Electrolytic Cleaning as Part of Surgical Reconstructive Peri-Implantitis Treatment: A Case Series
Abstract
1. Introduction
2. Study Design
3. Patient Information
4. Timeline and Diagnostic Assessment
5. Therapeutic Intervention
5.1. Patient Diagnostics
5.2. Non-Surgical Pre-Treatment
5.3. Surgical Treatment
6. Follow-Up
7. Outcomes
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Schwarz, F.; Cordaro, L.; Derks, J.; Hämmerle, C.H.F.; Heitz-Mayfield, L.J.; Hernández-Alfaro, F.; Meijer, H.J.A.; Naenni, N.; Ortiz-Vigón, A.; et al. Regeneration of alveolar ridge defects. Consensus report of group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 277–286. [Google Scholar] [CrossRef] [PubMed]
- De Waal, Y.C.M.; Raghoebar, G.M.; Meijer, H.J.A.; Winkel, E.G.; Van Winkelhoff, A.J. Implant decontamination with 2% chlorhexidine during surgical peri-implantitis treatment: A randomized, double-blind, controlled trial. Clin. Oral Implant. Res. 2015, 26, 1015–1023. [Google Scholar] [CrossRef]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Stewart, R.E.; Van Winkelhoff, A.J.; Meijer, H.J.A.; Raghoebar, G.M. Erythritol air polishing in the surgical treatment of peri-implantitis: A randomized controlled trial. Clin. Oral Implant. Res. 2022, 33, 184–196. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Heitz, F.; Koong, B.; Huang, T.; Chivers, P. Surgical peri-implantitis treatment with and without guided bone regeneration. A randomized controlled trial. Clin. Oral Implant. Res. 2023, 34, 892–910. