Short-Term Effects of Visceral Manual Therapy on Autonomic Nervous System Modulation in Individuals with Clinically Based Bruxism: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
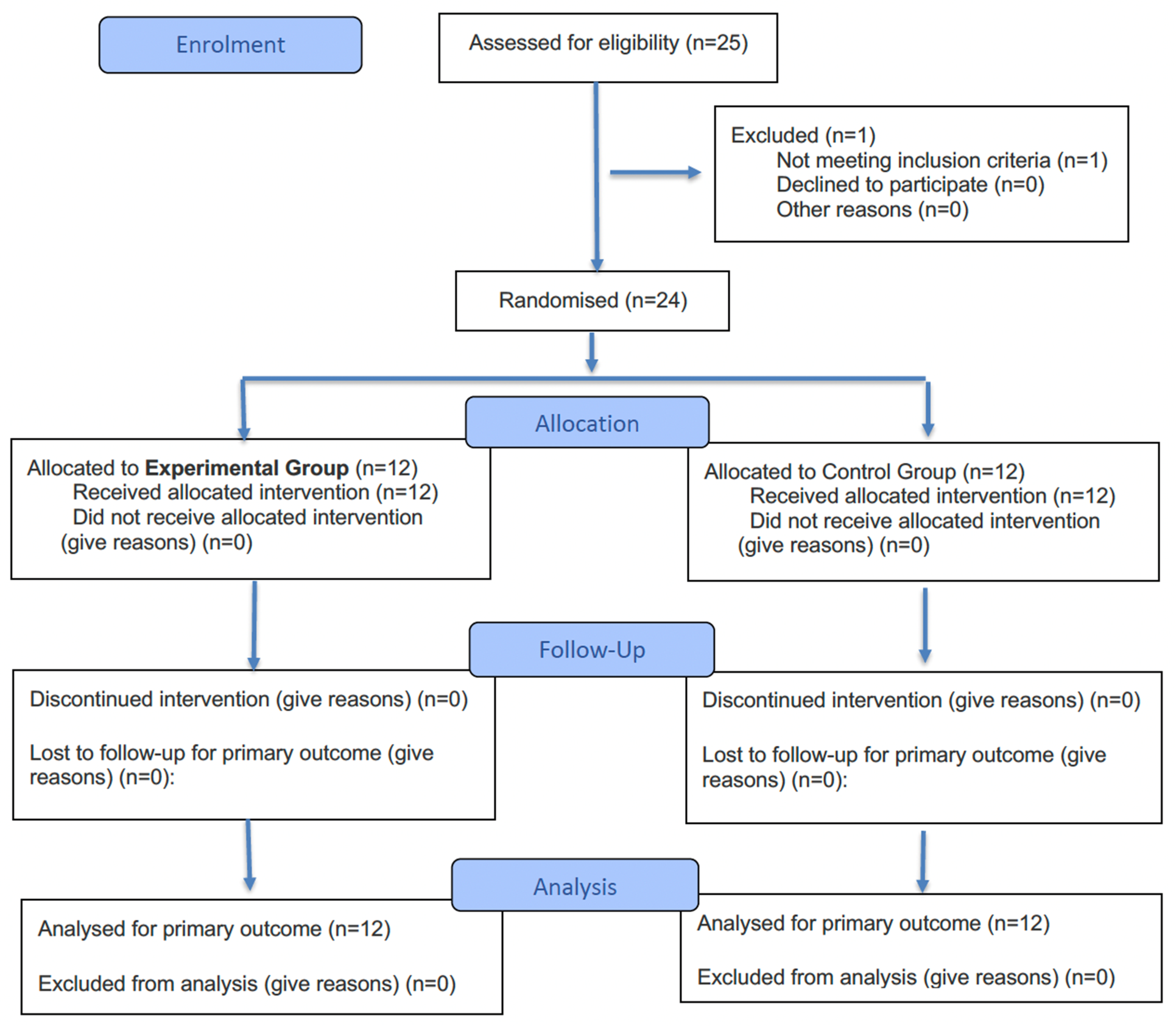
2.3. Randomization and Blinding
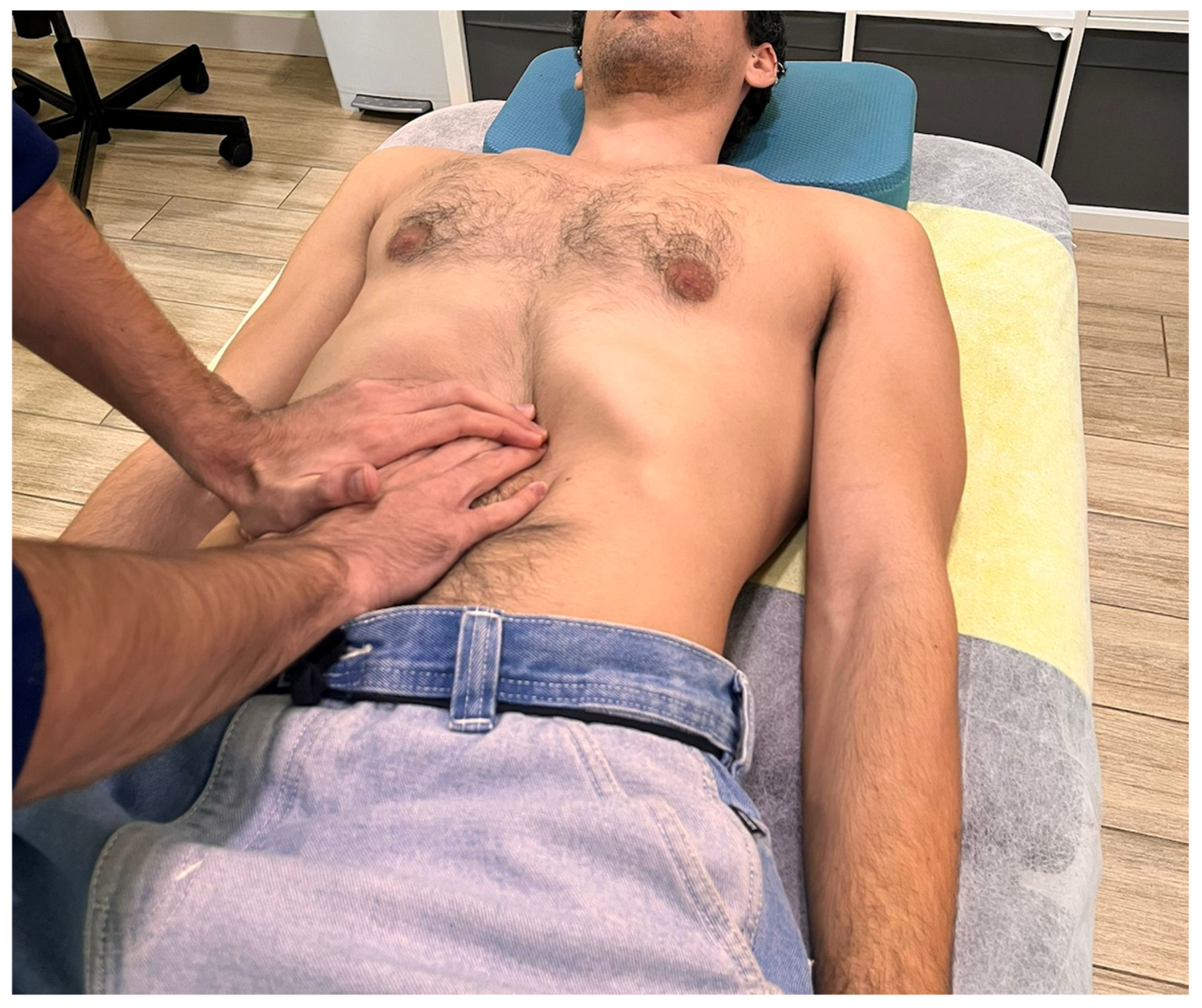
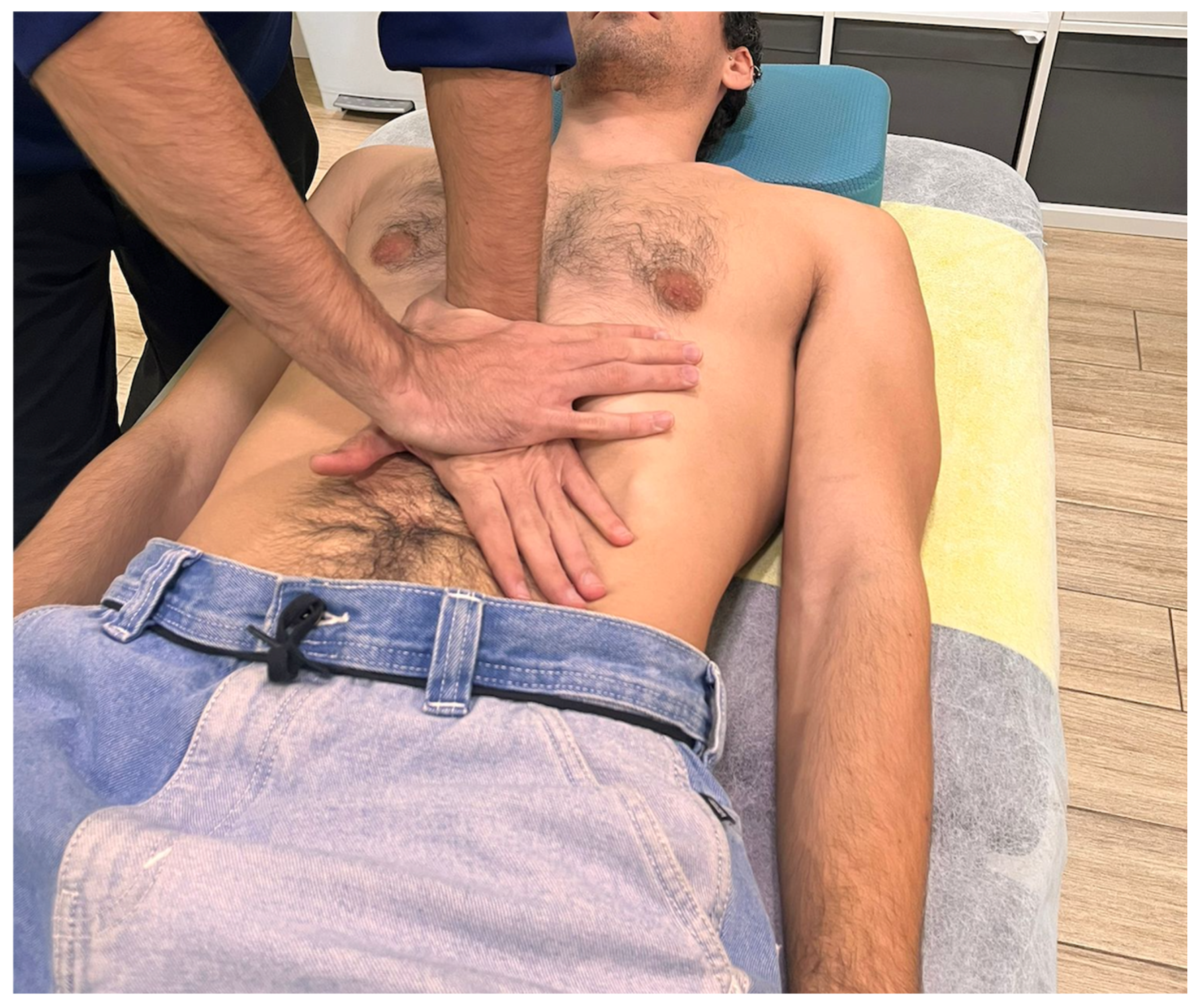
2.4. Interventions
2.5. Outcome Measures
2.5.1. Primary Measure—HRV
2.5.2. Secondary Measures
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. Primary Outcome—HRV
3.2. Secondary Outcomes
4. Discussion
4.1. Study Limitations
4.2. Possible Applications of Research and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANS | Autonomic nervous system |
| VMT | Visceral manual therapy |
| HRV | Heart rate variability |
| TMD | Temporomandibular disorder |
| HRV-RMSSD | Root mean square of the successive differences |
| HRV-SDNN | Standard deviation of the normal-to-normal interbeat intervals |
| PPT | Pressure pain threshold |
| MT | Manual therapy |
| ANOVA | Analysis of variance |
References
- Verhoeff, M.C.; Lobbezoo, F.; Ahlberg, J.; Bender, S.; Bracci, A.; Colonna, A.; Dal Fabbro, C.; Durham, J.; Glaros, A.G.; Häggman-Henrikson, B.; et al. Updating the Bruxism Definitions: Report of an International Consensus Meeting. J. Oral Rehabil. 2025, 0, 1–8. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International Consensus on the Assessment of Bruxism: Report of a Work in Progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- Ionfrida, J.A.; Stiller, H.L.; Kämmerer, P.W.; Walter, C. Dental Implant Failure Risk in Patients with Bruxism—A Systematic Review and Meta-Analysis of the Literature. Dent. J. 2025, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Pająk, A.; Wójcicki, M. Global Prevalence of Sleep Bruxism and Awake Bruxism in Pediatric and Adult Populations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4259. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Bogunia-Kubik, K.; Mazur, G.; Danel, D.; Smardz, J.; Wojakowska, A.; Poreba, R.; Dratwa, M.; Chaszczewska-Markowska, M.; Winocur, E.; et al. Genetic Basis of Sleep Bruxism and Sleep Apnea-Response to a Medical Puzzle. Sci. Rep. 2020, 10, 7497. [Google Scholar] [CrossRef] [PubMed]
