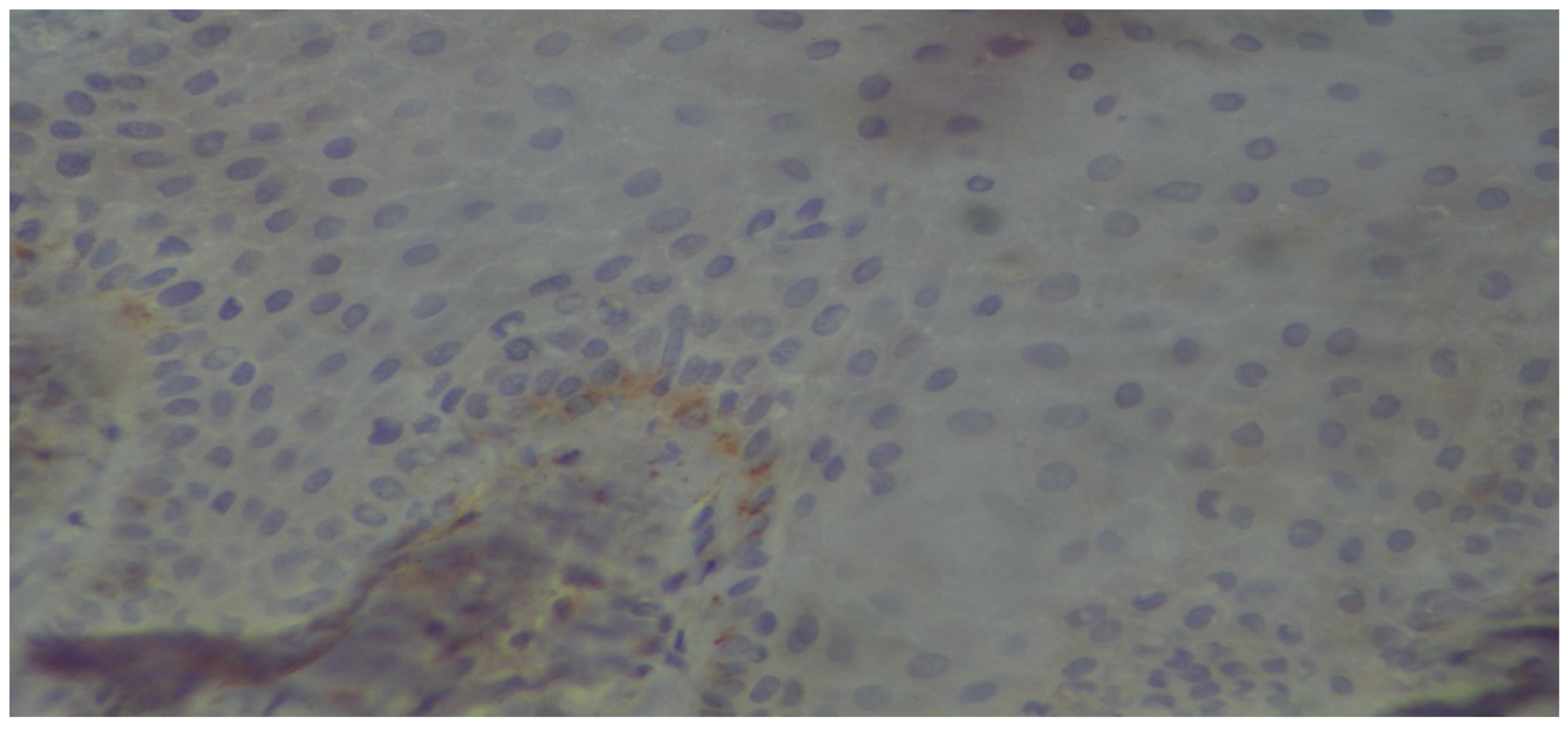

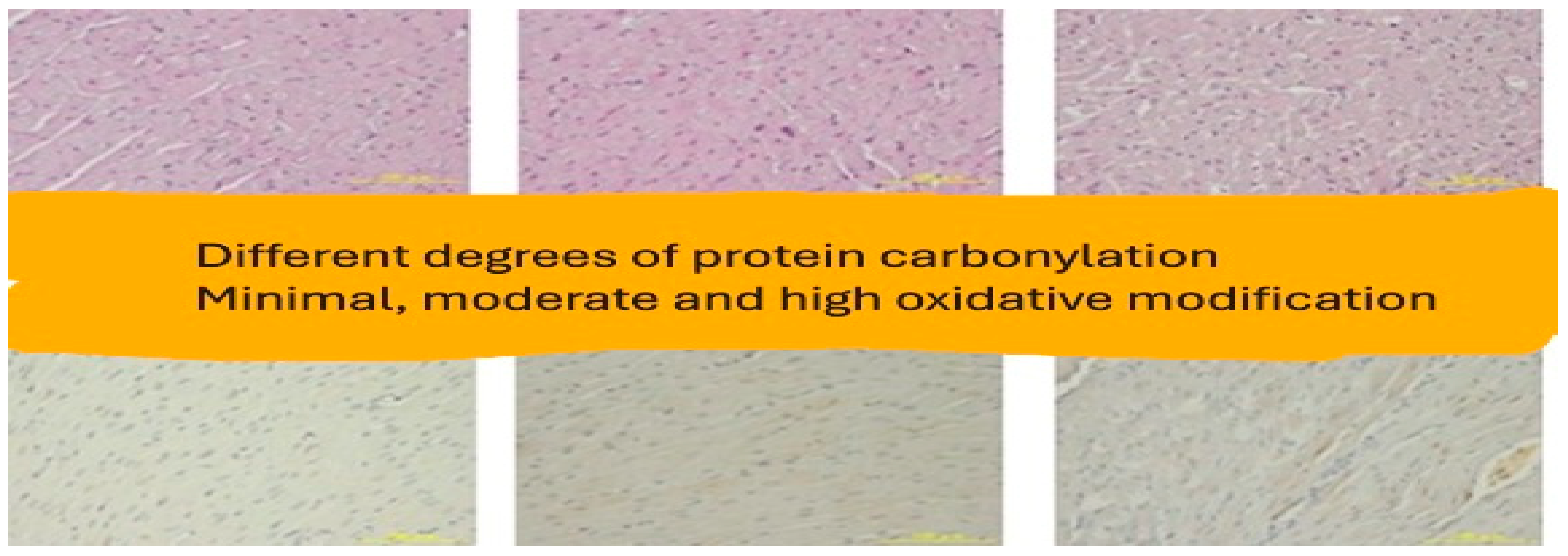
4. Discussion
5. Conclusions
- There is histopathological evidence for the first time of the carbonylation of proteins as a measure of oxidative damage and oxidative stress in both periodontal and diabetes diseases.
- Periodontitis patients with DM2 exhibited higher amounts of carbonylated proteins than patients without it, displaying an additive, though minor, effect in the prevalence and possibly pathogenesis of these two diseases.
- The introduced DNP antibody immunohistochemical method stands out to be a worthy exploratory alternative to assess protein carbonylation in gingival tissue biopsies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, E.M.; Matthews, J.B.; Halloran, D.J.O.; Griffiths, H.R.; Chapple, I.L. Oxidative and inflammatory status in Type 2 diabetes patients with periodontitis: Periodontitis and Diabetes Inflammatory Status. J. Clin. Periodontol. 2011, 38, 894–901. [Google Scholar] [CrossRef]
- Cheng, Z.; Meade, J.; Mankia, K.; Emery, P.; Devine, D.A. Periodontal disease and periodontal bacteria as triggers for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 19–30. [Google Scholar] [CrossRef]
- Buczko, P.; Zalewska, A.; Szarmach, I. Saliva and oxidative stress in oral cavity and in some systemic disorders. J. Physiol. Pharmacol. 2015, 66, 3–9. [Google Scholar] [PubMed]
- Carcuac, O.; Berglundh, T. Composition of Human Peri-implantitis and Periodontitis Lesions. J. Dent. Res. 2014, 93, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Milward, M.R.; Dietrich, T. The Prevalence of Inflammatory Periodontitis Is Negatively Associated with Serum Antioxidant Concentrations. J. Nutr. 2007, 137, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Demmer, R.T.; Papapanou, P.N. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontology 2000 2010, 53, 28–44. [Google Scholar] [CrossRef]
- Linden, G.J.; Lyons, A.; Scannapieco, F.A. Periodontal systemic associations: Review of the evidence. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S8–S19. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Genco, R.; on behalf of Working Group 2 of the Joint EFP/AAP Workshop*. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S106–S112. [Google Scholar] [CrossRef]
- Sánchez-León, M.E.; Loaeza-Reyes, K.J.; Matias-Cervantes, C.A.; Mayoral-Andrade, G.; Pérez-Campos, E.L.; Pérez-Campos-Mayoral, L.; Hernández-Huerta, M.T.; Zenteno, E.; Pérez-Cervera, Y.; Pina-Canseco, S. LOX-1 in Cardiovascular Disease: A Comprehensive Molecular and Clinical Review. Int. J. Mol. Sci. 2024, 25, 5276. [Google Scholar] [CrossRef]
- Acharya, A.B.; Thakur, S.; Muddapur, M.V. Effect of scaling and root planing on serum interleukin-10 levels and glycemic control in chronic periodontitis and type 2 diabetes mellitus. J. Indian Soc. Periodontol. 2015, 19, 188–193. [Google Scholar] [CrossRef]