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Ortiz-Vigón, A.; Guerrero, A.; Donati, M.; Bressan, E.; Ghensi, P.; Schaller, D.; Tomasi, C.; Karlsson, K.; Abrahamsson, I.; et al. Reconstructive surgical therapy of peri-implantitis: A multicenter randomized controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 921–944. [Google Scholar] [CrossRef]
- Donos, N.; Calciolari, E.; Ghuman, M.; Baccini, M.; Sousa, V.; Nibali, L. The efficacy of bone reconstructive therapies in the management of peri-implantitis. A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 285–316. [Google Scholar] [CrossRef]
- Sahrmann, P.; Ronay, V.; Hofer, D.; Attin, T.; Jung, R.E.; Schmidlin, P.R. In vitro cleaning potential of three different implant debridement methods. Clin. Oral Implant. Res. 2015, 26, 314–319. [Google Scholar] [CrossRef]
- Wei, M.C.T.; Tran, C.; Meredith, N.; Walsh, L.J. Effectiveness of implant surface debridement using particle beams at differing air pressures. Clin. Exp. Dent. Res. 2017, 3, 148–153. [Google Scholar] [CrossRef]
- Gosau, M.; Hahnel, S.; Schwarz, F.; Gerlach, T.; Reichert, T.E.; Bürgers, R. Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Clin. Oral Implant. Res. 2010, 21, 866–872. [Google Scholar] [CrossRef]