- La Touche, R.; Fernández-De-Las-Peñas, C.; Fernández-Carnero, J.; Escalante, K.; Angulo-Díaz-Parreño, S.; Paris-Alemany, A.; Cleland, J.A. The Effects of Manual Therapy and Exercise Directed at the Cervical Spine on Pain and Pressure Pain Sensitivity in Patients with Myofascial Temporomandibular Disorders. J. Oral Rehabil. 2009, 36, 644–652. [Google Scholar] [CrossRef]
- Takashima, M.; Arai, Y.; Kawamura, A.; Hayashi, T.; Takagi, R. Quantitative Evaluation of Masseter Muscle Stiffness in Patients with Temporomandibular Disorders Using Shear Wave Elastography. J. Prosthodont. Res. 2017, 61, 432–438. [Google Scholar] [CrossRef]
- Smardz, J.; Martynowicz, H.; Wojakowska, A.; Wezgowiec, J.; Danel, D.; Mazur, G.; Wieckiewicz, M. Lower Serotonin Levels in Severe Sleep Bruxism and Its Association with Sleep, Heart Rate, and Body Mass Index. J. Oral Rehabil. 2022, 49, 422–429. [Google Scholar] [CrossRef]
- Nota, A.; Pittari, L.; Paggi, M.; Abati, S.; Tecco, S. Correlation between Bruxism and Gastroesophageal Reflux Disorder and Their Effects on Tooth Wear. A Systematic Review. J. Clin. Med. 2022, 11, 1107. [Google Scholar] [CrossRef]
- Azario De Holanda, G.; Azario De Holanda, T.; Casarin, M. Are Sleep and Awake Bruxism Associated with Sleep Quality and Duration in Adults? A Systematic Review and Meta-Analysis. Sleep Med. 2025, 129, 175–186. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chon, S.; Auh, Q.S.; Verhoeff, M.C.; Lobbezoo, F. Clinical, Psychological, and Hematological Factors Predicting Sleep Bruxism in Patients with Temporomandibular Disorders. Sci. Rep. 2025, 15, 19148. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.M.; Singh, P.; Khrimian, L.; Morgan, D.A.; Chowdhury, S.; Arteaga-Solis, E.; Horvath, T.L.; Domingos, A.I.; Marsland, A.L.; Yadav, V.K.; et al. Mediation of the Acute Stress Response by the Skeleton. Cell Metab. 2019, 30, 890–902.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Chen, J.; Liu, Y.; Pan, L.L.; Guo, Y.X.; Zhang, Z.M.; Li, Q.; Chen, Y.J. Regulation of CeA-Vme Projection in Masseter Hyperactivity Caused by Restraint Stress. Front. Cell. Neurosci. 