- Rodriguez, I.R. Rapid analysis of oxysterols by HPLC and UV spectroscopy. BioTechniques 2004, 36, 952–958. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidation of low-density lipoproteins: Questions of initiation, propagation, and the effect of antioxidants. Am. J. Clin. Nutr. 1995, 61, 670S–677S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2020, 84, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Wiebkin, O.W.; Thonard, J.C. The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J. Periodontal Res. 1984, 19, 390–400. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited Review: Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods for Their Quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Ahmed, S.; Adamidis, A.; Jan, L.C.; Gibbons, N.; Mattana, J. Dexamethasone attenuates oxidation of extracellular matrix proteins by human monocytes. Exp. Mol. Pathol. 2003, 75, 137–143. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef]
- Yao, H.; Rahman, I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol. Appl. Pharmacol. 2011, 254, 72–85. [Google Scholar] [CrossRef]
- Curtis, J.M.; Hahn, W.S.; Long, E.K.; Burrill, J.S.; Arriaga, E.A.; Bernlohr, D.A. Protein carbonylation and metabolic control systems. Trends Endocrinol. Metab. 2012, 23, 399–406. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Roth, J. Mechanisms of Disease: A ‘DAMP’ view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 2007, 3, 382–390. [Google Scholar] [CrossRef]
- Miller, Y.I.; Choi, S.-H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-Specific Epitopes Are Danger-Associated Molecular Patterns Recognized by Pattern Recognition Receptors of Innate Immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Castronovo, V. Laminin receptors and laminin-binding proteins during tumor invasion and metastasis. Invasion Metastasis 1993, 13, 1–30. [Google Scholar] [PubMed]
- De Arcangelis, A.; Neuville, P.; Boukamel, R.; Lefebvre, O.; Kedinger, M.; Simon-Assmann, P. Inhibition of laminin alpha 1-chain expression leads to alteration of basement membrane assembly and cell differentiation. J. Cell Biol. 1996, 133, 417–430. [Google Scholar] [CrossRef]
- Kostidou, E.; Koliakos, G.; Paletas, K.; Kaloyianni, M. Monocyte Attachment and Migration through Collagen IV in Diabetes Mellitus. Mol. Cells 2008, 25, 452–456. [Google Scholar] [CrossRef]
- Kostidou, E.; Koliakos, G.; Alamdari, D.H.; Paletas, K.; Tsapas, A.; Kaloyianni, M. Enhanced laminin carbonylation by monocytes in diabetes mellitus. Clin. Biochem. 2007, 40, 671–679. [Google Scholar] [CrossRef]