- Schlee, M.; Wang, H.; Stumpf, T.; Brodbeck, U.; Bosshardt, D.; Rathe, F. Treatment of Periimplantitis with Electrolytic Cleaning versus Mechanical and Electrolytic Cleaning: 18-Month Results from a Randomized Controlled Clinical Trial. J. Clin. Med. 2021, 10, 3475. [Google Scholar] [CrossRef] [PubMed]
- Ratka, C.; Weigl, P.; Henrich, D.; Koch, F.; Schlee, M.; Zipprich, H. The Effect of In Vitro Electrolytic Cleaning on Biofilm-Contaminated Implant Surfaces. J. Clin. Med. 2019, 8, 1397. [Google Scholar] [CrossRef] [PubMed]
- Assunção, M.A.; Botelho, J.; Machado, V.; Proença, L.; Matos, A.P.A.; Mendes, J.J.; Bessa, L.J.; Taveira, N.; Santos, A. Dental Implant Surface Decontamination and Surface Change of an Electrolytic Method versus Mechanical Approaches: A Pilot In Vitro Study. J. Clin. Med. 2023, 12, 1703. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Peña, P. Electrolytic Surface Decontamination in the Reconstructive Therapy of Peri-Implantitis: Single-Center Outcomes. Int. J. Periodontics Restor. Dent. 2025, 45, 185–198. [Google Scholar] [CrossRef]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Belser, U.C.; Grütter, L.; Vailati, F.; Bornstein, M.M.; Weber, H.; Buser, D. Outcome Evaluation of Early Placed Maxillary Anterior Single-Tooth Implants Using Objective Esthetic Criteria: A Cross-Sectional, Retrospective Study in 45 Patients With a 2- to 4-Year Follow-Up Using Pink and White Esthetic Scores. J. Periodontol. 2009, 80, 140–151. [Google Scholar] [CrossRef]