2024, 18, 1509020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Liu, Y.; Wang, J.; Li, Q.; Zhang, Z.M.; Tu, T.; Lei, R.; Zhang, M.; Chen, Y.J. Activation of the Mesencephalic Trigeminal Nucleus Contributes to Masseter Hyperactivity Induced by Chronic Restraint Stress. Front. Cell. Neurosci. 2022, 16, 841133. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Liu, K.Y.; Elliott, T.; Knowles, M.; Howard, R. Heart Rate Variability in Relation to Cognition and Behavior in Neurodegenerative Diseases: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 73, 101539. [Google Scholar] [CrossRef]
- Michalek-Zrabkowska, M.; Martynowicz, H.; Wieckiewicz, M.; Smardz, J.; Poreba, R.; Mazur, G. Cardiovascular Implications of Sleep Bruxism—A Systematic Review with Narrative Summary and Future Perspectives. J. Clin. Med. 2021, 10, 2245. [Google Scholar] [CrossRef]
- Silvani, A.; Dampney, R.; Swoap, S.; Macefield, V.G.; Joyner, M.; Billman, G.E. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front. Physiol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache 2016, 56, 71–78. [Google Scholar] [CrossRef]
- Albagieh, H.; Alomran, I.; Binakresh, A.; Alhatarisha, N.; Almeteb, M.; Khalaf, Y.; Alqublan, A.; Alqahatany, M. Occlusal Splints-Types and Effectiveness in Temporomandibular Disorder Management. Saudi Dent. J. 2023, 35, 70–79. [Google Scholar] [CrossRef]
- Minakuchi, H.; Fujisawa, M.; Abe, Y.; Iida, T.; Oki, K.; Okura, K.; Tanabe, N.; Nishiyama, A. Managements of Sleep Bruxism in Adult: A Systematic Review. Jpn. Dent. Sci. Rev. 2022, 58, 124–136. [Google Scholar] [CrossRef]
- Fernández-Núñez, T.; Amghar-Maach, S.; Gay-Escoda, C. Efficacy of Botulinum Toxin in the Treatment of Bruxism: Systematic Review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e416–e424. [Google Scholar] [CrossRef]
- Kadıoğlu, M.B.; Sezer, M.; Elbasan, B. Effects of Manual Therapy and Home Exercise Treatment on Pain, Stress, Sleep, and Life Quality in Patients with Bruxism: A Randomized Clinical Trial. Medicina 2024, 60, 2007. [Google Scholar] [CrossRef] [PubMed]
