Blue Photosensitizer, Red Light, Clear Results: An Integrative Review of the Adjunctive Periodontal Treatment with Methylene Blue in Antimicrobial Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibity Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Search Strategy
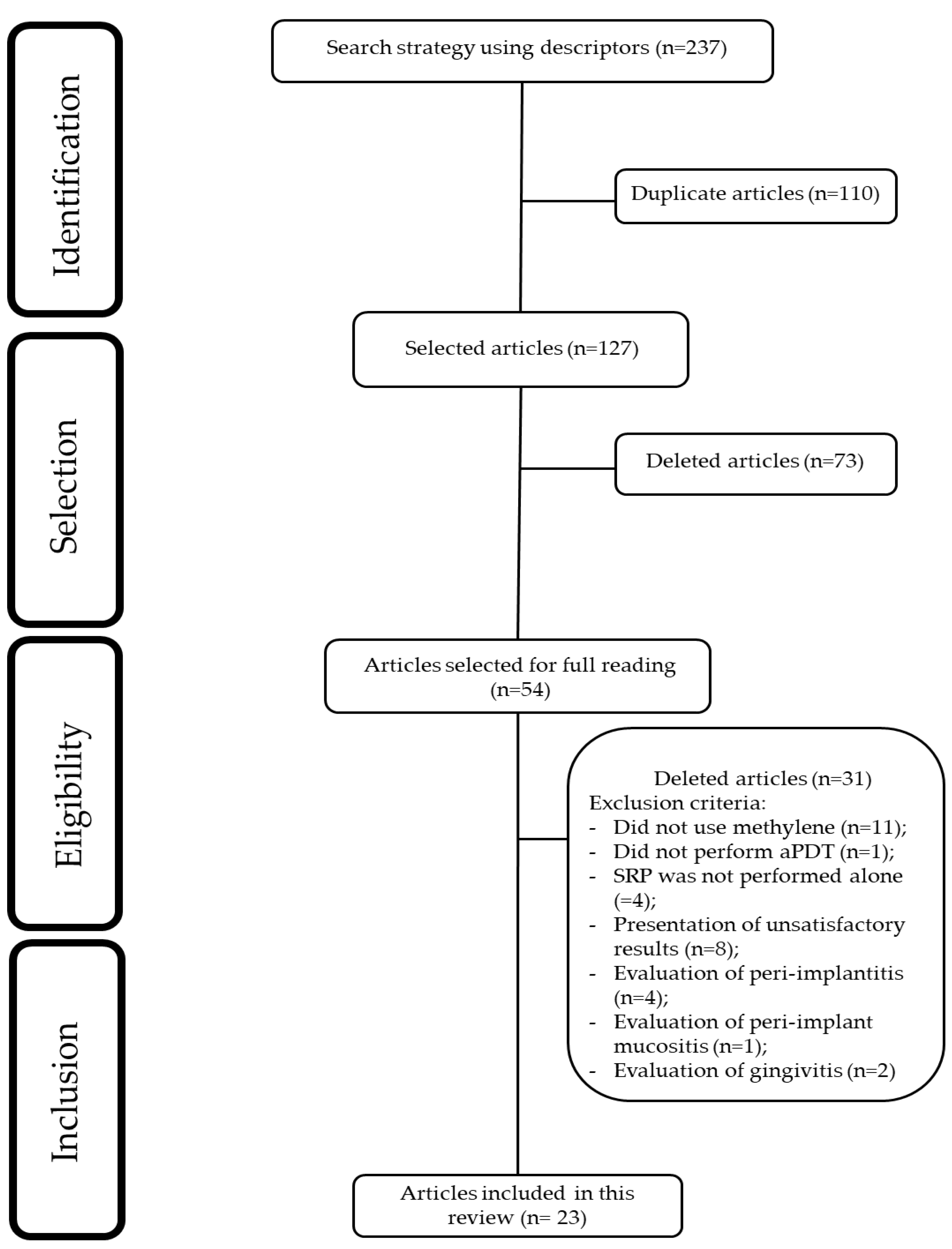
2.4. Study Selection
2.5. Data Collection Process
2.6. Risk of Bias
2.7. Data Analysis
3. Results
3.1. Clinical Studies Included in This Review
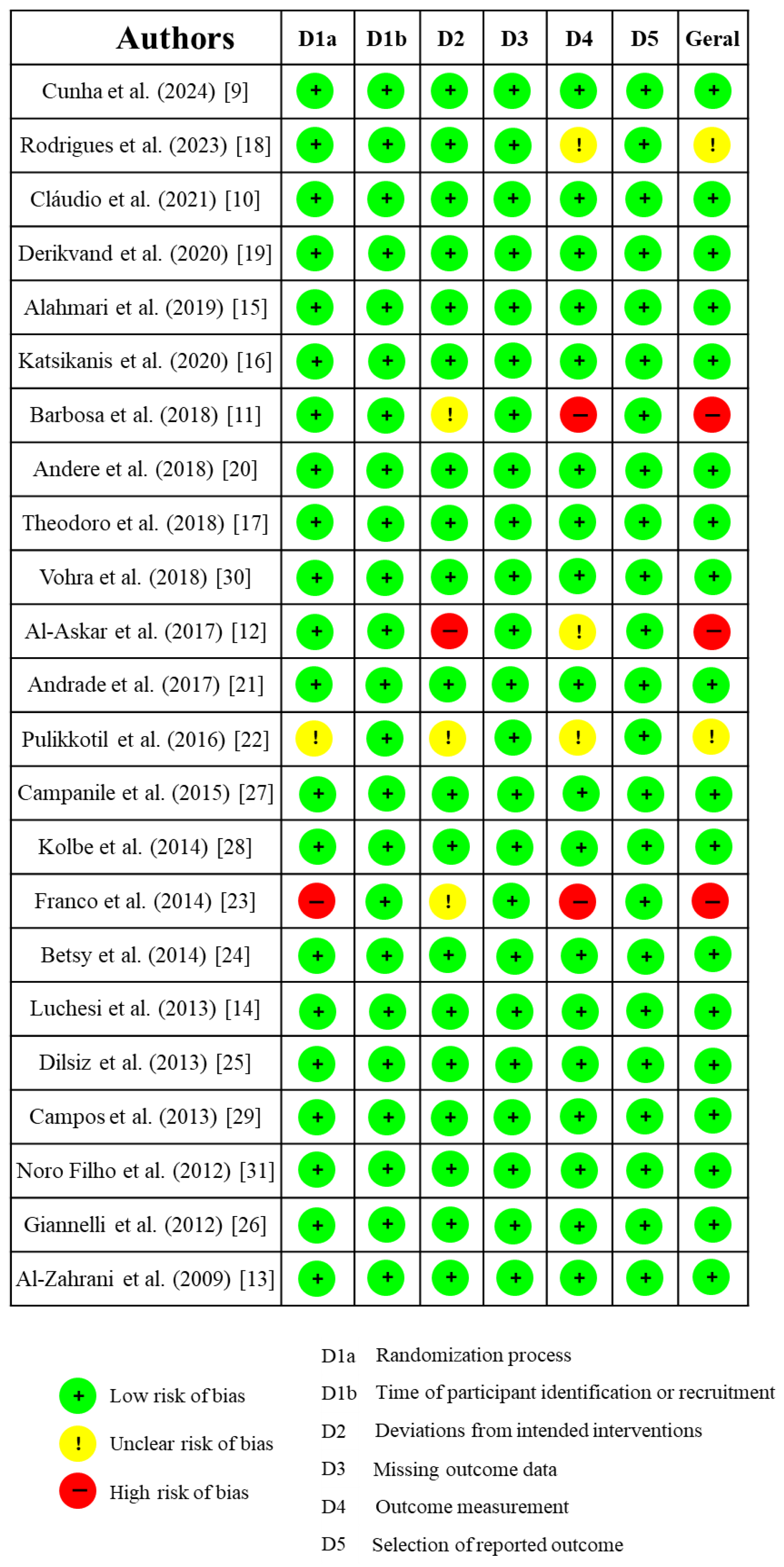
3.2. Risk of Bias Assessment
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Passanezi, E.; Damante, C.A.; de Rezende, M.L.; Greghi, S.L. Lasers in periodontal therapy. Periodontol. 2000 2015, 67, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Figuero, E.; Roldán, S.; Serrano, J.; Escribano, M.; Martín, C.; Preshaw, P.M. Efficacy of adjunctive therapies in patients with gingival inflammation: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 125–143. [Google Scholar] [CrossRef]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 176–198. [Google Scholar] [CrossRef]
- Gois, M.M.; Kurachi, C.; Santana, E.J.; Mima, E.G.; Spolidório, D.M.; Pelino, J.E.; Salvador Bagnato, V. Susceptibility of Staphylococcus aureus to porphyrin-mediated photodynamic antimicrobial chemotherapy: An in vitro study. Lasers Med. Sci. 2010, 25, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 827, 293–302. [Google Scholar] [CrossRef]
- Figueiredo-Godoi, L.M.A.; Garcia, M.T.; Pinto, J.G.; Ferreira-Strixino, J.; Faustino, E.G.; Pedroso, L.L.C.; Junqueira, J.C. Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii. Antibiotics 2022, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.N.; Teichert, M.C.; Sievert, C.E.; Biel, M.A. Effect of Cat on the photobactericidal efficacy of methylene blue and toluidine blue against gram-negative bacteria and the dye affinity for lipopolysaccharides. Lasers Surg. Med. 2006, 38, 946–954. [Google Scholar] [CrossRef]
- Cunha, P.O.; Gonsales, I.R.; Greghi, S.L.A.; Sant’ana, A.C.P.; Honório, H.M.; Negrato, C.A.; Zangrando, M.S.R.; Damante, C.A. Adjuvant antimicrobial photodynamic therapy improves periodontal health and reduces inflammatory cytokines in patients with type 1 diabetes mellitus. J. Appl. Oral Sci. 2024, 32, e20240258. [Google Scholar] [CrossRef]
- Cláudio, M.M.; Nuernberg, M.A.A.; Rodrigues, J.V.S.; Belizário, L.C.G.; Batista, J.A.; Duque, C.; Garcia, V.G.; Theodoro, L.H. Effects of multiple sessions of antimicrobial photodynamic therapy (aPDT) in the treatment of periodontitis in patients with uncompensated type 2 diabetes: A randomized controlled clinical study. Photodiagnosis Photodyn. Ther. 2021, 35, 102451. [Google Scholar] [CrossRef]
- Barbosa, F.I.; Araújo, P.V.; Machado, L.J.C.; Magalhães, C.S.; Guimarães, M.M.M.; Moreira, A.N. Effect of photodynamic therapy as an adjuvant to non-surgical periodontal therapy: Periodontal and metabolic evaluation in patients with type 2 diabetes mellitus. Photodiagnosis Photodyn. Ther. 2018, 22, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Al-Askar, M.; Al-Kheraif, A.A.; Ahmed, H.B.; Kellesarian, S.V.; Malmstrom, H.; Javed, F. Effectiveness of mechanical debridement with and without adjunct antimicrobial photodynamic therapy in the treatment of periodontal inflammation among patients with prediabetes. Photodiagnosis Photodyn. Ther. 2017, 20, 91–94. [Google Scholar] [CrossRef]
- Al-Zahrani, M.S.; Bamshmous, S.O.; Alhassani, A.A.; Al-Sherbini, M.M. Short-term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J. Periodontol. 2009, 80, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Luchesi, V.H.; Pimentel, S.P.; Kolbe, M.F.; Ribeiro, F.V.; Casarin, R.C.; Nociti, F.H., Jr.; Sallum, E.A.; Casati, M.Z. Photodynamic therapy in the treatment of class II furcation: A randomized controlled clinical trial. J. Clin. Periodontol. 2013, 40, 781–788. [Google Scholar] [CrossRef] [PubMed]