- Tomasi, C.; Regidor, E.; Ortiz-Vigón, A.; Derks, J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 340–356. [Google Scholar] [CrossRef]
- Aghazadeh, A.; Persson, R.G.; Renvert, S. Impact of bone defect morphology on the outcome of reconstructive treatment of peri-implantitis. Int. J. Implant. Dent. 2020, 6, 33. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Sculean, A.; Nart, J.; Wang, H. Defect angle as prognostic indicator in the reconstructive therapy of peri-implantitis. Clin. Implant. Dent. Relat. Res. 2023, 25, 992–999. [Google Scholar] [CrossRef]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and treatment of peri-implant diseases-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50, 4–76. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, H.; Yun, P.; Kim, Y. Survival analysis of implants after surgical treatment of peri-implantitis based on bone loss severity and surgical technique: A retrospective study. BMC Oral Health 2023, 23, 308. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Citterio, F.; Romandini, M.; Romano, F.; Mariani, G.M.; Buduneli, N.; Aimetti, M. Surface decontamination protocols for surgical treatment of peri-implantitis: A systematic review with meta-analysis. Clin. Oral Implant. Res. 2022, 33, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Louropoulou, A.; Slot, D.E.; Van der Weijden, F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: A systematic review. Clin. Oral Implant. Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef]
