The Effectiveness and Complication Rate of Resorbable Biopolymers in Oral Surgery: A Systematic Review
Abstract
1. Introduction
- Osteoconductive: Serving as a scaffold for new bone growth, guiding the regenerative processes of native bone.
- Osteoinductive: Stimulating the formation of new osteoblasts to accelerate regeneration.
- Osteopromotive: Enhancing the osteoinductive effect of other grafts or membranes [15].
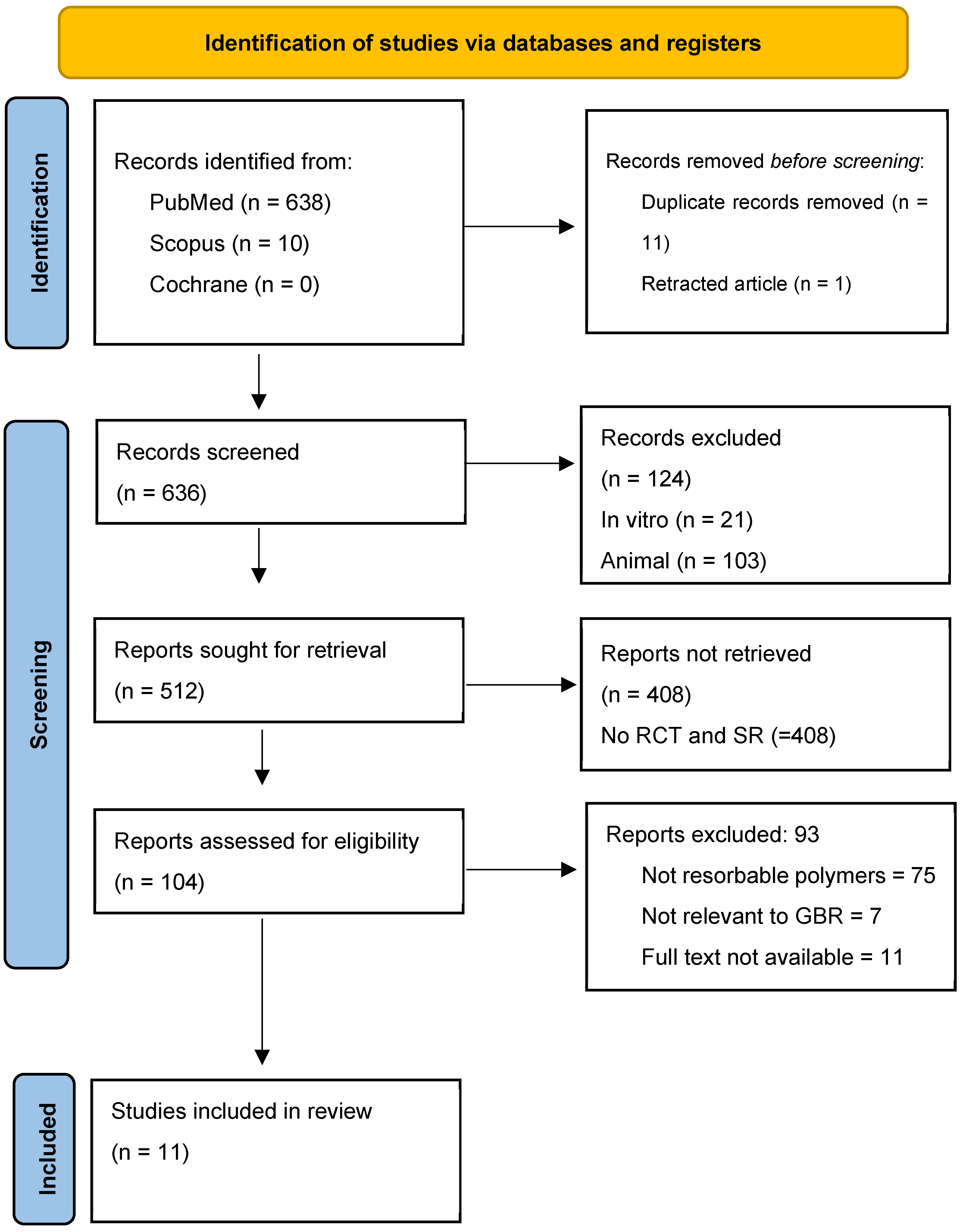
2. Materials and Methods
2.1. Search Strategy
- POPULATION: Patients requiring a bone augmentation procedure for implant dentistry.