- AlAhmari, F.; Ahmed, H.B.; Al-Kheraif, A.A.; Javed, F.; Akram, Z. Effectiveness of scaling and root planning with and without adjunct antimicrobial photodynamic therapy in the treatment of chronic periodontitis among cigarette-smokers and never-smokers: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2019, 25, 247–252. [Google Scholar] [CrossRef]
- Katsikanis, F.; Strakas, D.; Vouros, I. The application of antimicrobial photodynamic therapy (aPDT, 670 nm) and diode laser (940 nm) as adjunctive approach in the conventional cause-related treatment of chronic periodontal disease: A randomized controlled split-mouth clinical trial. Clin. Oral Investig. 2020, 24, 1821–1827. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Assem, N.Z.; Longo, M.; Alves, M.L.F.; Duque, C.; Stipp, R.N.; Vizoto, N.L.; Garcia, V.G. Treatment of periodontitis in smokers with multiple sessions of antimicrobial photodynamic therapy or systemic antibiotics: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2018, 22, 217–222. [Google Scholar] [CrossRef]
- Rodrigues, R.D.; Araujo, N.S.; Filho, J.M.P.; Vieira, C.L.Z.; Ribeiro, D.A.; Dos Santos, J.N.; Cury, P.R. Photodynamic therapy as adjunctive treatment of single-rooted teeth in patients with grade C periodontitis: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2023, 44, 103776. [Google Scholar] [CrossRef]
- Derikvand, N.; Ghasemi, S.S.; Safiaghdam, H.; Piriaei, H.; Chiniforush, N. Antimicrobial Photodynamic Therapy with Diode laser and Methylene blue as an adjunct to scaling and root planning: A clinical trial. Photodiagnosis Photodyn. Ther. 2020, 31, 101818. [Google Scholar] [CrossRef]
- Andere, N.M.R.B.; Dos Santos, N.C.C.; Araujo, C.F.; Mathias, I.F.; Rossato, A.; de Marco, A.C.; Santamaria, M., Jr.; Jardini, M.A.N.; Santamaria, M.P. Evaluation of the local effect of nonsurgical periodontal treatment with and without systemic antibiotic and photodynamic therapy in generalized aggressive periodontitis. A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2018, 24, 115–120. [Google Scholar] [CrossRef]
- Andrade, P.V.C.; Euzebio Alves, V.T.; de Carvalho, V.F.; De Franco Rodrigues, M.; Pannuti, C.M.; Holzhausen, M.; De Micheli, G.; Conde, M.C. Photodynamic therapy decrease immune-inflammatory mediators levels during periodontal maintenance. Lasers Med. Sci. 2017, 32, 9–17. [Google Scholar] [CrossRef]
- Pulikkotil, S.J.; Toh, C.G.; Mohandas, K.; Leong, K. Effect of photodynamic therapy adjunct to scaling and root planing in periodontitis patients: A randomized clinical trial. Aust. Dent. J. 2016, 61, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.J.; Pogue, R.E.; Sakamoto, L.H.; Cavalcante, L.L.; Carvalho, D.R.; de Andrade, R.V. Increased expression of genes after periodontal treatment with photodynamic therapy. Photodiagnosis Photodyn. Ther. 2014, 11, 41–47. [Google Scholar] [CrossRef]
- Betsy, J.; Prasanth, C.S.; Baiju, K.V.; Prasanthila, J.; Subhash, N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: A randomized controlled clinical trial. J. Clin. Periodontol. 2014, 41, 573–581. [Google Scholar] [CrossRef]
- Dilsiz, A.; Canakci, V.; Aydin, T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2013, 84, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Formigli, L.; Lorenzini, L.; Bani, D. Combined photoablative and photodynamic diode laser therapy as an adjunct to non-surgical periodontal treatment: A randomized split-mouth clinical trial. J. Clin. Periodontol. 2012, 39, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Campanile, V.S.M.; Giannopoulou, C.; Campanile, G.; Cancela, J.A.; Mombelli, A. Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: Clinical, microbiological, and local biological effects. Lasers Med. Sci. 2015, 30, 27–34. [Google Scholar] [CrossRef]
- Kolbe, M.F.; Ribeiro, F.V.; Luchesi, V.H.; Casarin, R.C.; Sallum, E.A.; Nociti, F.H., Jr.; Ambrosano, G.M.; Cirano, F.R.; Pimentel, S.P.; Casati, M.Z. Photodynamic therapy during supportive periodontal care: Clinical, microbiologic, immunoinflammatory, and patient-centered performance in a split-mouth randomized clinical trial. J. Periodontol. 2014, 85, e277–e286. [Google Scholar] [CrossRef]