- Barão, V.A.; Mathew, M.T.; Assunção, W.G.; Yuan, J.C.; Wimmer, M.A.; Sukotjo, C. The role of lipopolysaccharide on the electrochemical behavior of titanium. J. Dent. Res. 2011, 90, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, Y.; Ling, Z.; Zhao, C.; Xu, A.; He, F. The biofilm removal effect and osteogenic potential on the titanium surface by electrolytic cleaning: An in vitro comparison of electrolytic parameters and five techniques. Clin. Oral Implant. Res. 2024, 35, 454–466. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennström, J.L.; Berglundh, T. Risk for recurrence of disease following surgical therapy of peri-implantitis-A prospective longitudinal study. Clin. Oral Implant. Res. 2020, 31, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, B.; Choi, S.; Kim, Y. Long-term outcomes after peri-implantitis treatment and their influencing factors: A retrospective study. J. Periodontal Implant. Sci. 2021, 52, 194–205. [Google Scholar] [CrossRef]
- Serino, G.; Wada, M.; Mameno, T.; Renvert, S. Two- and ten-year follow-up of patients responding and non-responding to the surgical treatment of peri-implantitis: A retrospective evaluation. Clin. Oral Implant. Res. 2021, 32, 410–421. [Google Scholar] [CrossRef]