- Kostidou, E.; Topouridou, K.; Daniilidis, A.; Kaloyianni, M.; Koliakos, G. Oxidized laminin-1 induces increased monocyte attachment and expression of ICAM-1 in endothelial cells. Int. J. Exp. Pathol. 2009, 90, 630–637. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; Anderson, K.R.; Topper, J.N. The Critical Role of Mechanical Forces in Blood Vessel Development, Physiology and Pathology. J. Vasc. Surg. 1999, 29, 1104–1151. [Google Scholar] [CrossRef]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Shacter, E. Quantification and significance of protein oxidation in biological samples*. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wehr, N.B. Protein carbonylation: Avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox Rep. 2009, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H. Oxidized low-density lipoprotein as a biomarker of in vivo oxidative stress: From atherosclerosis to periodontitis. J. Clin. Biochem. Nutr. 2012, 51, 1–8. [Google Scholar] [CrossRef]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Albandar, J.M. Measurement and Distribution of Periodontal Diseases. In Burt and Eklund’s Dentistry, Dental Practice, and the Community, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–188. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S.; et al. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef]
- Hawes, D.; Shi, S.-R.; Dabbs, D.J.; Taylor, C.R.; Cote, R.J. Chapter 5: Immunohistochemistry. In Modern Surgical Pathology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 48–70. [Google Scholar]
- Fauzi, M.F.A.; Ahmad, W.S.H.M.W.; Jamaluddin, M.F.; Lee, J.T.H.; Khor, S.Y.; Looi, L.M.; Abas, F.S.; Aldahoul, N. Allred Scoring of ER-IHC Stained Whole-Slide Images for Hormone Receptor Status in Breast Carcinoma. Diagnostics 2022, 12, 3093. [Google Scholar] [CrossRef]
- Dentino, A.; Lee, S.; Mailhot, J.; Hefti, A.F. Principles of periodontology. Periodontology 2000 2013, 61, 16–53. [Google Scholar] [CrossRef]
- Brugués, A.; Bromuri, S.; Barry, M.; del Toro, Ó.J.; Mazurkiewicz, M.R.; Kardas, P.; Pegueroles, J.; Schumacher, M. Processing Diabetes Mellitus Composite Events in MAGPIE. J. Med. Syst. 2016, 40, 44. [Google Scholar] [CrossRef]
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Pinson, M.; Hoffman, W.H.; Garnick, J.J.; Litaker, M.S. Periodontal disease and type diabetes mellitus in children and adolescents. J. Clin. Periodontol. 1995, 22, 118–123. [Google Scholar] [CrossRef]
- Page, R.C.; Offenbacher, S.; Schroeder, H.E.; Seymour, G.J.; Kornman, K.S. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontology 2000 1997, 14, 216–248. [Google Scholar] [CrossRef]
- Soskolne, W.A.; Klinger, A. The Relationship Between Periodontal Diseases and Diabetes: An Overview. Ann. Periodontol. 2001, 6, 91–98. [Google Scholar] [CrossRef]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The Role of Oxidative Stress in the Pathogenesis of Type 2 Diabetes Mellitus Micro- and Macrovascular Complications: Avenues for a Mechanistic-Based Therapeutic Approach. Curr. Diabetes Rev. 2011, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kawaharada, R. Advanced Glycation End Products and Oxidative Stress in a Hyperglycaemic Environment. In Fundamentals of