- INTERVENTION: Bone augmentation using synthetic resorbable polymers.
- COMPARISON: Comparison among different types of resorbable polymers.
- OUTCOME: Effective bone augmentation (with evidence of effective volume measures in the interval time included) and reported complications related to the procedure.
- Human RCT studies focusing on regenerative surgery with at least a minimum number of 10 subjects included.
- No restriction for the anatomical site.
- Use of synthetic biopolymers.
2.2. Risk of Bias Assessment
3. Results
3.1. Are Resorbable Biopolymers Used for Regenerative Surgery?
3.2. Are Resorbable Biopolymers Performing Better than Conventional Materials?
3.3. Are There Any Complications Reported?
4. Discussion
Complications Associated with Resorbable Biopolymers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| GBR | Guided Bone Regeneration |
| GTR | Guided Tissue Regeneration |
| RCT | Randomized Controlled Trial |
| PLA | Polylactic Acid |
| PCL | Polycaprolactone |
| PEG | Polyethylene Glycol |
| PRGF | Platelet-Rich Growth Factors |
| PRF | Platelet-Rich Fibrin |
| PRP | Platelet-Rich Plasma |
| TCP | Tricalcium Phosphate |
| MMP | Matrix Metalloproteinases |
| ORC | Oxidized Regenerated Cellulose |
| PDLA | Poly-D-Lactic Acid |
| ABM/P-15 | Anorganic Bone Matrix/Peptide-15. |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PDL | Periodontal Ligament |
| CA | Citric Acid |
References
- Åkerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D-Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yadav, D.; Garg, R.K.; Ahlawat, A.; Chhabra, D. 3D printable biomaterials for orthopedic implants: Solution for sustainable and circular economy. Resour. Policy 2020, 68, 101767. [Google Scholar] [CrossRef]
- Thompson, D.M.; Rohrer, M.D.; Prasad, H.S. Comparison of bone grafting materials in human extraction sockets: Clinical, histologic, and histomorphometric evaluations. Implant. Dent. 2006, 15, 89–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zwahlen, R.A.; Cheung, L.K.; Zheng, L.W.; Chow, R.L.; Li, T.; Schuknecht, B.; Grätz, K.W.; Weber, F.E. Comparison of two resorbable membrane systems in bone regeneration after removal of wisdom teeth: A randomized-controlled clinical pilot study. Clin. Oral Implants Res. 2009, 20, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Mijiritsky, E.; Canullo, L.; Menini, M.; Caponio, V.C.A.; Grassi, A.; Gobbato, L.; Baldi, D. An Analysis of Different Techniques Used to Seal Post-Extractive Sites-A Preliminary Report. Dent. J. 2022, 10, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Angelis, N.; Kassim, Z.; Mohd Yusof, E.; Yumang, C.; Menini, M. Bone Augmentation Techniques with Customized Titanium Meshes: A Systematic Review of Randomized Clinical Trials. Open Dent. J. 2023, 17, e187421062302201. [Google Scholar] [CrossRef]
- Menini, M.; Canullo, L.; Iacono, R.; Triestino, A.; Caponio, V.C.A.; Savadori, P.; Pesce, P.; Pedetta, A.; Guerra, F. Effect of Different Graft Material Consistencies in the Treatment of Minimal Bone Dehiscence: A Retrospective Pilot Study. Dent. J. 2024, 12, 198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rakhmatia, Y.D.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Koyano, K. Fibroblast attachment onto novel titanium mesh membranes for guided bone regeneration. Odontology 2015, 103, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Greco, G.B.; Cinci, L.; Pieri, L. Horizontal-guided Bone Regeneration using a Titanium Mesh and an Equine Bone Graft. J. Contemp. Dent. Pract. 2015, 16, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restorative Dent. 2011, 31, 265–273. [Google Scholar] [PubMed]
- Meza-Mauricio, J.; Furquim, C.P.; Dos Reis, L.D.; Maximiano, M.M.; Mendoza-Azpur, G.; Muniz, F.W.; Rasperini, G.; Faveri, M. How efficacious is the combination of substitute bone graft with autogenous bone graft in comparison with substitute bone graft alone in the horizontal bone gain? A systematic review and meta-analysis. J. Clin. Exp. Dent. 2022, 14, e678–e688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ram, A.; Duncan, W.J.; Coates, D.E.; Nobakht, S.; Tkatchenko, T.; Milne, T.J. Bone remodelling marker expression in grafted and ungrafted sheep tooth extraction sockets: A comparative randomised study. Arch. Oral Biol. 2023, 153, 105738. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Menini, M.; Canullo, L.; Khijmatgar, S.; Modenese, L.; Gallifante, G.; Del Fabbro, M. Radiographic and Histomorphometric Evaluation of Biomaterials Used for Lateral Sinus Augmentation: A Systematic Review on the Effect of Residual Bone Height and Vertical Graft Size on New Bone Formation and Graft Shrinkage. J. Clin. Med. 2021, 10, 4996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5 (Suppl. S1), S125–S127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, D.B.; Deshpande, N.C.; Dandekar, S.A. Comparative evaluation of platelet-rich fibrin membrane and collagen membrane along with demineralized freeze-dried bone allograft in Grade II furcation defects: A randomized controlled study. J. Indian. Soc. Periodontol. 2018, 22, 322–327. [Google Scholar] [PubMed]
- Kim, J.; McBride, S.; Tellis, B.; Alvarez-Urena, P.; Song, Y.H.; Dean, D.D.; Sylvia, V.L.; Elgendy, H.; Ong, J.; Hollinger, J.O. Rapid-prototyped PLGA/β-TCP/hydroxyapatite nanocomposite scaffolds in a rabbit femoral defect model. Biofabrication 2012, 4, 025003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. Multifunctional cellulose-based biomaterials for dental applications: A sustainable approach to oral health and regeneration. Ind. Crops Prod. 2023, 203, 117142. [Google Scholar] [CrossRef]
- Emecen, P.; Akman, A.C.; Hakki, S.S.; Hakki, E.E.; Demiralp, B.; Tözüm, T.F.; Nohutcu, R.M. ABM/P-15 modulates proliferation and mRNA synthesis of growth factors of periodontal ligament cells. Acta Odontol. Scand. 2009, 67, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Tommasato, G.; Pesce, P.; Ravidà, A.; Khijmatgar, S.; Sculean, A.; Galli, M.; Antonacci, D.; Canullo, L. Sealing materials for post-extraction site: A systematic review and network meta-analysis. Clin. Oral Investig. 2022, 26, 1137–1154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F. Biodegradable Polymers for Biomedical Additive Manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Simeone, D.; Torge, D.; Macchiarelli, G.; Marchetti, E. Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials 2022, 15, 8284. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cucchi, A.; Vignudelli, E.; Fiorino, A.; Pellegrino, G.; Corinaldesi, G. Vertical ridge augmentation (VRA) with Ti-reinforced d-PTFE membranes or Ti meshes and collagen membranes: 1-year results of a randomized clinical trial. Clin. Oral Implant. Res. 2021, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.-M.; Sufaru, I.-G.; Teslaru, S.; Ghiciuc, C.M.; Stafie, C.S. Finding the Perfect Membrane: Current Knowledge on Barrier Membranes in Regenerative Procedures: A Descriptive Review. Appl. Sci. 2022, 12, 1042. [Google Scholar] [CrossRef]
- Shahdad, S.; Gamble, E.; Matani, J.; Zhang, L.; Gambôa, A. Randomized clinical trial comparing PEG-based synthetic to porcine-derived collagen membrane in the preservation of alveolar bone following tooth extraction in anterior maxilla. Clin. Oral Implant. Res. 2020, 31, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Hälg, G.A.; Thoma, D.S.; Hämmerle, C.H. A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clin. Oral Implant. Res. 2009, 20, 162–168. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, N.; Pesce, P.; Poedjiastoeti, W.; Suwandi, T.; Tjandrawinata, R.; Bagnasco, F.; Menini, M. 3D-Printable Biopolymers for Socket Preservation Technique: Soft Tissues Response: A Pilot Randomised Clinical Study. Dent. J. 2024, 12, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saini, A.; Singh, M.; Lal, N.; Dixit, J. Assessment of combination techniques in enhancing the regenerative potential of tricalcium phosphate graft in treatment of infrabony periodontal defects. Indian J. Dent. Res. 2011, 22, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Shetty, S.K.; Garg, G.; Pai, S. Clinical effectiveness of autologous platelet-rich plasma and Peptide-enhanced bone graft in the treatment of intrabony defects. J. Periodontol. 2009, 80, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Pereira, I.; Costa, F.; Brandão, A.; Pereira, J.E.; Maurício, A.C.; Santos, J.D.; Amaro, I.; Falacho, R.; Coelho, R.; et al. Randomized clinical study of injectable dextrin-based hydrogel as a carrier of a synthetic bone substitute. Clin. Oral Investig. 