- Harper, B.; Price, P.; Steele, M. The Efficacy of Manual Therapy on HRV in Those with Long-Standing Neck Pain: A Systematic Review. Scand. J. Pain 2023, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Eguaras, N.; Rodríguez-López, E.S.; Lopez-Dicastillo, O.; Franco-Sierra, M.Á.; Ricard, F.; Oliva-Pascual-Vaca, Á. Effects of Osteopathic Visceral Treatment in Patients with Gastroesophageal Reflux: A Randomized Controlled Trial. J. Clin. Med. 2019, 8, 1738. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hurtado, I.; Arguisuelas, M.D.; Almela-Notari, P.; Cortés, X.; Barrasa-Shaw, A.; Campos-González, J.C.; Lisón, J.F. Effects of Diaphragmatic Myofascial Release on Gastroesophageal Reflux Disease: A Preliminary Randomized Controlled Trial. Sci. Rep. 2019, 9, 7273. [Google Scholar] [CrossRef]
- Alanazi, M.S.; Degenhardt, B.; Kelley-Franklin, G.; Jacobson, E.; Fritz, S.; Kettner, N.; Kremen, V.; Lipke, L.; Reed, W.R.; Reed, W.R. Autonomic Nervous System and Viscera-Related Responses to Manual Therapy: A Narrative Overview. Int. J. Osteopath. Med. 2024, 54, 100735. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 Statement: Updated Guideline for Reporting Randomized Trials. Nat. Med. 2025, 31, 1776–1783. [Google Scholar] [CrossRef]
- Henssen, D.J.H.A.; Derks, B.; van Doorn, M.; Verhoogt, N.C.; Staats, P.; Vissers, K.; Van Cappellen van Walsum, A.M. Visualizing the Trigeminovagal Complex in the Human Medulla by Combining Ex-Vivo Ultra-High Resolution Structural MRI and Polarized Light Imaging Microscopy. Sci. Rep. 2019, 9, 11305. [Google Scholar] [CrossRef]
- Ricard, F. Tratado de Osteopatía Visceral y Medicina Interna. Sistema Digestivo, 2nd ed.; Editorial Medica Panamericana: Madrid, Spain, 2016. [Google Scholar]
- Marizeiro, D.F.; Florêncio, A.C.L.; Nunes, A.C.L.; Campos, N.G.; Lima, P.O.d.P. Immediate Effects of Diaphragmatic Myofascial Release on the Physical and Functional Outcomes in Sedentary Women: A Randomized Placebo-Controlled Trial. J. Bodyw. Mov. Ther. 2018, 22, 924–929. [Google Scholar] [CrossRef]