Glycosylation; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Abbass, M.M.; Korany, N.S.; Salama, A.H.; Dmytryk, J.J.; Safiejko-Mroczka, B. The relationship between receptor for advanced glycation end products expression and the severity of periodontal disease in the gingiva of diabetic and non diabetic periodontitis patients. Arch. Oral Biol. 2012, 57, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Bhattacharyya, I.; Farkhondeh-Kish, F.; Perez, F.M.; Caudle, R.M.; Heft, M.W. Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: A study utilizing immunohistochemistry and RT-PCR. J. Clin. Periodontol. 2005, 32, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Grimm, W.-D.; Shchetinin, E.; Bobryshev, D.; Sirak, S. Quantifying analysis of advanced glycosylation end products (ages) expression in periodontitis patients with diabetes type II. Med. News North Cauc. 2015, 10, 178–183. [Google Scholar] [CrossRef]
- Rajeev, K.; Karthika, R.; Mythili, R.; Krishnan, V.; Nirmal, M. Role of receptors of advanced glycation end-products (RAGE) in type 2 diabetic and non-diabetic individuals with chronic periodontal disease: An immunohistochemical study. J. Investig. Clin. Dent. 2011, 2, 287–292. [Google Scholar] [CrossRef]
- Chopra, A.; Jayasinghe, T.N.; Eberhard, J. Are Inflamed Periodontal Tissues Endogenous Source of Advanced Glycation End-Products (AGEs) in Individuals with and without Diabetes Mellitus? A Systematic Review. Biomolecules 2022, 12, 642. [Google Scholar] [CrossRef]
- Doria, E.; Buonocore, D.; Focarelli, A.; Marzatico, F. Relationship between Human Aging Muscle and Oxidative System Pathway. Oxidative Med. Cell. Longev. 2012, 2012, 830257. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein Oxidation and Aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Chida, A.S.; Rahman, I. Redox modifications of protein–thiols: Emerging roles in cell signaling. Biochem. Pharmacol. 2006, 71, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Stadtman, E.R. Oxidative modification of proteins during aging. Exp. Gerontol. 2001, 36, 1495–1502. [Google Scholar] [CrossRef]
- Baraibar, M.A.; Liu, L.; Ahmed, E.; Friguet, B. Protein Oxidative Damage at the Crossroads of Cellular Senescence, Aging, and Age-Related Diseases. Oxidative Med. Cell. Longev. 2012, 2012, 919832. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Sculley, D.V.; Langley-Evans, S.C. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin. Sci. 2003, 105, 167–172. [Google Scholar] [CrossRef]
- Baltacıoğlu, E.; Akalın, F.A.; Alver, A.; Değer, O.; Karabulut, E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Arch. Oral Biol. 2008, 53, 716–722. [Google Scholar] [CrossRef]