2023, 27, 979–994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kusirisin, T.; Suwanprateeb, J.; Buranawat, B. Polycaprolactone versus collagen membrane and 1-year clinical outcomes: A randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2023, 25, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Mihatovic, I.; Cordaro, L.; Windisch, P.; Friedmann, A.; Blanco Carrion, J.; Sanz Sanchez, I.; Hallman, M.; Quirynen, M.; Hammerle, C.H.F. Comparison of a polyethylene glycol membrane and a collagen membrane for the treatment of bone dehiscence defects at bone level implants-A prospective, randomized, controlled, multicenter clinical trial. Clin. Oral Implant. Res. 2020, 31, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Santana, R.; Gyurko, R.; Kanasi, E.; Xu, W.P.; Dibart, S. Synthetic polymeric barrier membrane associated with blood coagulum, human allograft, or bovine bone substitute for ridge preservation: A randomized, controlled, clinical and histological trial. Int. J. Oral Maxillofac. Surg. 2019, 48, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Arunjaroensuk, S.; Panmekiate, S.; Pimkhaokham, A. The Stability of Augmented Bone Between Two Different Membranes Used for Guided Bone Regeneration Simultaneous with Dental Implant Placement in the Esthetic Zone. Int. J. Oral Maxillofac. Implant. 2018, 33, 206–216. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, N.; Amaroli, A.; Sabbieti, M.G.; Cappelli, A.; Lagazzo, A.; Pasquale, C.; Barberis, F.; Agas, D. Tackling Inequalities in Oral Health: Bone Augmentation in Dental Surgery through the 3D Printing of Poly(ε-caprolactone) Combined with 20% Tricalcium Phosphate. Biology 2023, 12, 536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venkatesan, J.; Kim, S.K. Nano-hydroxyapatite composite biomaterials for bone tissue engineering—A review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ma, B.J.; Shao, J.L.; Ge, S.H. Advances in the Application of Hydroxyapatite Composite Materials in Bone Tissue Engineering. Sichuan Da Xue Xue Bao Yi Xue Ban 2021, 52, 357–363. (In Chinese) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehrotra, D.; Dwivedi, R.; Nandana, D.; Singh, R.K. From injectable to 3D printed hydrogels in maxillofacial tissue engineering: A review. J. Oral Biol. Craniofac. Res. 2020, 10, 680–689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, J.X.; Raghavan, P.; Desai, T.A. Harnessing Biomaterials for Immunomodulatory-Driven Tissue Engineering. Regen. Eng. Transl. Med. 2023, 9, 224–239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Montagna, F.; Maiullaro, T.; Sannino, A.; Maffezzoli, A. 3D printing of hydroxyapatite polymer-based composites for bone tissue engineering. J. Polym. Eng. 2017, 37, 741–746. [Google Scholar] [CrossRef]
- Nyika, J.; Mwema, F.M.; Mahamood, R.M.; Akinlabi, E.T.; Jen, T.C. Advances in 3D printing materials processing-environmental impacts and alleviation measures. Adv. Mater. Process. Technol. 2021, 8 (Suppl. S3), 1275–1285. [Google Scholar] [CrossRef]
| Author | Article Title | Description | Number of Patients | Time of Follow Up | Key Findings | Complications Reported | Conclusions |
|---|---|---|---|---|---|---|---|
| Shahdad et al. [28] | Randomized clinical trial comparing PEG-based synthetic to porcine-derived collagen membrane in the preservation of alveolar bone following tooth extraction in anterior maxilla | The aim of this randomized controlled clinical trial was to compare alveolar bone preservation covered by either a synthetic membrane or a porcine collagen membrane. | 30 | 6 months | PEG membrane resulted in significantly lower percentage loss in both labial and coronal dimensions compared to porcine collagen. Implant placement was comparable in both groups. | No major complications reported. | Use of PEG membrane led to a better preservation of ridge dimensions following extraction. |
| Jung et al. [29] | A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants | The aim of this study was to evaluate a synthetic resorbable hydrogel membrane based on polyethylene glycol (PEG) that could provide vertical bone fill comparable to that of a standard collagen membrane. | 37 | 6 months | Vertical bone gain after 6 months was similar between PEG and collagen membranes (5.63 mm vs. 4.25 mm). PEG showed slightly more soft tissue issues but allowed for simplified application. | More soft tissue complications with PEG membrane (e.g., delayed/incomplete healing) but allowed for simplified application. All complications were resolved uneventfully. | PEG membrane achieved comparable regenerative outcomes to collagen with easier handling despite minor soft tissue complications. |