- Loro, F.L.; Martins, R.; Ferreira, J.B.; de Araujo, C.L.P.; Prade, L.R.; Both, C.B.; Nobre, J.C.N.; Monteiro, M.B.; Dal Lago, P. Validation of a Wearable Sensor Prototype for Measuring Heart Rate to Prescribe Physical Activity: Cross-Sectional Exploratory Study. JMIR Biomed. Eng. 2024, 9, e57373. [Google Scholar] [CrossRef]
- Schaffarczyk, M.; Rogers, B.; Reer, R.; Gronwald, T. Validity of the Polar H10 Sensor for Heart Rate Variability Analysis during Resting State and Incremental Exercise in Recreational Men and Women. Sensors 2022, 22, 6536. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, P.; Shrestha, L.; Mahotra, N.B. Validity of Elite-HRV Smartphone Application for Measuring Heart Rate Variability Compared to Polar V800 Heart Rate Monitor. J. Nepal Health Res. Counc. 2022, 19, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Himariotis, A.T.; Coffey, K.F.; Noel, S.E.; Cornell, D.J. Validity of a Smartphone Application in Calculating Measures of Heart Rate Variability. Sensors 2022, 22, 9883. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Bourdillon, N.; Yazdani, S.; Vesin, J.M.; Schmitt, L.; Millet, G.P. RMSSD Is More Sensitive to Artifacts Than Frequency-Domain Parameters: Implication in Athletes’ Monitoring. J. Sports Sci. Med. 2022, 21, 260. [Google Scholar] [CrossRef]
- Laborde, S.; Ackermann, S.; Borges, U.; D’Agostini, M.; Giraudier, M.; Iskra, M.; Mosley, E.; Ottaviani, C.; Salvotti, C.; Schmaußer, M.; et al. Leveraging Vagally Mediated Heart Rate Variability as an Actionable, Noninvasive Biomarker for Self-Regulation: Assessment, Intervention, and Evaluation. Policy Insights Behav. Brain Sci. 2023, 10, 212–220. [Google Scholar] [CrossRef]
- Besson, C.; Baggish, A.L.; Monteventi, P.; Schmitt, L.; Stucky, F.; Gremeaux, V. Assessing the Clinical Reliability of Short-Term Heart Rate Variability: Insights from Controlled Dual-Environment and Dual-Position Measurements. Sci. Rep. 2025, 15, 5611. [Google Scholar] [CrossRef]
- Chaves, T.C.; Nagamine, H.M.; De Sousa, L.M.; De Oliveira, A.S.; Grossi, D.B. Intra- and Interrater Agreement of Pressure Pain Threshold for Masticatory Structures in Children Reporting Orofacial Pain Related to Temporomandibular Disorders and Symptom-Free Children. J. Orofac. Pain 2007, 21, 133–142. [Google Scholar]