- Pradeep, A.; Ramchandraprasad, M.; Bajaj, P.; Rao, N.; Agarwal, E.; Ar, P.; Ns, R. Protein carbonyl: An oxidative stress marker in gingival crevicular fluid in healthy, gingivitis, and chronic periodontitis subjects. Contemp. Clin. Dent. 2013, 4, 27–31. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ngo, L.Q.; Promsudthi, A.; Surarit, R. Salivary oxidative stress biomarkers in chronic periodontitis and acute coronary syndrome. Clin. Oral Investig. 2017, 21, 2345–2353. [Google Scholar] [CrossRef]
- Su, H.; Gornitsky, M.; Velly, A.M.; Yu, H.; Benarroch, M.; Schipper, H.M. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free. Radic. Biol. Med. 2009, 46, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Sayre, L.M.; Anderson, V.E.; Harris, P.L.; Beal, M.F.; Kowall, N.; Perry, G. Cytochemical Demonstration of Oxidative Damage in Alzheimer Disease by Immunochemical Enhancement of the Carbonyl Reaction with 2,4-Dinitrophenylhydrazine. J. Histochem. Cytochem. 1998, 46, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Zelasko, D.A.; Harris, P.L.R.; Perry, G.; Salomon, R.G.; Smith, M.A. 4-Hydroxynonenal-Derived Advanced Lipid Peroxidation End Products Are Increased in Alzheimer’s Disease. J. Neurochem. 1997, 68, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Richey Harris, P.L.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread Peroxynitrite-Mediated Damage in Alzheimer’s Disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [CrossRef]
- Liu, L.; Marcocci, L.; Wong, C.M.; Park, A.-M.; Suzuki, Y.J. Serotonin-mediated protein carbonylation in the right heart. Free Radic. Biol. Med. 2008, 45, 847–854. [Google Scholar] [CrossRef]
- Wong, C.M.; Cheema, A.K.; Zhang, L.; Suzuki, Y.J. Protein Carbonylation as a Novel Mechanism in Redox Signaling. Circ. Res. 2008, 102, 310–318. [Google Scholar] [CrossRef]
- Wong, C.M.; Marcocci, L.; Liu, L.; Suzuki, Y.J. Cell Signaling by Protein Carbonylation and Decarbonylation. Antioxidants Redox Signal. 2010, 12, 393–404. [Google Scholar] [CrossRef]
- Requena, J.R.; Chao, C.-C.; Levine, R.L.; Stadtman, E.R. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 69–74. [Google Scholar] [CrossRef]




| Group | Gingival Samples | Diabetes Status | Periodontal Diagnosis |
|---|---|---|---|
| Periodontal disease and diabetes (Test Group 1) | 13 | Type II (11), Type I (2) | Periodontitis (Stages I–IV, Grades A–C) |
| Periodontal disease only (Test Group 2) | 10 | – | Periodontitis (Stages I–IV, Grades A–C) |
| *Experimental control | 2 | – | Non-diabetic and non-periodontitis patients |
| (a) | ||||||
| Patient ID | Age (yrs) | Sample IDs | HbA1c (%) | Diabetes Type | Periodontal Diagnosis (2017) | BoP (%) |
| Pat 1 | 77 | 1, 2 | 8.4 | II | Stage IV, Grade C | 45 |
| Pat 2 | 59 | 4, 5 | 6.5 | I | Stage III, Grade C | 30 |
| Pat 3 | 70 | 6, 7, 8 | 6.0 | II | Stage IV, Grade C | 40 |
| Pat 4 | 59 | 11 | 6.1 | II | Stage IV, Grade C | 50 |
| Pat 5 | 65 | 3 | 6.3 | II | Stage III, Grade C | 40 |
| Pat 6 | 55 | 18, 19, 22 | 9.1 | II | Stage IV, Grade C | 60 |
| Pat 7 | 64 | 25 | 6.6 | II | Stage III, Grade C | 35 |
| (b) | ||||||