- Kaiser, F.; Schwarnweber, D.; Bierbaum, S.; Wolf-Brandstetter, C. Success and side effects of different treatment options in the low current attack of bacterial biofilms on titanium implants. Bioelectrochemistry 2020, 133, 107485. [Google Scholar] [CrossRef]

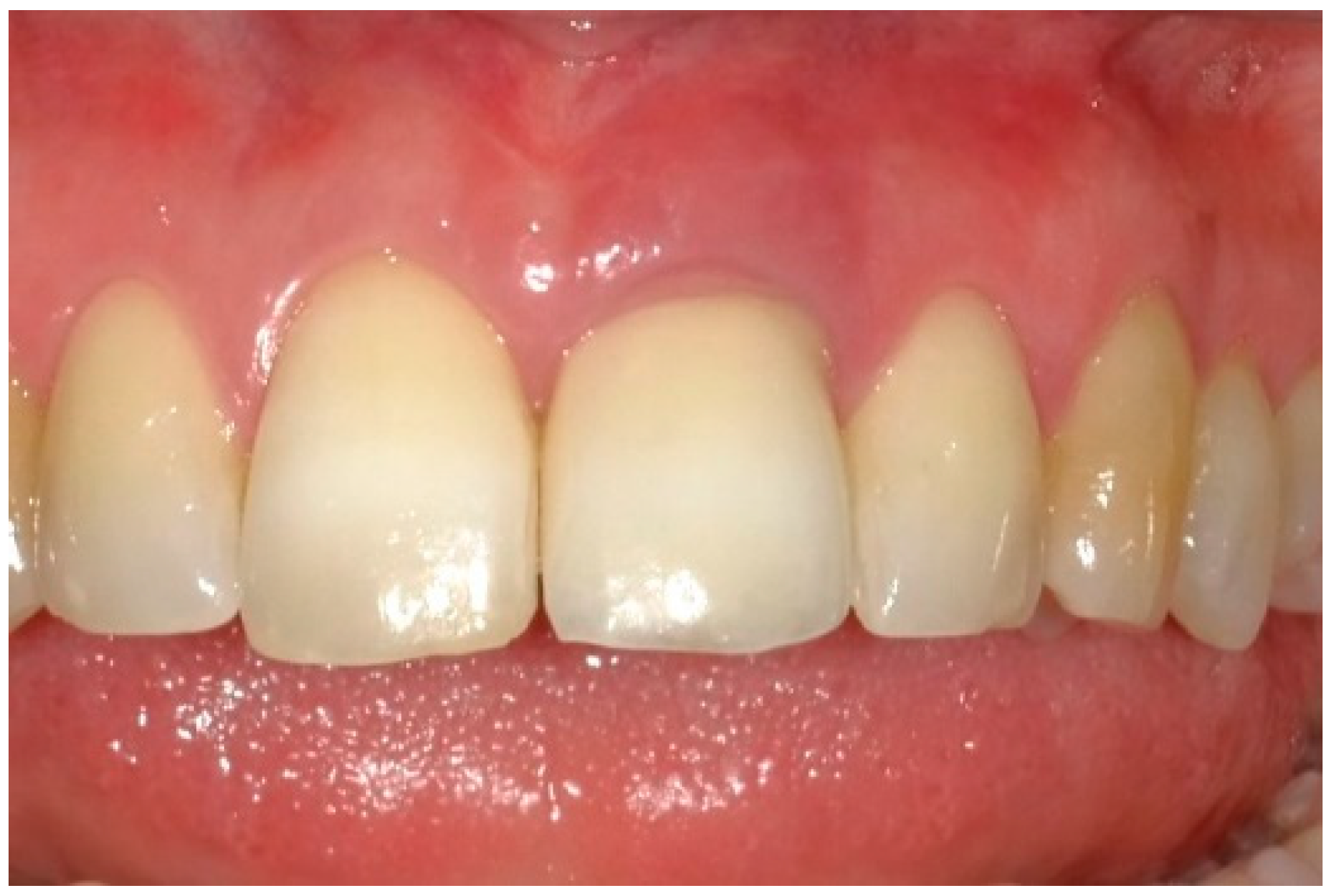
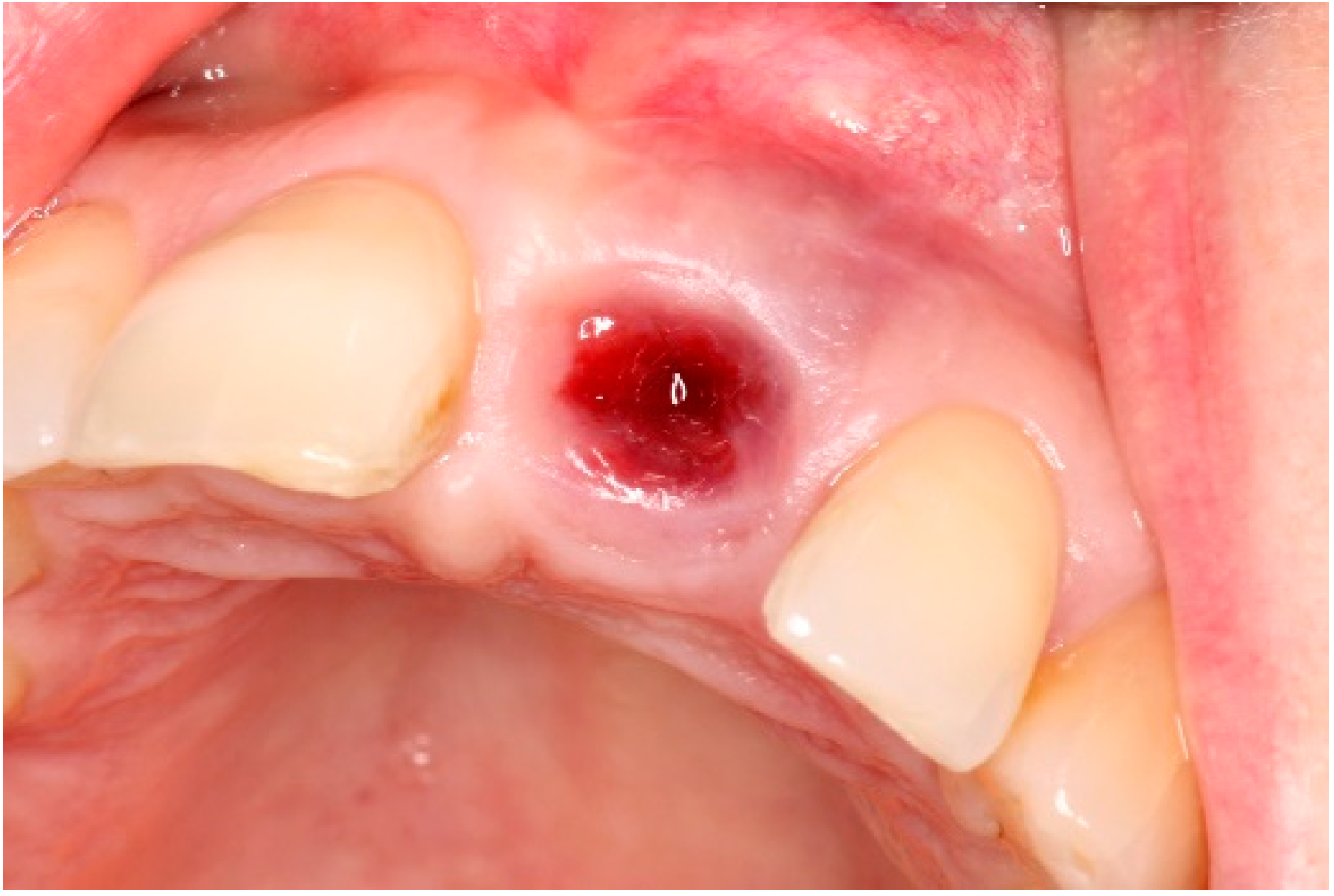
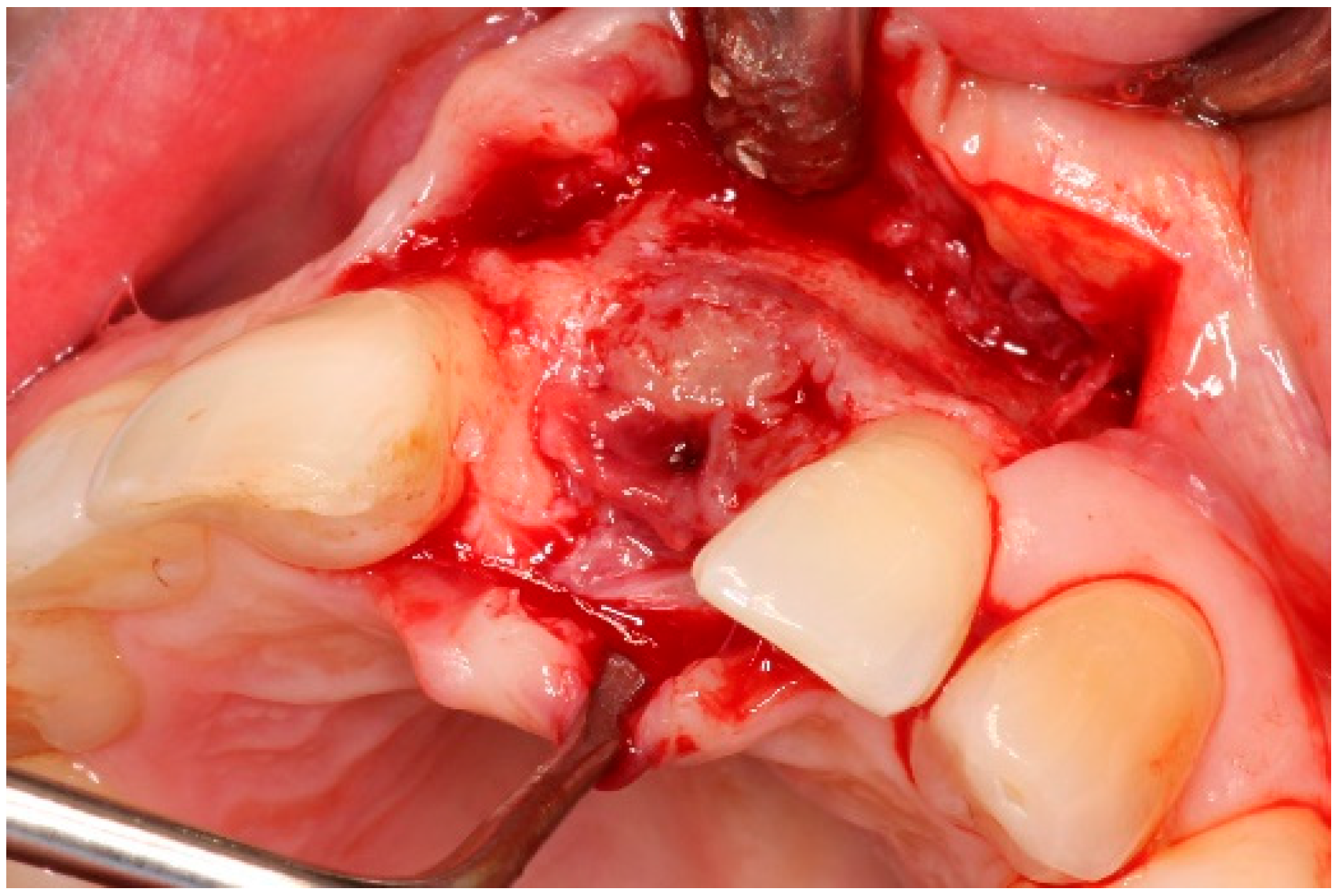
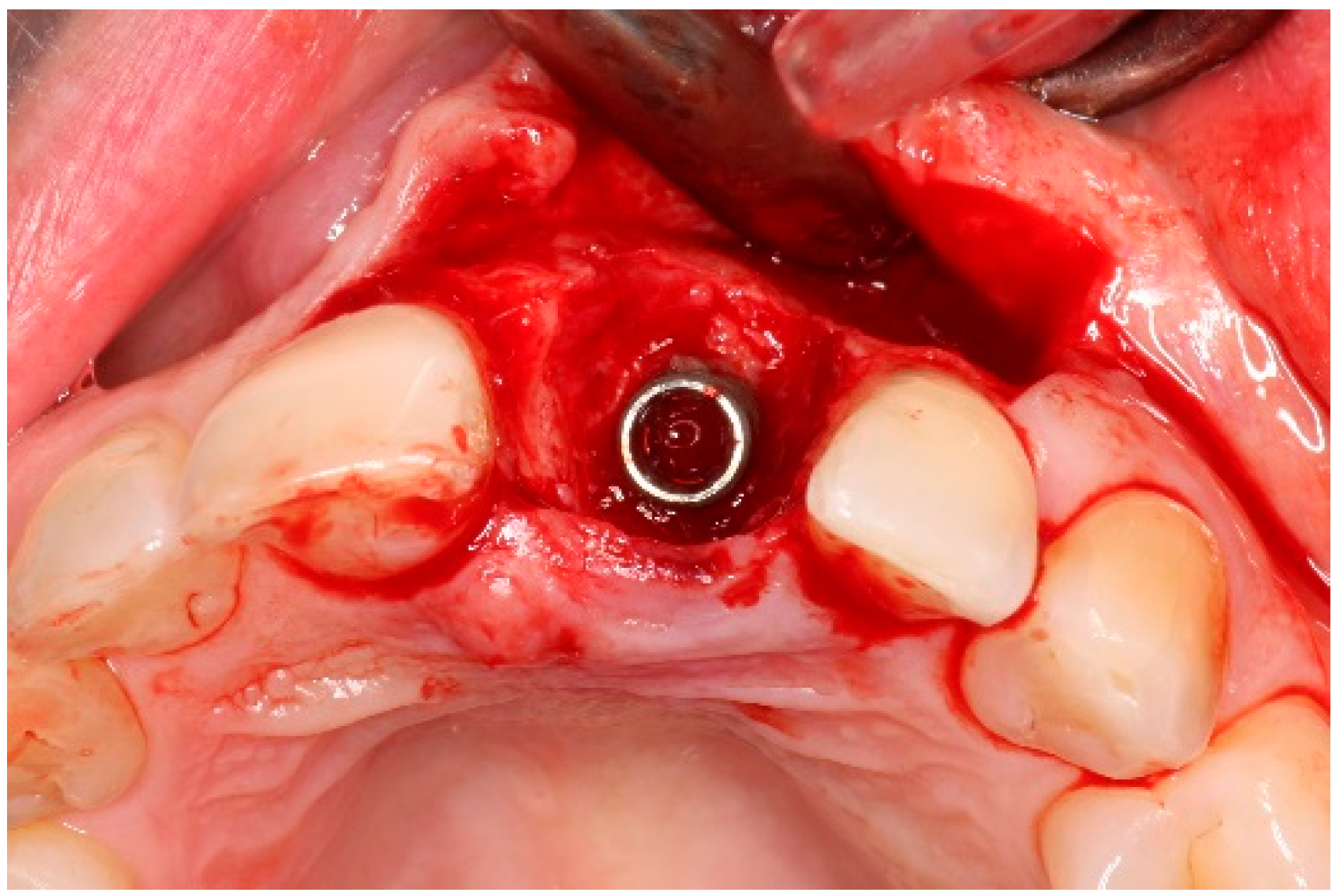
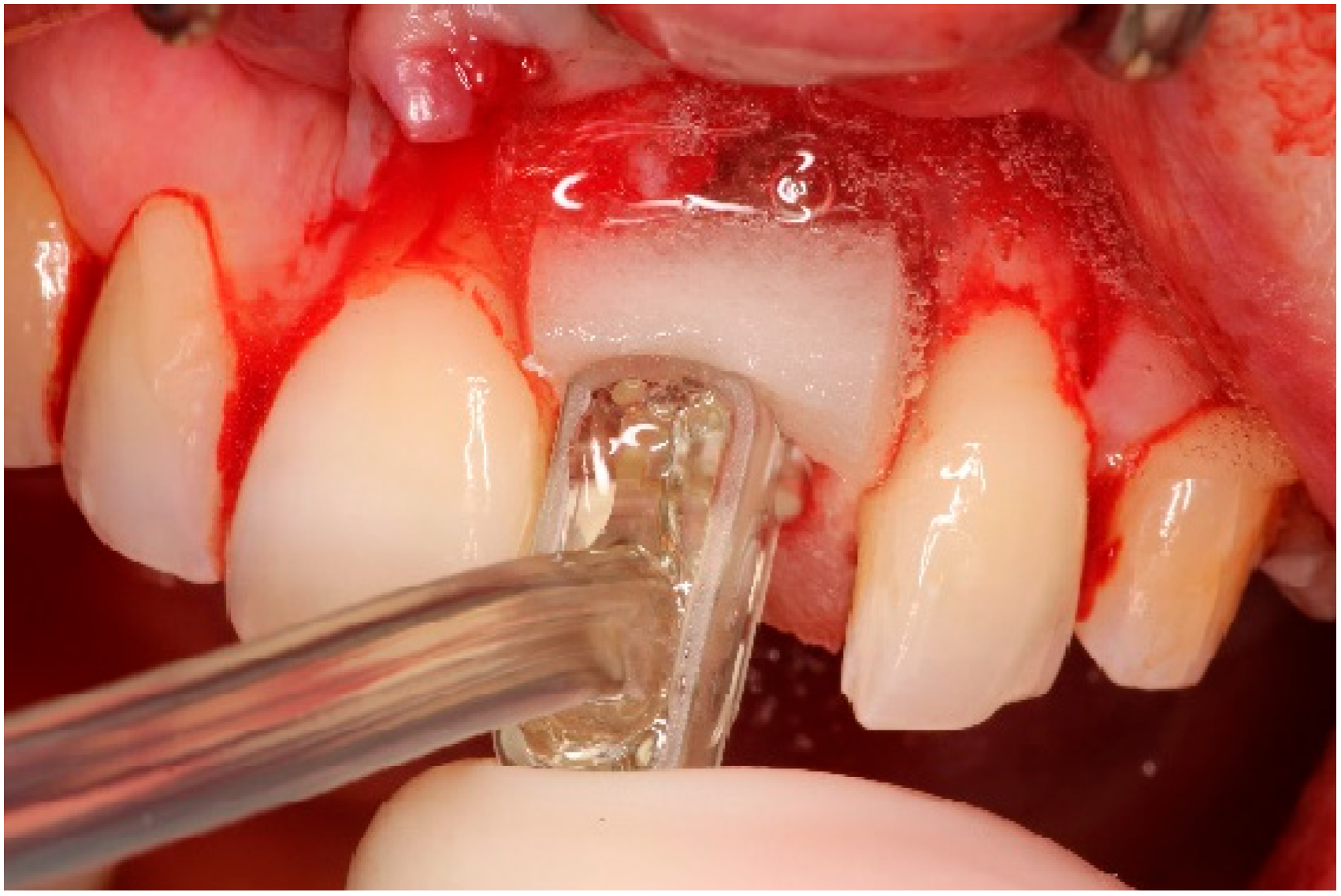

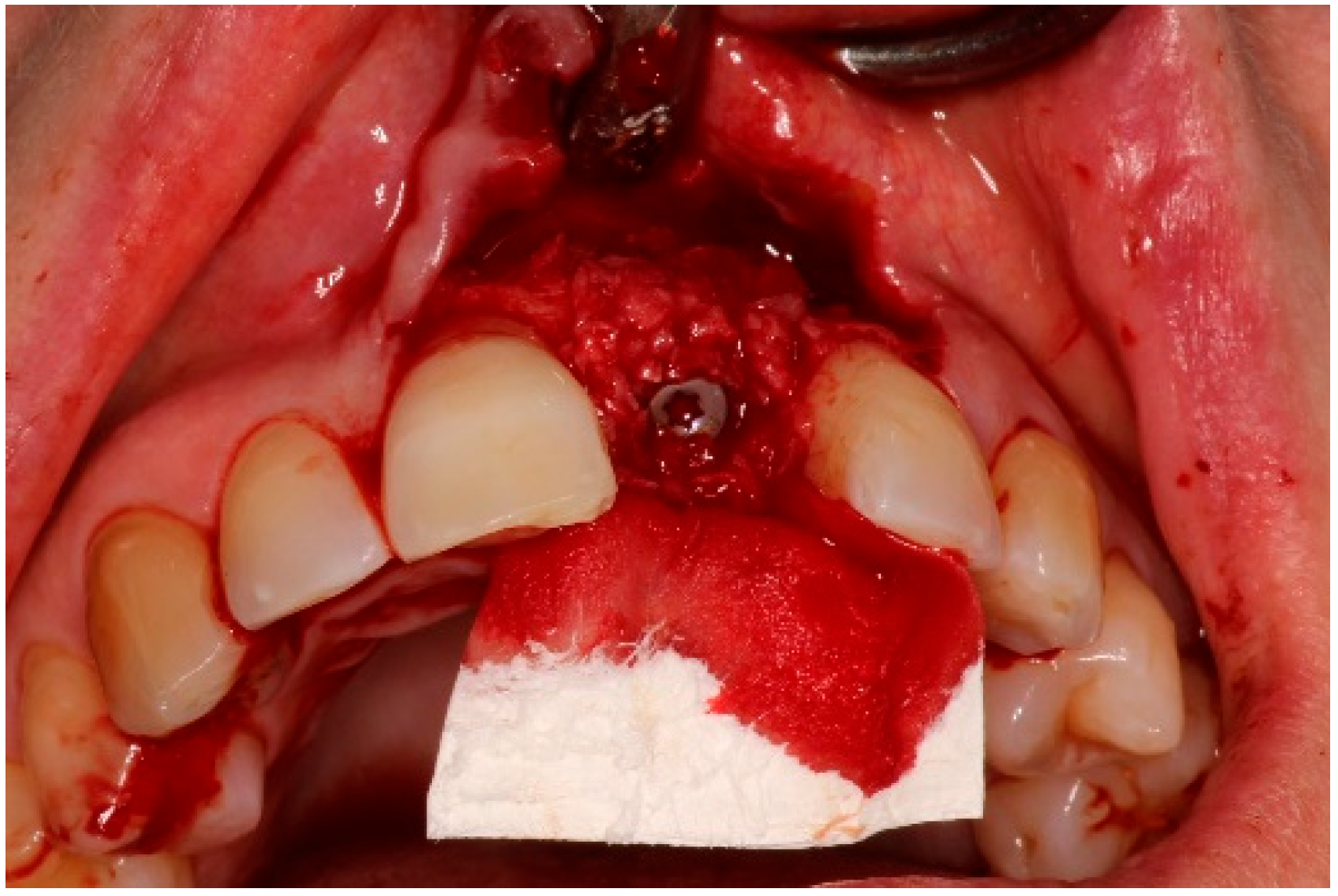
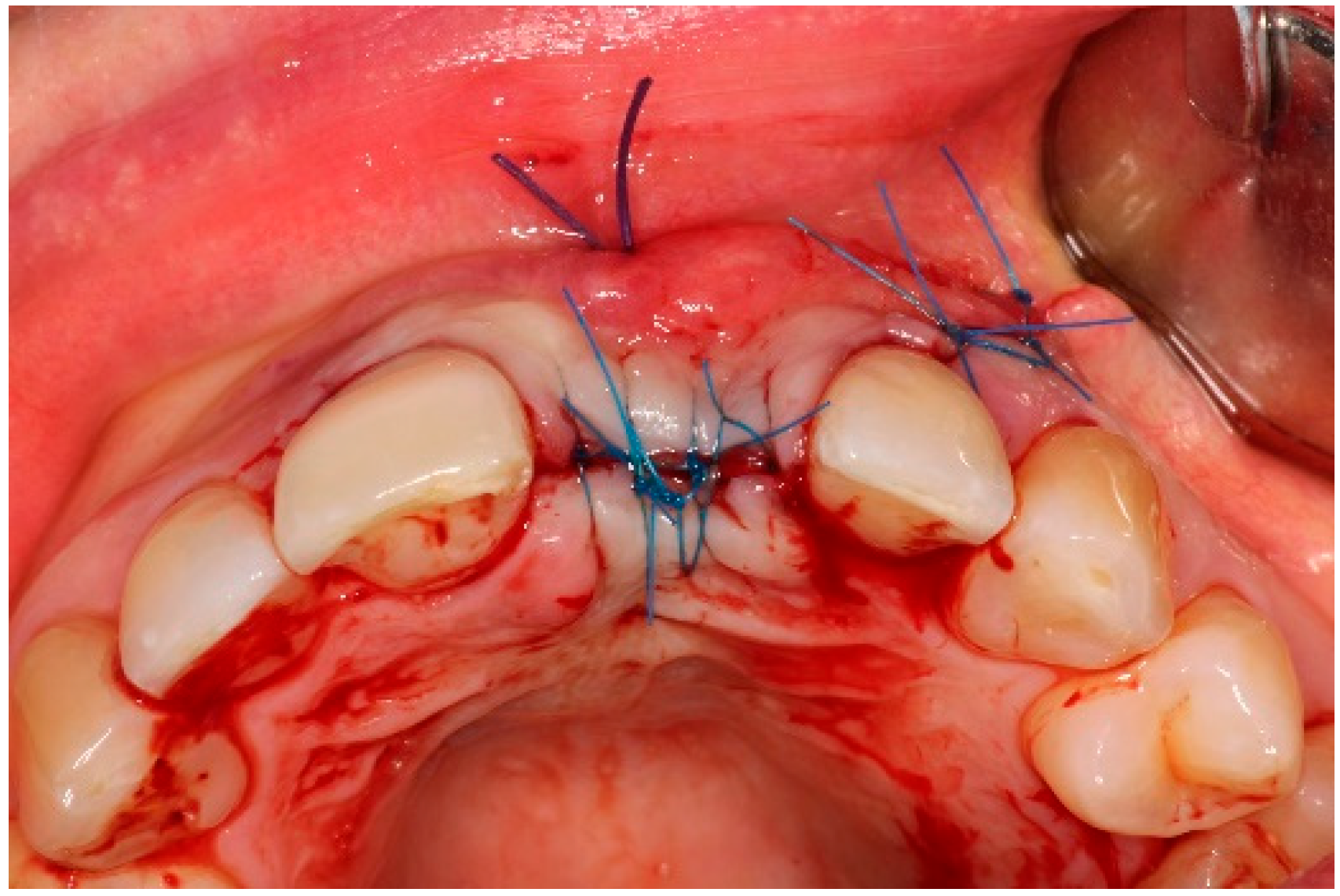
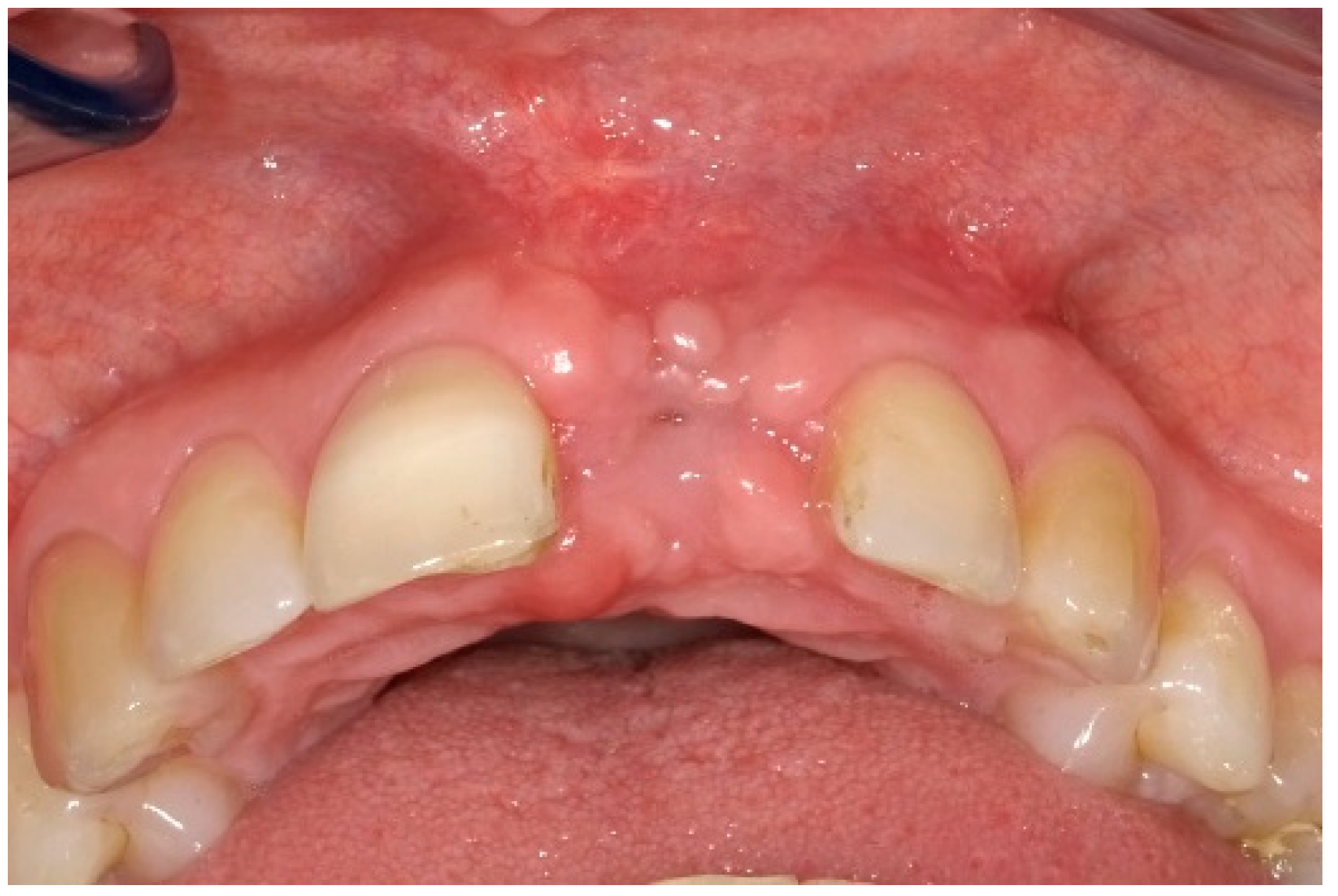


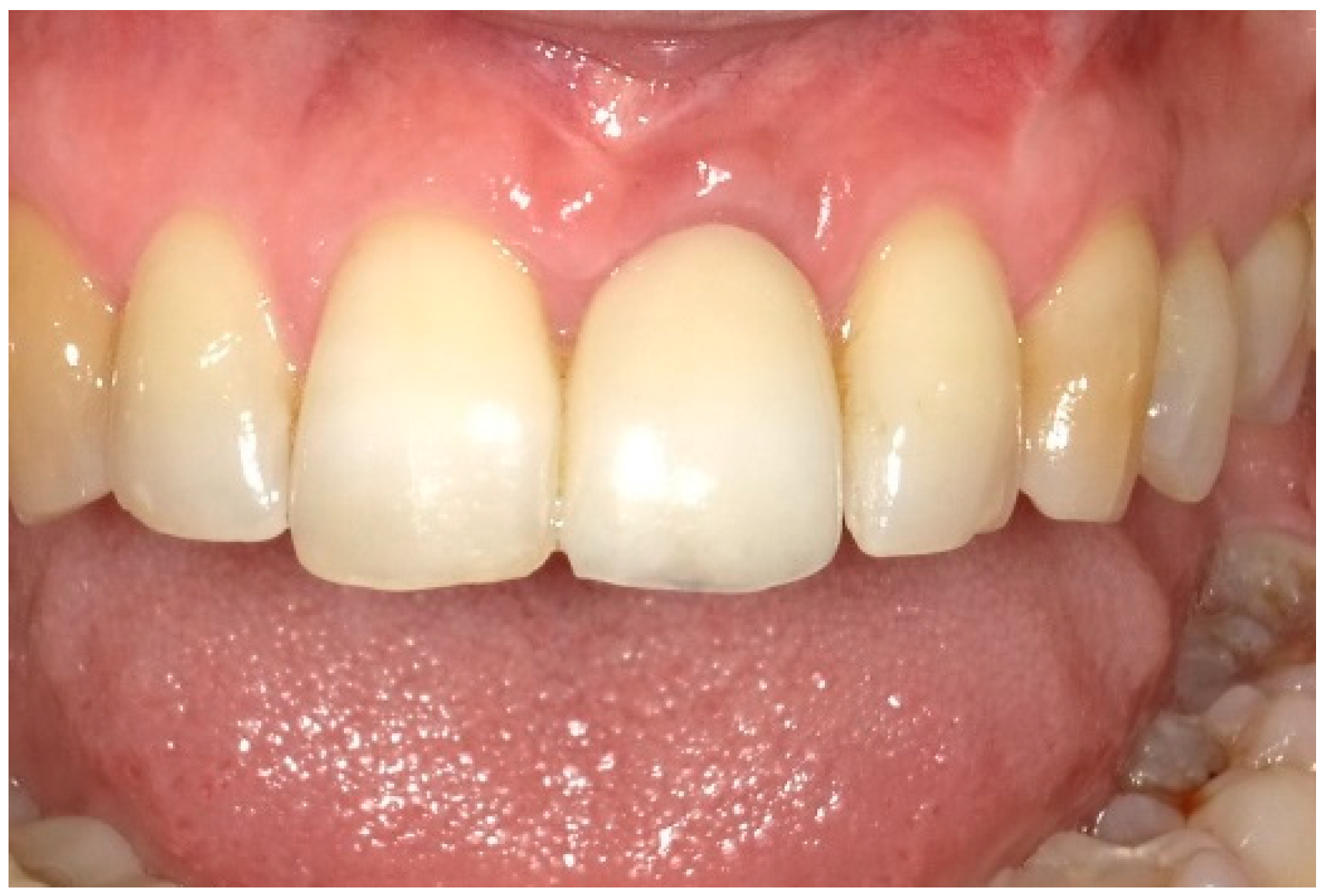
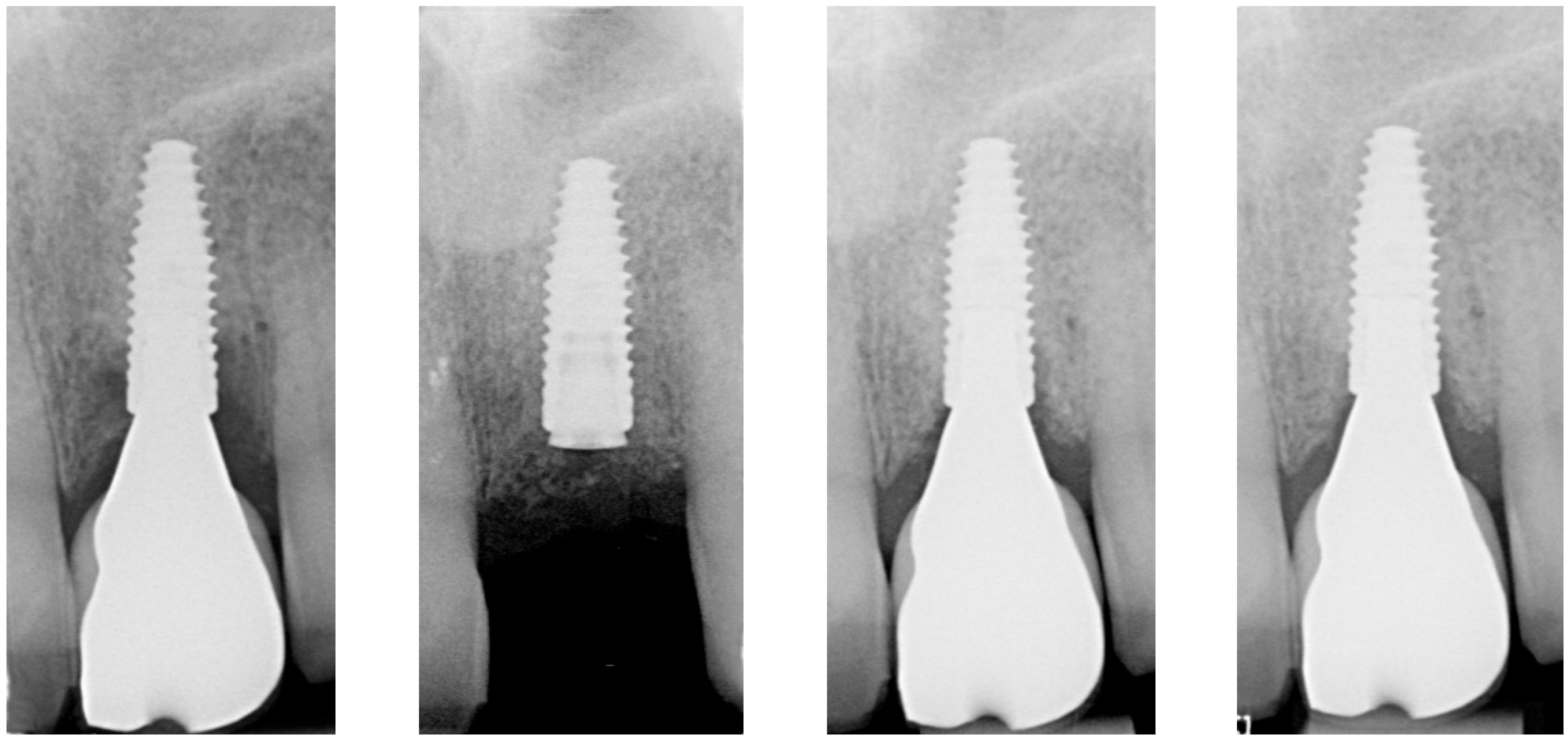
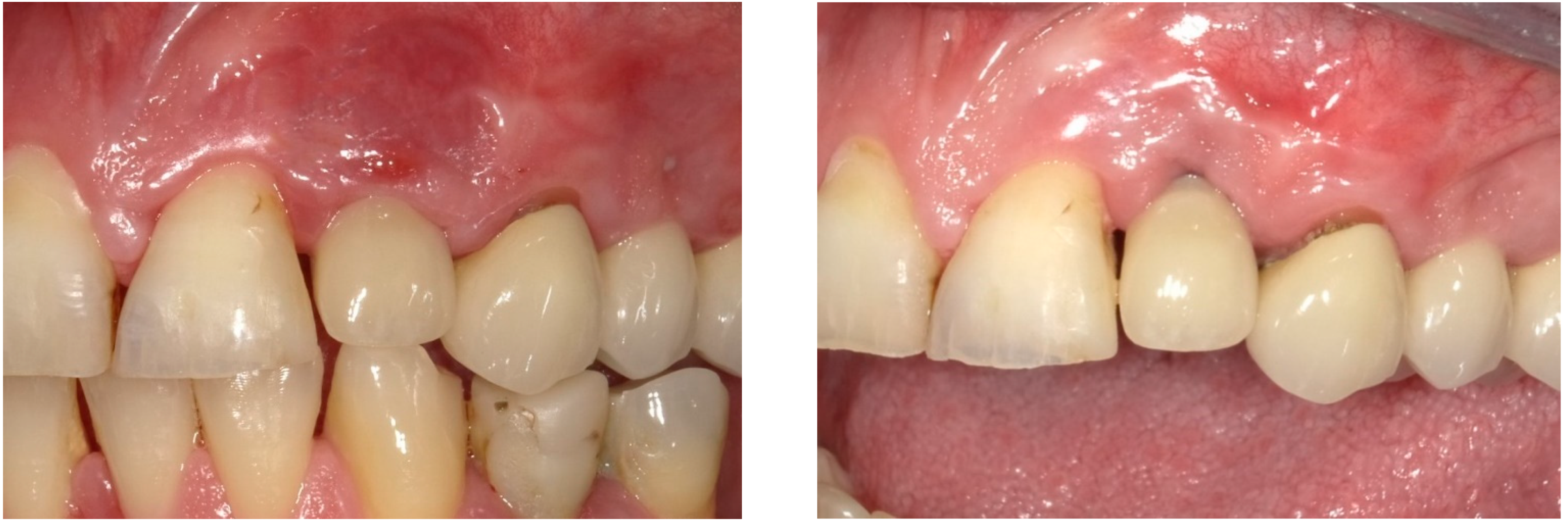
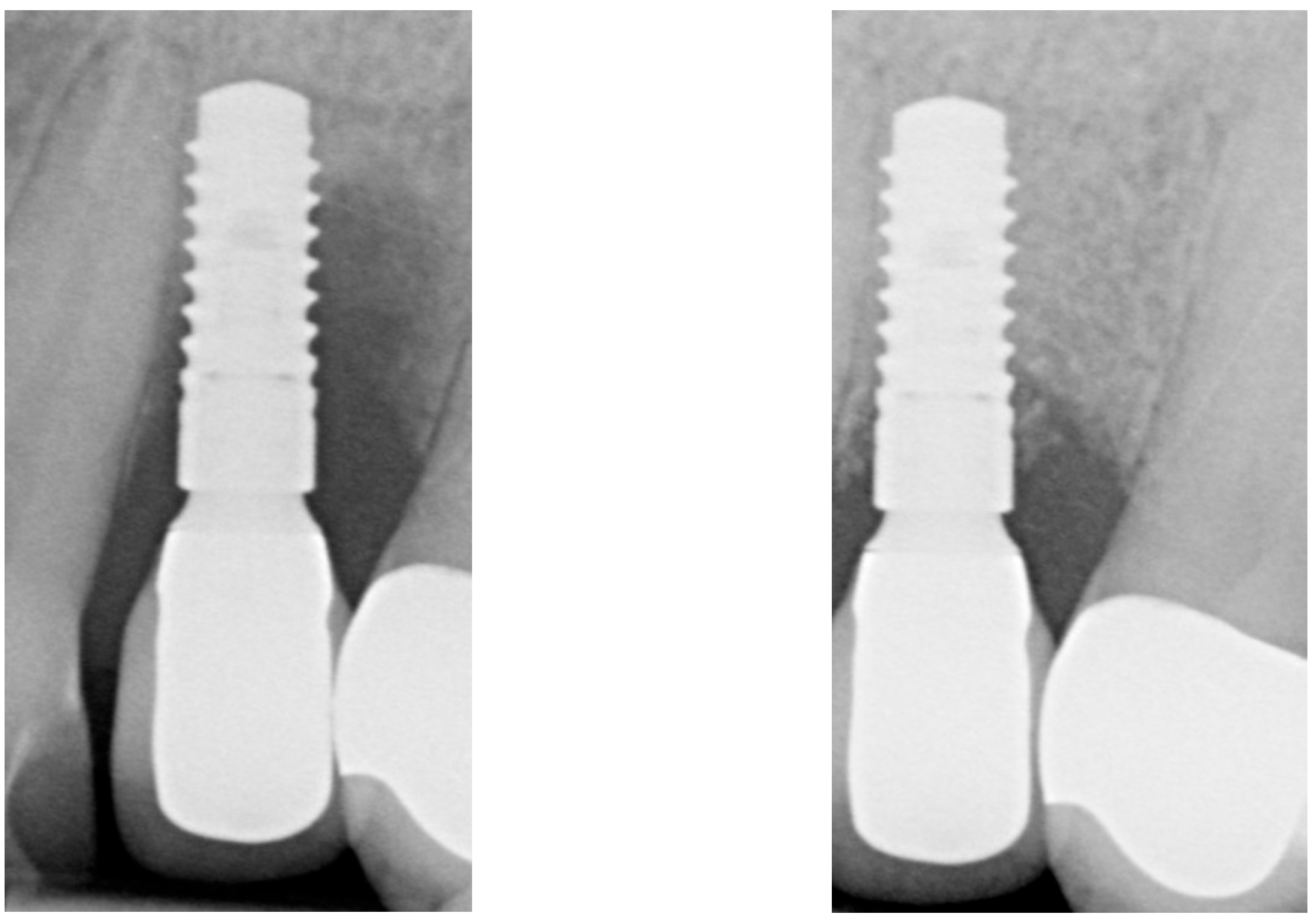

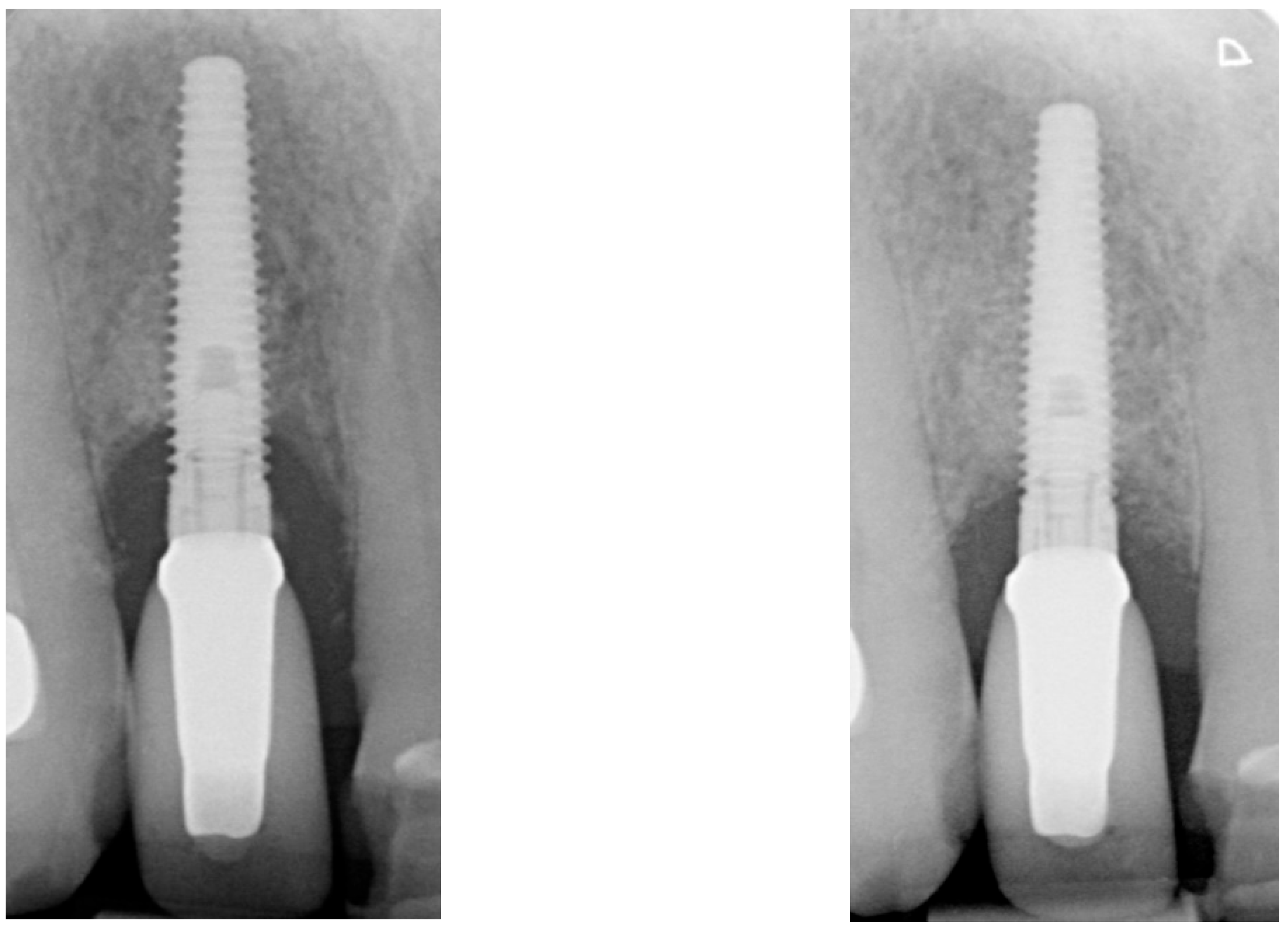
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Gender (female/male) | Female | Male | Female |
| Age at inclusion (years) | 48 | 65 | 57 |
| Implant diameter (mm) | 3.3 | 3.5 | 4.1 |
| Implant length (mm) | 10.0 | 16.0 | 12.0 |
| Implant position | 22 | 12 | 21 |
| Suprastructure | Screw-retained crown | Screw-retained crown | Screw-retained crown |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Mean peri-implant pocket depth at Tpre (mm) | 8.5 | 8.5 | 8.5 |
| Mean peri-implant pocket depth at T9 (mm) | 3.2 | 3.5 | 3.0 |
| Mean peri-implant pocket depth at T12 (mm) | 3.2 | 4.0 | 3.0 |
| Deepest peri-implant pocket at Tpre (mm) | 11.0 | 10.0 | 9.0 |
| Deepest peri-implant pocket at T9 (mm) | 4.0 | 6.0 | 4.0 |