- Yao, Y.; Cai, B.; Fan, S.; Yang, H.X.; Zhang, Y.X.; Xu, L.L. The Association between Forward Head Posture and Masticatory Muscle Pressure Pain Thresholds in Patients with Temporomandibular Joint Dissorders: A Cross-Sectional Observational Study. Clin. Oral Investig. 2023, 27, 353–360. [Google Scholar] [CrossRef]
- Lettner, J.; Królikowska, A.; Ramadanov, N.; Oleksy, Ł.; Hakam, H.T.; Becker, R.; Prill, R. Evaluating the Reliability of MyotonPro in Assessing Muscle Properties: A Systematic Review of Diagnostic Test Accuracy. Medicina 2024, 60, 851. [Google Scholar] [CrossRef]
- Posta, D.D.; Paternostro, F.; Costa, N.; Branca, J.J.V.; Guarnieri, G.; Morelli, A.; Pacini, A.; Campi, G. Evaluating Biomechanical and Viscoelastic Properties of Masticatory Muscles in Temporomandibular Disorders: A Patient-Centric Approach Using MyotonPRO Measurements. Bioengineering 2025, 12, 97. [Google Scholar] [CrossRef]
- Rossettini, G.; Carlino, E.; Testa, M. Clinical Relevance of Contextual Factors as Triggers of Placebo and Nocebo Effects in Musculoskeletal Pain. BMC Musculoskelet. Disord. 2018, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.M.; Muniz de Souza, H.C.; Viana, R.; Neves, V.R.; Dornelas de Andrade, A. Immediate Effects of Rib Mobilization and Diaphragm Release Techniques on Cardiac Autonomic Control in Patients with Chronic Obstructive Pulmonary Disease: A Pilot Study. J. Chiropr. Med. 2020, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- McCoss, C.A.; Johnston, R.; Edwards, D.J.; Millward, C. Preliminary Evidence of Regional Interdependent Inhibition, Using a ‘Diaphragm Release’ to Specifically Induce an Immediate Hypoalgesic Effect in the Cervical Spine. J. Bodyw. Mov. Ther. 2017, 21, 362–374. [Google Scholar] [CrossRef]
- Márquez-Vera, A.; Polo-Ferrero, L.; Puente-González, A.S.; Méndez-Sánchez, R.; Blanco-Rueda, J.A. Immediate Effects of the Mandibular Muscle Energy Technique in Adults with Chronic Temporomandibular Disorder. Clin. Pract. 2024, 14, 2568–2579. [Google Scholar] [CrossRef]