| Zwahlen et al. [4] | A comparison of two resorbable membrane systems in bone regeneration after the removal of wisdom teeth: a randomized controlled clinical pilot study | Randomized, prospective, and partially blinded pilot study, 15 patients received biodegradable membrane system Inion GTR™ on one side and the double-layer resorbable membrane Bio-Gide® by Geistlich on the other. | 15 | 3–6 months | Bone biopsies showed similar new bone formation between Inion (synthetic) and Bio-Gide (xenogenic) membranes. CT and histological analysis revealed no statistically significant differences in bone regeneration or membrane performance. | Mild adverse events (wound infection, hematoma, late swelling) in three patients; healing generally uneventful. | Both membranes performed similarly in terms of safety and regenerative capacity. The main difference lies in the origin: synthetic vs. animal. |
| De Angelis et al. [30] | 3D-Printable Biopolymers for Socket Preservation Technique: Soft Tissues Response: A Pilot Randomised Clinical Study | Randomized study on the healing of post-extraction sites with 3D-printed biomaterials vs. control group. | 39 | 6 weeks | Both 3D-printed biopolymers (PLA+HA and PCL+β-TCP) significantly outperformed the open healing control group in soft tissue closure for posterior sockets (p < 0.05). Anterior sockets healed fully in all groups by 4 weeks, with no significant differences. | No complications reported. Healing was uneventful in all 39 patients. | 3D-printed biopolymer membranes effectively promote soft tissue closure after extraction, particularly in posterior sites. |
| Saini et al. [31] | Assessment of combination techniques in enhancing the regenerative potential of tricalcium phosphate graft in treatment of infrabony periodontal defects | The aim of the present study was to evaluate and compare the clinical outcomes after reconstructive surgery using tricalcium phosphate (TCP) alone; TCP and root conditioning with citric acid (CA); and TCP, CA, and an oxidized regenerated cellulose (ORC) membrane. | 39 | 6 months | All groups (TCP alone, TCP + CA, TCP + CA + ORC) showed significant improvements in PPD reduction, CAL gain, and defect fill. No significant intergroup differences. | No significant complications reported. | TCP combination therapies are at least as effective as TCP alone for infrabony defect regeneration. |
| Pradeep et al. [32] | Clinical effectiveness of autologous platelet-rich plasma and Peptide-enhanced bone graft in the treatment of intrabony defects | This study was conducted to compare the effectiveness of two regenerative techniques (autologous PRP plus ABM/P-15 versus autologous PRP alone) in the treatment of intraosseous defects in humans, analyzing clinical and radiological parameters. | 28 | 9 months | PRP + ABM/P-15 showed statistically significant improvement in clinical and radiologic outcomes versus PRP alone. Greater bone fill observed on CT in the test group. | No significant complications reported. | PRP + ABM/P-15 is more effective than PRP alone for treating intrabony defects. Larger studies needed. |
| Machado et al. [33] | Randomized clinical study of injectable dextrin-based hydrogel as a carrier of a synthetic bone substitute | The aim of this randomized controlled clinical trial was to compare alveolar ridge preservation outcomes using a synthetic bone substitute alone or combined with a dextrin-based injectable hydrogel. | 12 | 6 months | DEXGEL Bone showed greater granule resorption, better handling, and improved implant primary stability. Similar bone volume and density compared to control. | No local or systemic complications observed. | DEXGEL Bone is a safe and effective injectable carrier that enhances bone substitute handling and implant stability. |
| Kusirisin et al. [34] | Polycaprolactone versus collagen membrane and 1-year clinical outcomes: a randomized controlled trial | The aim of this randomized controlled clinical trial was to evaluate and compare the outcomes of guided bone regeneration using a bilayered polycaprolactone membrane versus a collagen membrane over a 1-year period following implant placement. | 24 | 1 year | PCL membrane showed similar buccal bone thickness (BBT) and soft tissue dimensional change (STC) outcomes compared to collagen membrane; no statistically significant differences at 1-year follow-up. | Four early membranes exposed were found in the test group and three in the control group at 2 weeks after surgery. No other biological complications were seen during the study periods. | PCL membrane provides comparable outcomes to collagen membrane for GBR with simultaneous implant placement; further studies with larger sample size are needed. |
