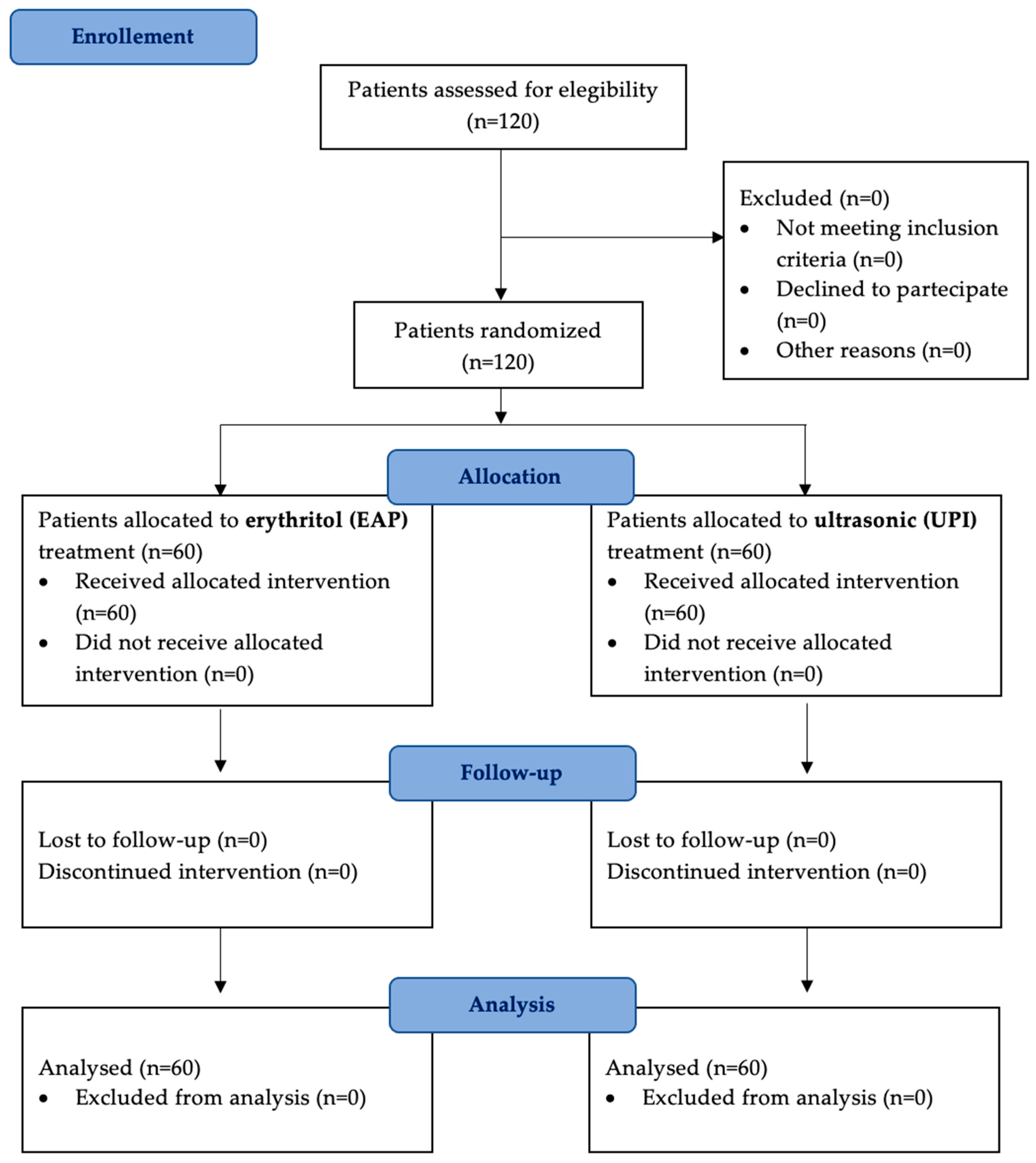
Figure 1.
A flow diagram illustrating patient selection, randomization, and follow-up. A total of 120 patients were enrolled and randomly assigned to two treatment groups: erythritol-based air-polishing (Group EAP) and ultrasonic instrumentation with PEEK inserts (Group UPI). Clinical parameters (PPD, BOP, PI) were assessed at baseline (T0), six months (T1), and twelve months (T2). No patients were lost to follow-up.