- Campos, G.N.; Pimentel, S.P.; Ribeiro, F.V.; Casarin, R.C.; Cirano, F.R.; Saraceni, C.H.; Casati, M.Z. The adjunctive effect of photodynamic therapy for residual pockets in single-rooted teeth: A randomized controlled clinical trial. Lasers Med. Sci. 2013, 28, 317–324. [Google Scholar] [CrossRef]
- Vohra, F.; Akram, Z.; Bukhari, I.A.; Sheikh, S.A.; Javed, F. Short-term effects of adjunctive antimicrobial photodynamic therapy in obese patients with chronic periodontitis: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2018, 21, 10–15. [Google Scholar] [CrossRef]
- Noro Filho, G.A.; Casarin, R.C.; Casati, M.Z.; Giovani, E.M. PDT in non-surgical treatment of periodontitis in HIV patients: A split-mouth, randomized clinical trial. Lasers Surg. Med. 2012, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Jervøe-Storm, P.M.; Bunke, J.; Worthington, H.V.; Needleman, I.; Cosgarea, R.; MacDonald, L.; Walsh, T.; Lewis, S.R.; Jepsen, S. Adjunctive antimicrobial photodynamic therapy for treating periodontal and peri-implant diseases. Cochrane Database Syst. Rev. 2024, 7, CD011778. [Google Scholar] [CrossRef] [PubMed]
- Alasqah, M.N. Efficacy of methylene blue-mediated antimicrobial photodynamic therapy on clinical and radiographic outcomes among patients with periodontal diseases: A systematic review and meta-analysis of randomized controlled trials. Photodiagnosis Photodyn. Ther. 2024, 46, 104000. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Hyder, T.; Al-Hamoudi, N.; Binshabaib, M.S.; Alharthi, S.S.; Hanif, A. Efficacy of photodynamic therapy versus antibiotics as an adjunct to scaling and root planing in the treatment of periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 19, 86–92. [Google Scholar] [CrossRef]
- Xue, D.; Tang, L.; Bai, Y.; Ding, Q.; Wang, P.; Zhao, Y. Clinical efficacy of photodynamic therapy adjunctive to scaling and root planing in the treatment of chronic periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 18, 119–127. [Google Scholar] [CrossRef]
- Xue, D.; Zhao, Y. Clinical effectiveness of adjunctive antimicrobial photodynamic therapy for residual pockets during supportive periodontal therapy: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 17, 127–133. [Google Scholar] [CrossRef]
- Vohra, F.; Akram, Z.; Safii, S.H.; Vaithilingam, R.D.; Ghanem, A.; Sergis, K.; Javed, F. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review. Photodiagnosis Photodyn. Ther. 2016, 13, 139–147. [Google Scholar] [CrossRef]
- Javed, F.; Qadri, T.; Ahmed, H.B.; Al-Hezaimi, K.; Corbet, F.E.; Romanos, G.E. Is photodynamic therapy with adjunctive non-surgical periodontal therapy effective in the treatment of periodontal disease under immunocompromised conditions? J. Coll. Physicians Surg. Pak. 2013, 23, 731–736. [Google Scholar]
- Sgolastra, F.; Petrucci, A.; Gatto, R.; Marzo, G.; Monaco, A. Photodynamic therapy in the treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2013, 28, 669–682. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Graziani, F.; Gatto, R.; Monaco, A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: A meta-analysis. Lasers Med. Sci. 2010, 25, 605–613. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Shah, P.S.; Tenenbaum, H.C.; Goldberg, M.B. The effect of photodynamic therapy for periodontitis: A systematic review and meta-analysis. J. Periodontol. 2010, 81, 4–14. [Google Scholar] [CrossRef]