| Deepest peri-implant pocket at T12 (mm) | 5.0 | 6.0 | 4.0 |
| Peri-implant plaque at Tpre (%) | 0.0 | 16.7 | 0.0 |
| Peri-implant plaque at T9 (%) | 0.0 | 66.7 | 0.0 |
| Peri-implant plaque at T12 (%) | 16.7 | 16.7 | 0.0 |
| Peri-implant BS at Tpre (%) | 100.0 | 100.0 | 100.0 |
| Peri-implant BS at T9 (%) | 100.0 | 33.3 | 16.7 |
| Peri-implant BS at T12 (%) | 16.7 | 83.3 | 33.3 |
| Peri-implant SS at Tpre (%) | 16.7 | 66.7 | 50.0 |
| Peri-implant SS at T9 (%) | 0.0 | 0.0 | 0.0 |
| Peri-implant SS at T12 (%) | 0.0 | 0.0 | 0.0 |
| Midfacial mucosa level at Tpre (mm) | 3.0 | 2.0 | 5.0 |
| Midfacial mucosa level at T9 (mm) | 5.0 | 6.0 | 6.0 |
| Midfacial mucosa level at T12 (mm) | 5.0 | 6.0 | 6.0 |
| Midfacial keratinized tissue width at Tpre (mm) | 3.0 | 7.0 | 5.0 |
| Midfacial keratinized tissue width at T9 (mm) | 3.0 | 3.0 | 3.0 |
| Midfacial keratinized tissue width at T12 (mm) | 3.0 | 3.0 | 2.0 |
| Radiographic bone level at Tpre mesial (mm) | 5.6 | 3.8 | 1.7 |
| Radiographic bone level at Tpre distal (mm) | 7.6 | 4.2 | 3.2 |
| Radiographic bone level at T2 mesial (mm) | 0.4 | 3.5 | 0.0 |
| Radiographic bone level at T2 distal (mm) | 1.8 | 3.9 | 0.0 |
| Radiographic bone level at T6 mesial (mm) | 2.8 | 3.4 | 1.1 |
| Radiographic bone level at T6 distal (mm) | 3.5 | 0.7 | 2.5 |
| Radiographic bone level at T12 mesial (mm) | 2.3 | 2.5 | 1.7 |
| Radiographic bone level at T12 distal (mm) | 3.5 | 1.5 | 2.5 |