- Machado, N.A.G.; Costa, Y.M.; Quevedo, H.M.; Stuginski-Barbosa, J.; Valle, C.M.; Bonjardim, L.R.; Garib, D.G.; Conti, P.C.R. The Association of Self-Reported Awake Bruxism with Anxiety, Depression, Pain Threshold at Pressure, Pain Vigilance, and Quality of Life in Patients Undergoing Orthodontic Treatment. J. Appl. Oral Sci. 2020, 28, e20190407. [Google Scholar] [CrossRef]
- Jiménez-Silva, A.; Peña-Durán, C.; Tobar-Reyes, J.; Frugone-Zambra, R. Sleep and Awake Bruxism in Adults and Its Relationship with Temporomandibular Disorders: A Systematic Review from 2003 to 2014. Acta Odontol. Scand. 2017, 75, 36–58. [Google Scholar] [CrossRef]
- Lee, K.; Chon, S. Assessments of Muscle Thickness and Tonicity of the Masseter and Sternocleidomastoid Muscles and Maximum Mouth Opening in Patients with Temporomandibular Disorder. Healthcare 2021, 9, 1640. [Google Scholar] [CrossRef]
- Taş, S.; Kaynak, B.A.; Salkin, Y.; Karakoç, Z.B.; Dağ, F. An Investigation of the Changes in Mechanical Properties of the Orofacial and Neck Muscles between Patients with Myogenous and Mixed Temporomandibular Disorders. Cranio J. Craniomandib. Pract. 2024, 42, 150–159. [Google Scholar] [CrossRef]

| Experimental Group n = 12 | Control Group n = 12 | |
|---|---|---|
| Age, y, mean (SD) [Range] | 38.5 (9.64) [21–55] | 38.5 (11.92) [25–64] |
| Sex, female, n (%) | 5 (41.66%) | 6 (50%) |
| Height, cm, mean (SD) [Range] | 168.25 (8.42) [154–178] | 170.00 (10.89) [156–187] |
| Weight, Kg, mean (SD) [Range] | 75.89 (19.42) [46.30–104.10] | 89.97 (22.27) [55–133] |
| BMI, Kg/cm2, mean (SD) [Range] | 26.40 (4.67) [19.52–33.23] | 30.96 (6.55) [22.60–43.96] |
| Within-Group Differences | Between-Group Differences | Effect Size | ||
|---|---|---|---|---|
| Experimental Group | Control Group | |||
| HRV–SDNN | ||||
| Baseline (T1) | 39.64 (12.01) | 38.76 (23.26) | ||
| Post-first intervention (T2) | 58.25 (24.00) | 38.34 (21.79) | ||
| Pre-second intervention (T3) | 41.21 (21.14) | 32.50 (9.97) | ||
| Post-second intervention (T4) | 48.21 (14.75) | 32.51 (15.49) | ||
| 4-week follow-up (T5) | 41.21 (21.14) | 32.50 (9.97) | ||
| Change T1 to T2 | −18.61 (15.96) | 0.42 (4.23) | 0.001 (−15.43 to −2.76) | 0.42 (0.10–0.62) |