- Savović, J.; Turner, R.M.; Mawdsley, D.; Jones, H.E.; Beynon, R.; Higgins, J.P.T.; Sterne, J.A.C. Association Between Risk-of-Bias Assessments and Results of Randomized Trials in Cochrane Reviews: The ROBES Meta-Epidemiologic Study. Am. J. Epidemiol. 2018, 187, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.M.; Theodoro, L.H.; Bosco, A.F.; Nagata, M.J.; Bonfante, S.; Garcia, V.G. Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J. Periodontol. 2008, 79, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Prates, R.A.; Yamada, A.M.; Suzuki, L.C.; França, C.M.; Cai, S.; Mayer, M.P.; Ribeiro, A.C.; Ribeiro, M.S. Histomorphometric and microbiological assessment of photodynamic therapy as an adjuvant treatment for periodontitis: A short-term evaluation of inflammatory periodontal conditions and bacterial reduction in a rat model. Photomed. Laser Surg. 2011, 29, 835–844. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Suaid, F.A.; de Andrade, P.F.; Oliveira, F.S.; Novaes, A.B., Jr.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F.; Souza, S.L. Adjunctive effect of antimicrobial photodynamic therapy to nonsurgical periodontal treatment in smokers: A randomized clinical trial. Lasers Med. Sci. 2015, 30, 617–625. [Google Scholar] [CrossRef]
- Lulic, M.; Leiggener Görög, I.; Salvi, G.E.; Ramseier, C.A.; Mattheos, N.; Lang, N.P. One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: A proof-of-principle randomized-controlled clinical trial. J. Clin. Periodontol. 2009, 36, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Greghi, S.L.A.; Sant’Ana, A.C.P.; Zangrando, M.S.R.; Damante, C.A. Multiple Sessions of Antimicrobial Photodynamic Therapy Improve Periodontal Outcomes in Patients with Down Syndrome: A 12-Month Randomized Clinical Trial. Dent. J. 2025, 13, 33. [Google Scholar] [CrossRef]
- Moro, M.G.; de Carvalho, V.F.; Godoy-Miranda, B.A.; Kassa, C.T.; Horliana, A.C.R.T.; Prates, R.A. Efficacy of antimicrobial photodynamic therapy (aPDT) for nonsurgical treatment of periodontal disease: A systematic review. Lasers Med. Sci. 2021, 36, 1573–1590. [Google Scholar] [CrossRef]
- Malgikar, S.; Harinath, R.; Vidya, S.; Satyanarayana; Vikram, R.; Julieta, J. Clinical effects of photodynamic and low-level laser therapies as an adjunct to scaling and root planing of chronic periodontitis: A split-mouth randomized controlled clinical trial. Indian J. Dent. Res. 2016, 27, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Annaji, S.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Bharmappa, R.; Kamath, V. Efficacy of Photodynamic Therapy and Lasers as an Adjunct to Scaling and Root Planing in the Treatment of Aggressive Periodontitis—A Clinical and Microbiologic Short Term Study. J. Clin. Diagn. Res. JCDR 2016, 10, ZC08–ZC12. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.; Corbet, E.F.; Jin, L. Combined photodynamic and low-level laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. J. Periodontal Res. 2011, 46, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Damante, C.A. Laser parameters in systematic reviews. J. Clin. Periodontol. 2021, 48, 550–552. [Google Scholar] [CrossRef] [PubMed]
| Patient Profile | Number of Articles (n) | Reference |
|---|---|---|
| Diabetes | 5 | [9,10,11,12,13] |
| Furcation lesion | 1 | [14] |
| Smokers | 3 | [15,16,17] |
| Periodontitis | 9 | [18,19,20,21,22,23,24,25,26] |
| Residual periodontal pockets | 3 | [27,28,29] |
| Different patient profiles (Obesity and HIV) | 2 | [30] (Obesity); [31] (HIV) |
| Authors, Year and Participant (n) | Wavelength | Laser Parameters | Optic Fiber | Concentration of Dye | Repetition | Main Results |
|---|---|---|---|---|---|---|
| Cunha et al. (2024) (n = 38) [9] | 650 | 100 mW/80 s | Optic fiber (d = 600 μm) | 10 mg/mL | 3 sessions | SRP group presented greater values of PD (p < 0.05). There was a significant reduction in TNF-α in crevicular fluid of patients treated by aPDT (p < 0.05) |
| Rodrigues et al. (2023) (n = 14) [18] | 660 | 100 mW/0.25 mW/cm2/14.94 J/cm2/10 s | NR | 1% | 2 sessions | aPDT promoted better results of PD after 3 months. There was 18% less probability of presenting a final PD > 4 mm compared to SRP. |
| Cláudio et al. (2021) ** (n = 34) [10] | 660 | 157 J/cm2/100 mW/50 s | Optic fiber (d = 0.03 cm2) | 10 mg/mL | 3 sessions | aPDT presented a reduction in NRP after 3 and 6 months (p < 0.05). |