| Case 1 | Case 2 | Case 3 | ||||
|---|---|---|---|---|---|---|
| Tpre | T12 | Tpre | T12 | Tpre | T12 | |
| Mesial papilla | 1 | 1 | 0 | 0 | 2 | 2 |
| Distal papilla | 1 | 1 | 0 | 0 | 2 | 1 |
| Curvature of the facial mucosa | 1 | 1 | 2 | 2 | 1 | 2 |
| Level of the facial mucosa | 0 | 2 | 2 | 1 | 1 | 2 |
| Root convexity/soft tissue color and texture | 0 | 1 | 2 | 2 | 0 | 1 |
| Total | 3 | 6 | 6 | 5 | 6 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakkers, J.; Meijer, H.J.A.; de Waal, Y.C.M.; Raghoebar, G.M. Electrolytic Cleaning as Part of Surgical Reconstructive Peri-Implantitis Treatment: A Case Series. Dent. J. 2025, 13, 237. https://doi.org/10.3390/dj13060237
Hakkers J, Meijer HJA, de Waal YCM, Raghoebar GM. Electrolytic Cleaning as Part of Surgical Reconstructive Peri-Implantitis Treatment: A Case Series. Dentistry Journal. 2025; 13(6):237. https://doi.org/10.3390/dj13060237
Chicago/Turabian StyleHakkers, Jarno, Henny J. A. Meijer, Yvonne C. M. de Waal, and Gerry M. Raghoebar. 2025. "Electrolytic Cleaning as Part of Surgical Reconstructive Peri-Implantitis Treatment: A Case Series" Dentistry Journal 13, no. 6: 237. https://doi.org/10.3390/dj13060237
APA StyleHakkers, J., Meijer, H. J. A., de Waal, Y. C. M., & Raghoebar, G. M. (2025). Electrolytic Cleaning as Part of Surgical Reconstructive Peri-Implantitis Treatment: A Case Series. Dentistry Journal, 13(6), 237. https://doi.org/10.3390/dj13060237