| Change T3 to T4 | −11.61 (18.16) | −3.42 (8.58) | 0.172 (−13.64 to −1.39) | 0.08 (0.00–0.32) |
| HRV–RMSSD | ||||
| Baseline (T1) | 36.15 (14.73) | 40.63 (36.37) | ||
| Post-first intervention (T2) | 54.41 (25.86) | 40.59 (35.91) | ||
| Pre-second intervention (T3) | 33.56 (8.46) | 28.15 (14.60) | ||
| Post-second intervention (T4) | 45.93 (16.20) | 31.30 (19.16) | ||
| 4-week follow-up (T5) | 39.74 (24.00) | 34.09 (17.65) | ||
| Change T1 to T2 | −18.26 (−29.02 to −7.49) | 0.04 (−2.03 to 2.10) | 0.001 (−15.51 to −2.70) | 0.38 (0.07–0.59) |
| Change T3 to T4 | −12.37 (−22.46 to −2.29) | −3.15 (−7.80 to 1.50) | 0.08 (−13.24 to −2.28) | 0.13 (0.00- 0.38) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Rico, C.; Fricke-Comellas, H.; Heredia-Rizo, A.M.; Díaz-Mancha, J.A.; Rosado-Portillo, A.; Fernández-Seguín, L.M. Short-Term Effects of Visceral Manual Therapy on Autonomic Nervous System Modulation in Individuals with Clinically Based Bruxism: A Randomized Controlled Trial. Dent. J. 2025, 13, 325. https://doi.org/10.3390/dj13070325
Navarro-Rico C, Fricke-Comellas H, Heredia-Rizo AM, Díaz-Mancha JA, Rosado-Portillo A, Fernández-Seguín LM. Short-Term Effects of Visceral Manual Therapy on Autonomic Nervous System Modulation in Individuals with Clinically Based Bruxism: A Randomized Controlled Trial. Dentistry Journal. 2025; 13(7):325. https://doi.org/10.3390/dj13070325
Chicago/Turabian StyleNavarro-Rico, Cayetano, Hermann Fricke-Comellas, Alberto M. Heredia-Rizo, Juan Antonio Díaz-Mancha, Adolfo Rosado-Portillo, and Lourdes M. Fernández-Seguín. 2025. "Short-Term Effects of Visceral Manual Therapy on Autonomic Nervous System Modulation in Individuals with Clinically Based Bruxism: A Randomized Controlled Trial" Dentistry Journal 13, no. 7: 325. https://doi.org/10.3390/dj13070325
APA StyleNavarro-Rico, C., Fricke-Comellas, H., Heredia-Rizo, A. M., Díaz-Mancha, J. A., Rosado-Portillo, A., & Fernández-Seguín, L. M. (2025). Short-Term Effects of Visceral Manual Therapy on Autonomic Nervous System Modulation in Individuals with Clinically Based Bruxism: A Randomized Controlled Trial. Dentistry Journal, 13(7), 325. https://doi.org/10.3390/dj13070325