| Derikvand et al. (2020) (n = 50) [19] | 660 | 150 mW/60 s | NR | 100 μg/mL | Single | Reduction in PD at aPDT group after 3 and 6 months, in comparison to SRP group (p < 0.01). |
| Katsikanis et al. (2020) ** (n = 21) [16] | 670 | 350 mW/0.445 W/cm2/120 s | Diameter—1 cm | 1% | 3 sessions | Only PI presented statistically significant differences at baseline (p = 0.038) in SRP group. |
| Alahmari et al. (2019) (n = 83) [15] | 660 | 150 mW/75 mW/cm2/60 s | Optic fiber (d = 600 μm) | 0.005% | Single | Only PI in SRP group presented statistically significant differences (p < 0.05) after 1 month. PD and CAL were greater in S group When compared to NS group. |
| Barbosa et al. (2018) (n = 12) [11] | 660 | 40 mW/120 s/4.8 J | - | 10 mg/mL | Single | There was no difference between groups for PD and CAL (p > 0.05). aPDT group presented better results for PI after 1 month and BOP after 6 months. |
| Andere et al. (2018) (n = 36) [20] | 660 | 60 mW/129 J/cm2/60 s | Optic fiber | 10 mg/mL | Single | Group UPD + CLM + aPDT presented greater CAL values when compared to UPD and UPD + aPDT (p < 0.05). |
| Theodoro et al. (2018) (n = 51) [17] | 660 | 100 mW/160 J/cm2/48 s | Optic fiber (d= 0.03 cm2) | 10 mg/mL | 3 sessions | After 6 months, group MTZ + AMX and aPDT presented lower PD, greater CAL and less BOP, but without statistically significant differences between SRP and aPDT. |
| Vohra et al. (2018) ** (n = 52) [30] | 670 | 150 mW/60 s | Optic fiber (d = 0.6 mm) | 0.005% | Single | PI was better for SRP group after 1.5 and 3 months (p < 0.05). |
| Al-Askar et al. (2017) (n = 70) [12] | 670 | 150 mW/60 s | NR | 0.005% | Single | There was no difference between groups and periods. There was no difference in CBL in all groups at 3 and 6 months. |
| Andrade et al. (2017) (n = 28) [21] | 660 | 40 mW/90 s/90 J/cm2 | Optic Fiber (d = 200 μm) | 0.01% | Single | There were no differences between groups. There was a reduction in IL-8 in aPDT group after 3 months (p = 0.04). |
| Pulikkotil et al. (2016) (n = 20) [22] | Red LED (628 Hz) | 628 Hz/20 s | NR | NR | Single | There was a significant reduction in BOP after 3 months in aPDT group. (p < 0.01). There were no differences in A.a. quantification. |
| Campanile et al. (2015) (n = 28) [27] | 670 | 280 mW, ±0.2 dB | Optic fiber | NR | Twice a week | aPDT group presented reduction in PD after 3 and 6 months. There was a reduction in C reactive protein. There were no microbiological differences. |
| Kolbe et al. (2014) (n = 22) [28] | 660 | 0.06 W, 129 J/cm2, 60 s | Optic fiber (d = 600 μm) | 10 mg/mL | Single | There were no statistically significant differences in clinical parameters. Reduction in Pg., Aa., and inflammatory cytokines. |
| Franco et al. (2014) ** (n = 15) [23] | 660 | 0.06 W/cm2, 90 s, 5.4 Jcm2 | Optic fiber (d = 0.4 mm) | 0.01% | Once a week—total of 4 sessions | Reduction in BOP in aPDT group (p < 0.05). Increase in RANK/OPG and FGF-2 levels. |
| Betsy et al. (2014) (n = 88) [24] | 655 | 1 W, 0.06 W/cm2,60 s | Optic fiber (d = 0.5 mm) | 10 mg/mL | Single | Significant reductions in PD, CAL, BOP, PI and GI for aPDT group (p < 0.05). |
| Luchesi et al. (2013) (n = 37) [14] | 660 | 0.06 W, 129 J/cm2, 60 s | Optic fiber (d = 600 μm) | 10 mg/mL | Single | There were no statistically significant differences in clinical parameters. Reduction in Pg., Aa., and inflammatory cytokines up to 6 months. |
| Dilsiz et al. (2013) (n = 24) [25] | 808 | 0.1 W, 6 J, 60 s | Optic fiber (d = 300 μm) | 1% | Single | aPDT group presented reduction in PD and CAL after 6 months. (p < 0.05). |
| Campos et al. (2013) (n = 13) [29] | 660 | 0.06 W, 129 J/cm2, 60 s | Optic fiber (d = 600 μm) | 10 mg/mL | Single | aPDT group presented reduction in PD, CAL, and BOP after 6 months. |