| Patient ID | Age (yrs) | Sample IDs | Periodontal Diagnosis (2017) | BoP (%) | ||
| Pat 8 | 65 | 9 | Gingivitis | 20 | ||
| Pat 9 | 60 | 10 | Stage II, Grade B | 20 | ||
| Pat 10 | 48 | 12, 21 | Stage III, Grade B | 10 | ||
| Pat 11 | 58 | 13 | Stage III, Grade B | 35 | ||
| Pat 12 | 55 | 16 | Stage I, Grade B | 35 | ||
| Pat 13 | 43 | 17 | Stage IV, Grade C | 50 | ||
| Pat 14 | 57 | 20 | Stage II, Grade A | 30 | ||
| Pat 15 | 39 | 24 | Stage I, Grade C | 35 | ||
| Pat 16 | 70 | 23 | Stage IV, Grade C | 45 | ||
| Statistical Results | ||||
|---|---|---|---|---|
| Parameter | Patient Group | Sample Count | Mean ± SD | p-Value |
| % of Positive Inflammatory Cells | Periodontal Disease and Diabetes (Test) | 13 | 49.2 ± 35.97 | 0.036 |
| Periodontal Disease Only (Control) | 9 | 19.7 ± 34.40 | ||
| Allred Score (% Positive Cells × Intensity) | Periodontal Disease and Diabetes (Test) | 13 | 59.6 ± 49.52 | 0.036 |
| Periodontal Disease Only (Control) | 9 | 20.8 ± 34.21 | ||
| Staining Intensity | Periodontal Disease and Diabetes (Test Group) | Periodontal Disease Only (Control Group) | p-Value |
|---|---|---|---|
| Negative (0) | 2 (15.4%) | 5 (55.6%) | |
| Mild (1) | 9 (69.2%) | 3 (33.3%) | |
| Moderate (2) | 2 (15.4%) | 1 (11.1%) | |
| Chi-square test | 0.174 |
| Staining Pattern | Periodontal Disease and Diabetes (Test Group) | Periodontal Disease Only (Control Group) | p-Value |
|---|---|---|---|
| Negative | 9 (69.2%) | 7 (77.8%) | |
| Focal Mild Positivity | 2 (15.4%) | 1 (11.1%) | |
| Diffuse Mild | 1 (7.7%) | 1 (11.1%) | |
| Diffuse Moderate | 1 (7.7%) | 0 (0.0%) | |
| Non-applicable/Missing | 0 (0.0%) | 0 (0.0%) | |
| Chi-square test | 0.544 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efthymiou, A.; Anastasiadou, P.; Anagnostou, E.; Koliakos, G.; Kalfas, S.; Vouros, I. Detection of Protein Carbonylation in Gingival Biopsies from Periodontitis Patients with or Without Diabetes Mellitus—A Pilot Study. Dent. J. 2025, 13, 328. https://doi.org/10.3390/dj13070328
Efthymiou A, Anastasiadou P, Anagnostou E, Koliakos G, Kalfas S, Vouros I. Detection of Protein Carbonylation in Gingival Biopsies from Periodontitis Patients with or Without Diabetes Mellitus—A Pilot Study. Dentistry Journal. 2025; 13(7):328. https://doi.org/10.3390/dj13070328
Chicago/Turabian StyleEfthymiou, Alexandra, Pinelopi Anastasiadou, Eleftherios Anagnostou, George Koliakos, Sotirios Kalfas, and Ioannis Vouros. 2025. "Detection of Protein Carbonylation in Gingival Biopsies from Periodontitis Patients with or Without Diabetes Mellitus—A Pilot Study" Dentistry Journal 13, no. 7: 328. https://doi.org/10.3390/dj13070328
APA StyleEfthymiou, A., Anastasiadou, P., Anagnostou, E., Koliakos, G., Kalfas, S., & Vouros, I. (2025). Detection of Protein Carbonylation in Gingival Biopsies from Periodontitis Patients with or Without Diabetes Mellitus—A Pilot Study. Dentistry Journal, 13(7), 328. https://doi.org/10.3390/dj13070328