| Jung et al. [35] | Comparison of a polyethylene glycol membrane and a collagen membrane for the treatment of bone dehiscence defects at bone level implants—a prospective, randomized, controlled, multicenter clinical trial | The aim of this randomized controlled multicenter clinical trial was to evaluate and compare the clinical outcomes of guided bone regeneration using a polyethylene glycol membrane versus a collagen membrane in the treatment of bony dehiscence defects at bone level implants. | 117 | 18 months | PEG and collagen membranes both resulted in vertical bone fill (59.7% PEG vs. 64.4% BG). The non-inferiority of PEG could not be demonstrated; MBL slightly increased in both groups. | Soft tissue complications occurred in both PEG and collagen groups, without significant differences. | Both membranes supported bone regeneration, but PEG could not be shown to be non-inferior to collagen. |
| Santana et al. [36] | Synthetic polymeric barrier membrane associated with blood coagulum, human allograft, or bovine bone substitute for ridge preservation: a randomized, controlled, clinical and histological trial | The aim of this randomized controlled clinical and histological trial was to assess the extent of alveolar ridge preservation following socket grafting with blood coagulum, human allograft, or bovine bone substitute, each covered by a synthetic polyethylene glycol membrane. | 32 | 6 months | Use of AL + PEG membrane preserved ridge width (1.5 mm) better than BB (2.5 mm) or BC (2.3 mm). New bone formation was highest in BC (47.8%), followed by AL (33.3%) and BB (28.2%). | Post- surgical complications were minimal for all treatment modalities tested. | Alveolar bone preservation was best achieved using AL with PEG barrier. Different graft materials yielded different bone formation percentages. |
| Arunjaroensuk et al. [37] | The Stability of Augmented Bone Between Two Different Membranes Used for Guided Bone Regeneration Simultaneous with Dental Implant Placement in the Esthetic Zone | The aim of this randomized controlled clinical trial was to compare the effectiveness of a synthetic polylactic acid membrane versus a collagen membrane in maintaining buccal bone thickness following simultaneous guided bone regeneration and implant placement. | 48 | 6 months | Both PLA (synthetic) and collagen membranes showed comparable reductions in facial bone thickness at all levels (0–6 mm apical to implant shoulder). No statistically significant differences. | Minor complications of gingival inflammation and membrane exposure were observed in three cases in the test group and two cases in the control group, but all sites recovered uneventfully. | PLA membrane is as effective as collagen membrane in maintaining stable augmented bone in the esthetic zone. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabozzi, R.; Bianchetti, F.; Baldi, D.; Sanchez, C.Y.; Bagnasco, F.; De Angelis, N. The Effectiveness and Complication Rate of Resorbable Biopolymers in Oral Surgery: A Systematic Review. Dent. J. 2025, 13, 264. https://doi.org/10.3390/dj13060264
Fabozzi R, Bianchetti F, Baldi D, Sanchez CY, Bagnasco F, De Angelis N. The Effectiveness and Complication Rate of Resorbable Biopolymers in Oral Surgery: A Systematic Review. Dentistry Journal. 2025; 13(6):264. https://doi.org/10.3390/dj13060264
Chicago/Turabian StyleFabozzi, Riccardo, Francesco Bianchetti, Domenico Baldi, Catherine Yumang Sanchez, Francesco Bagnasco, and Nicola De Angelis. 2025. "The Effectiveness and Complication Rate of Resorbable Biopolymers in Oral Surgery: A Systematic Review" Dentistry Journal 13, no. 6: 264. https://doi.org/10.3390/dj13060264
APA StyleFabozzi, R., Bianchetti, F., Baldi, D., Sanchez, C. Y., Bagnasco, F., & De Angelis, N. (2025). The Effectiveness and Complication Rate of Resorbable Biopolymers in Oral Surgery: A Systematic Review. Dentistry Journal, 13(6), 264. https://doi.org/10.3390/dj13060264