| Noro Filho et al. (2012) (n = 12) [31] | 660 | 0.03 W, 0.428 W/cm2, 57.14 J/cm2, 133 s | Optic fiber (a = 0.07 cm2) | 0.01% | Single | aPDT presented reduction in PD after 6 months, BOP after 3 and 6 months. There were no differences in microbiological parameters. |
| Giannelli et al. (2012) (n = 26) [26] | 635 | 0.1 W, 120 s (60 s inside + 60 s outside) d = 0.6 mm | Optic fiber (d = 0.6 mm) | 0.3% | 4 to 10 sessions | aPDT presented reduction in PD, CAL, BOP and spirochetes after 12 months. |
| Al-Zahrani et al. (2009) (n = 45) [13] | 670 | 60 s | NR | 0.01% | Single | There were no significant differences. |
| Evaluation Time (Months) | Number of Articles (n) | Reference |
|---|---|---|
| 0 1, 3 and 6 | 3 | [9,11,24] |
| 0 and 3 | 4 | [13,18,23,29] |
| 0, 3 and 6 | 8 | [10,12,14,16,17,20,27,28] |
| 0, 1.5, 3 and 6 | 2 | [19,31] |
| 0, 1 and 3 | 2 | [15,22] |
| 0, 1.5 and 3 | 1 | [30] |
| 0, 3 and 12 | 1 | [21] |
| 0 and 6 | 1 | [25] |
| 0 and 12 | 1 | [26] |
| Randomization Process | ||
|---|---|---|
| Method Used | Number of Articles (n) | Reference |
| Coin toss | 3 | [15,22,30] |
| Computer-generated list | 8 | [13,14,20,25,27,28,29,31] |
| Computer-generated numbers | 1 | [9] |
| Random numbers | 1 | [24] |
| Online randomizer | 3 | [10,17] |
| Deck of cards | 1 | [18] |
| Lottery draw | 1 | [19] |
| Randomization chart | 1 | [16] |
| Computer software | 2 | [11,21] |
| Drawing lots from an opaque bag | 1 | [12] |
| Sealed opaque envelopes | 1 | [26] |
| Not reported | 1 | [23] |
| Authors | Patient Profile | PD | BOP (%) | CAL | PI (%) | GI | GR |
|---|---|---|---|---|---|---|---|
| Cunha et al. (2024) [9] | Periodontitis/Type 1 Diabetes Mellitus | SRP (p < 0.05) | NHE | NHE | NHE | - | - |
| Rodrigues et al. (2023) [18] | Periodontitis | aPDT (p = 0.02 at 3 months | - | NHE | - | - | NHE |
| Cláudio et al. (2021) [10] | Diabetes Mellitus | NHE | NHE | NHE | NHE | - | NHE |
| Derikvand et al. (2020) [19] | Periodontitis | aPDT (p < 0.01) at 3 and 6 months | - | - | NHE | NHE | - |
| Alahmari et al. (2019) [15] | Smokers | NHE | NHE | NHE | SRP (p < 0.01) at 1 month | - | - |
| Katsikanis et al. (2020) [16] | Moderate smoker | NHE | NHE | NHE | SRP (p = 0.038) at baseline | - | - |
| Barbosa et al. (2018) [11] | Periodontitis/Diabetes Mellitus | NHE | aPDT (p = 0.05) at 6 months | NHE | aPDT (p = 0.02) only at 1-month follow-up | - | - |
| Andere et al. (2018) [20] | Periodontitis | aPDT (p < 0.05) at 3 months | NHE | NHE | - | - | NHE |
| Theodoro et al. (2018) [17] | Smokers | NHE | NHE | NHE | - | - | - |
| Vohra et al. (2018) [30] | Obesity/Periodontitis | NHE | NHE | NHE | SRP (p < 0.01) at 1.5 months and 3 months | - | - |
| Al-Askar et al. (2017) [12] | Pre-diabetes | NHE | NHE | - | NHE | - | - |
| Andrade et al. (2017) [21] | Periodontitis | NHE | NHE | NHE | NHE | - | - |
| Pulikkotil et al. (2016) [22] | Periodontitis | NHE | aPDT (p < 0.01) at 3 months | NHE | NHE | - | - |
| Campanile et al. (2015) [27] | Residual pockets | aPDT (p = 0.04) at 3 months | NHE | NHE | NHE | NHE | - |
| Kolbe et al. (2014) [28] | Residual pockets | NHE | NHE | NHE | - | - | - |
| Franco et al. (2014) [23] | Periodontitis | NHE | aPDT (p < 0.05) | NHE | NHE | - | - |
| Betsy et al. (2014) [24] | Periodontitis | aPDT (p < 0.05) at 3 and 6 months | aPDT (p < 0.05) at 1 and 3 months | aPDT (p < 0.05) at 3 and 6 months | aPDT (p < 0.05) at 2 weeks | aPDT (p < 0.05) at 1 and 3 months | NHE |
| Luchesi et al. (2013) [14] | Furcation Class III | NHE | NHE | NHE | NHE | - | - |
| Dilsiz et al. (2013) [25] | Periodontitis | aPDT (p < 0.05) at 6 months | NHE | aPDT (p < 0.05) at 6 months | NHE | NHE | - |
| Campos et al. (2013) [29] | Residual pockets | aPDT (p < 0.05) at 3 months | aPDT (p < 0.05) at 3 months | aPDT (p < 0.05) at 3 months | - | - | - |
| Noro Filho et al. (2012) [31] | HIV | aPDT (p < 0.05) at 6 months | aPDT (p < 0.05) at 3 and 6 months | NHE | NHE | - | NHE |
| Giannelli et al. (2012) [26] | Periodontitis | aPDT (p < 0.001) at 12 months | aPDT (p < 0.001) at 12 months | aPDT (p < 0.001) at 12 months | |||
| Al-Zahrani et al. (2009) [13] | Diabetes Mellitus | NHE | NHE | NHE | NHE | - | - |
| Authors and Year | Selected Articles and Study Participants (n) | Conclusion |
|---|---|---|
| Jervøe-Storm et al. (2024) [32] | 50 selected articles (n = 1407) | The available evidence is quite limited, making it difficult to draw definitive conclusions about the superior clinical benefits of aPDT as an adjunctive therapy in the active treatment or maintenance of periodontitis. Furthermore, the data suggest that the observed improvements may be too small to hold clinical relevance. To enhance the reliability of these findings, it is essential to conduct large, well-designed, and rigorously evaluated randomized controlled trials (RCTs), taking into account the variability of outcomes over time. |
| Alasqah et al. (2024) [33] | 11 selected articles (n= 455) *** | Methylene blue-mediated antimicrobial photodynamic therapy (aPDT) resulted in statistically significant improvements in clinical parameters, including plaque index (PI), probing depth (PD), and bleeding on probing (BOP) in patients with periodontitis. However, no significant differences were observed in clinical attachment level (CAL) when compared to conventional treatment alone. Due to the heterogeneity of protocols and methodological limitations of the included studies, the authors recommend cautious interpretation of the findings and emphasize the need for further randomized clinical trials with standardized protocols and long-term follow-up to validate the efficacy of aPDT. |
| Salvi et al. (2020) [4] | 17 selected articles (n = 370) | The available evidence on adjunctive therapy with lasers and aPDT is limited to a small number of controlled studies, with notable variability in study designs. |
| Chambrone; Wang; Romanos, (2018) [34] | 26 selected articles (n = 686) | aPDT may provide additional clinical benefits in patients with periodontitis and peri-implantitis, particularly in reducing probing depth (PD) and improving clinical attachment level (CAL). However, these improvements were modest (generally less than 1 mm), and the quality of the available evidence was rated as low to moderate. Therefore, the authors recommend cautious interpretation of the findings and emphasize that most clinical recommendations in favor of aPDT are still primarily based on expert opinion. Further studies with greater methodological rigor are needed to definitively validate its efficacy. |
| Akram et al. (2017) [35] | 5 selected articles (n = 159) | Meta-analysis demonstrated a statistically significant gain in clinical attachment level (CAL) (WMD = 0.60; 95% CI: 0.25 to 0.95; p = 0.001), but not in probing pocket depth (PPD) reduction (WMD = 0.67; 95% CI: –0.36 to 1.71; p = 0.204), when comparing aPDT to adjunctive antibiotic therapy at follow-up. Whether aPDT is more effective than adjunctive antibiotic therapy in the treatment of periodontitis remains inconclusive, as the current body of evidence is weak. Caution is warranted in interpreting these findings due to the small sample size and high heterogeneity across studies. |
| Xue et al. (2017) [36] | 11 selected articles (n = 243) | aPDT provides short-term clinical benefits in patients with chronic periodontitis, primarily in the reduction in probing depth (PD) and, to a lesser extent, in clinical attachment level (CAL) gain. These effects were statistically significant at 3 months, but were not consistently sustained at 6 months. Furthermore, the benefits were more pronounced in non-smoking patients. |
| Xue; Zhao, (2017) [37] | 4 selected articles (n = 62) | aPDT provides additional clinical benefits in the treatment of residual periodontal pockets in patients with chronic periodontitis undergoing supportive periodontal therapy. Pooled data from four randomized clinical trials demonstrated statistically significant reductions in probing depth (MD = 0.69 mm) and gains in clinical attachment level (MD = 0.60 mm) when compared to scaling and root planing (SRP) alone. However, these effects were significant only in non-smoking patients, as studies including smokers did not reveal clinically relevant differences between treatment modalities |
| Vohra et al. (2016) [38] | 7 selected articles (n = 218) | In the use of aPDT for the treatment of aggressive periodontitis, the authors concluded that this therapeutic approach may be effective as an adjunct to SRP. However, further randomized clinical trials are needed to confirm these findings. |
| Javed et al. (2013) [39] | 6 selected articles (n = 615 and 270 *) | aPDT was analyzed as an adjunct to non-surgical periodontal therapy in immunocompromised patients. After review, only six articles were found, of which only one was a randomized clinical trial; the others were laboratory studies conducted in rats. Various factors, such as smoking and poor oral hygiene, may interfere with the outcomes, making it difficult to assess the effectiveness of this therapy. In conclusion, further studies are needed. |
| Sgolastra et al. (2013) [40] | 7 selected articles (n = 261) | After evaluating seven articles, the clinical outcomes were found to be modest, indicating a lack of scientific evidence and the need for further studies to assess the efficacy of aPDT as an adjunct to SRP. |
| Sgolastra et al. (2013) [41] | 14 selected articles (n = 389) | A more rigorous systematic review was recently published, including 14 studies, but without promising results. aPDT may have short-term effects, as the evidence does not indicate significant differences after six months. Therefore, the authors recommend conducting additional clinical trials with long-term follow-up. |
| Atieh, 2010 [42] | 4 selected articles (n = 161) | The analysis included only four articles, with a post-therapy follow-up of three weeks. The results showed a clinical gain of 0.29 mm in attachment level and a reduction of 0.11 mm in probing depth. The authors concluded that the use of aPDT may be beneficial, but they cautioned about the limitation of the small number of studies included. |
| Azarpazhooh et al. (2010) [43] | 5 selected articles (n = 74 and 62 **) | The review included five studies, three of which were similar to Atieh, 2010, [42] without distinguishing the types of periodontitis between them, considering studies with follow-up at 3 and 6 months. The results showed a minimal gain in clinical attachment (0.34 mm) and a reduction in probing depth (0.25 mm). The authors concluded that aPDT was not shown to be more effective. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, H.H.C.; Chicrala-Toyoshima, G.M.; Damante, C.A.; Ferreira, R. Blue Photosensitizer, Red Light, Clear Results: An Integrative Review of the Adjunctive Periodontal Treatment with Methylene Blue in Antimicrobial Photodynamic Therapy. Dent. J. 2025, 13, 289. https://doi.org/10.3390/dj13070289
Oliveira HHC, Chicrala-Toyoshima GM, Damante CA, Ferreira R. Blue Photosensitizer, Red Light, Clear Results: An Integrative Review of the Adjunctive Periodontal Treatment with Methylene Blue in Antimicrobial Photodynamic Therapy. Dentistry Journal. 2025; 13(7):289. https://doi.org/10.3390/dj13070289
Chicago/Turabian StyleOliveira, Higor Henrique Carvalho, Gabriela Moura Chicrala-Toyoshima, Carla Andreotti Damante, and Rafael Ferreira. 2025. "Blue Photosensitizer, Red Light, Clear Results: An Integrative Review of the Adjunctive Periodontal Treatment with Methylene Blue in Antimicrobial Photodynamic Therapy" Dentistry Journal 13, no. 7: 289. https://doi.org/10.3390/dj13070289
APA StyleOliveira, H. H. C., Chicrala-Toyoshima, G. M., Damante, C. A., & Ferreira, R. (2025). Blue Photosensitizer, Red Light, Clear Results: An Integrative Review of the Adjunctive Periodontal Treatment with Methylene Blue in Antimicrobial Photodynamic Therapy. Dentistry Journal, 13(7), 289. https://doi.org/10.3390/dj13070289