Figure 1.
A flow diagram illustrating patient selection, randomization, and follow-up. A total of 120 patients were enrolled and randomly assigned to two treatment groups: erythritol-based air-polishing (Group EAP) and ultrasonic instrumentation with PEEK inserts (Group UPI). Clinical parameters (PPD, BOP, PI) were assessed at baseline (T0), six months (T1), and twelve months (T2). No patients were lost to follow-up.
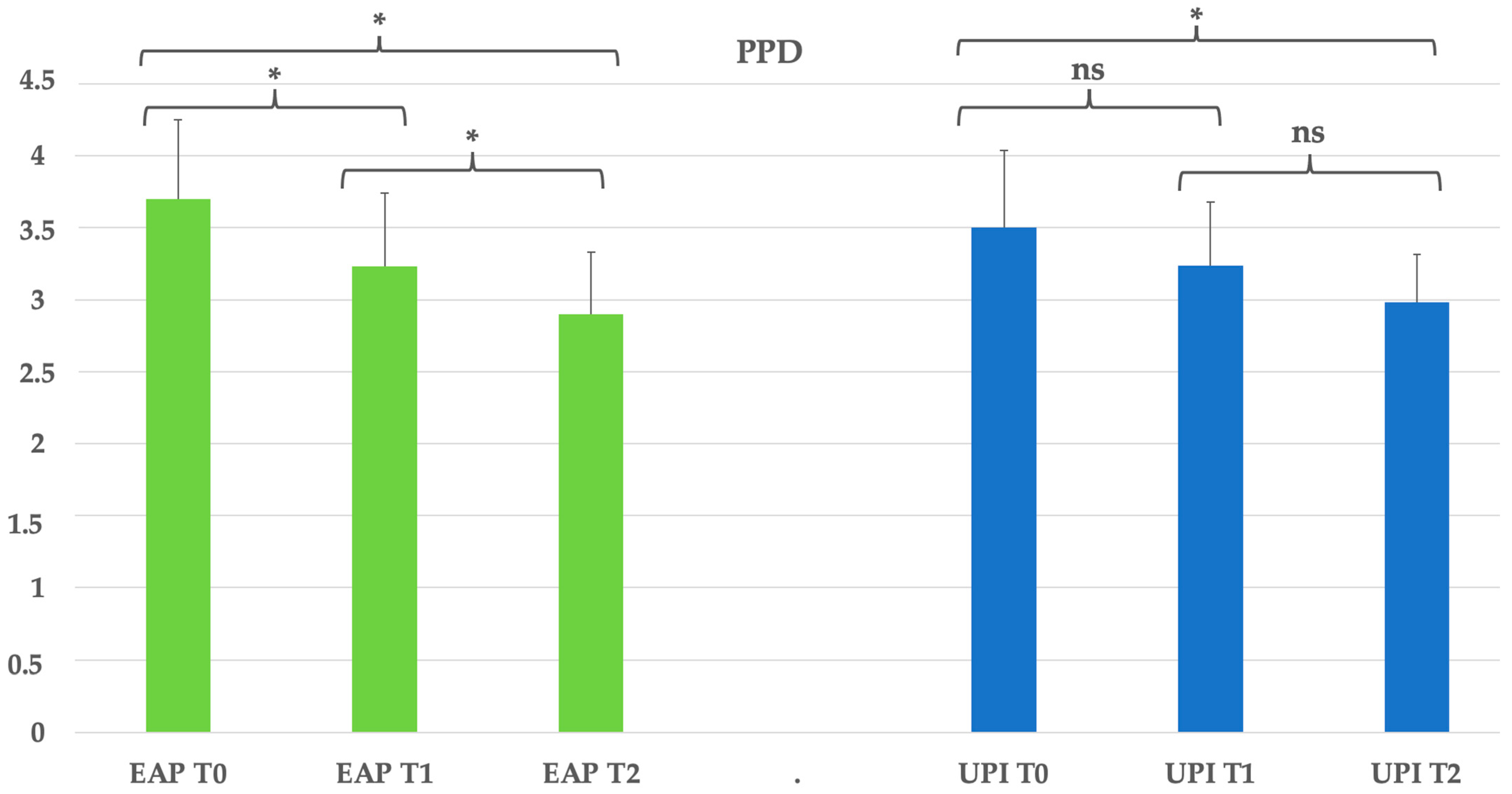
Figure 2.
Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 2.
Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
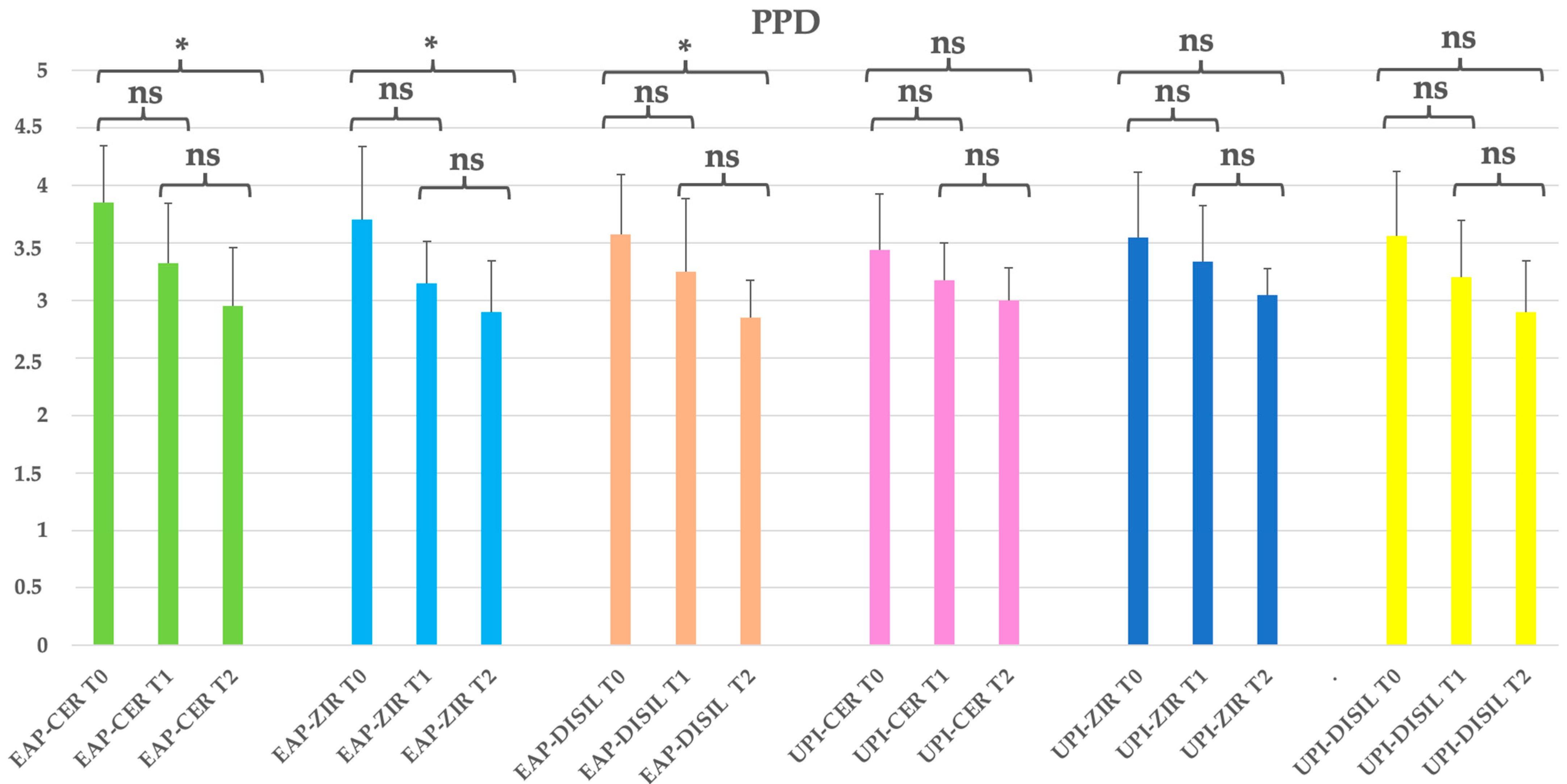
Figure 3.
EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 3.
EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 4.
Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 4.
Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 5.
EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 5.
EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
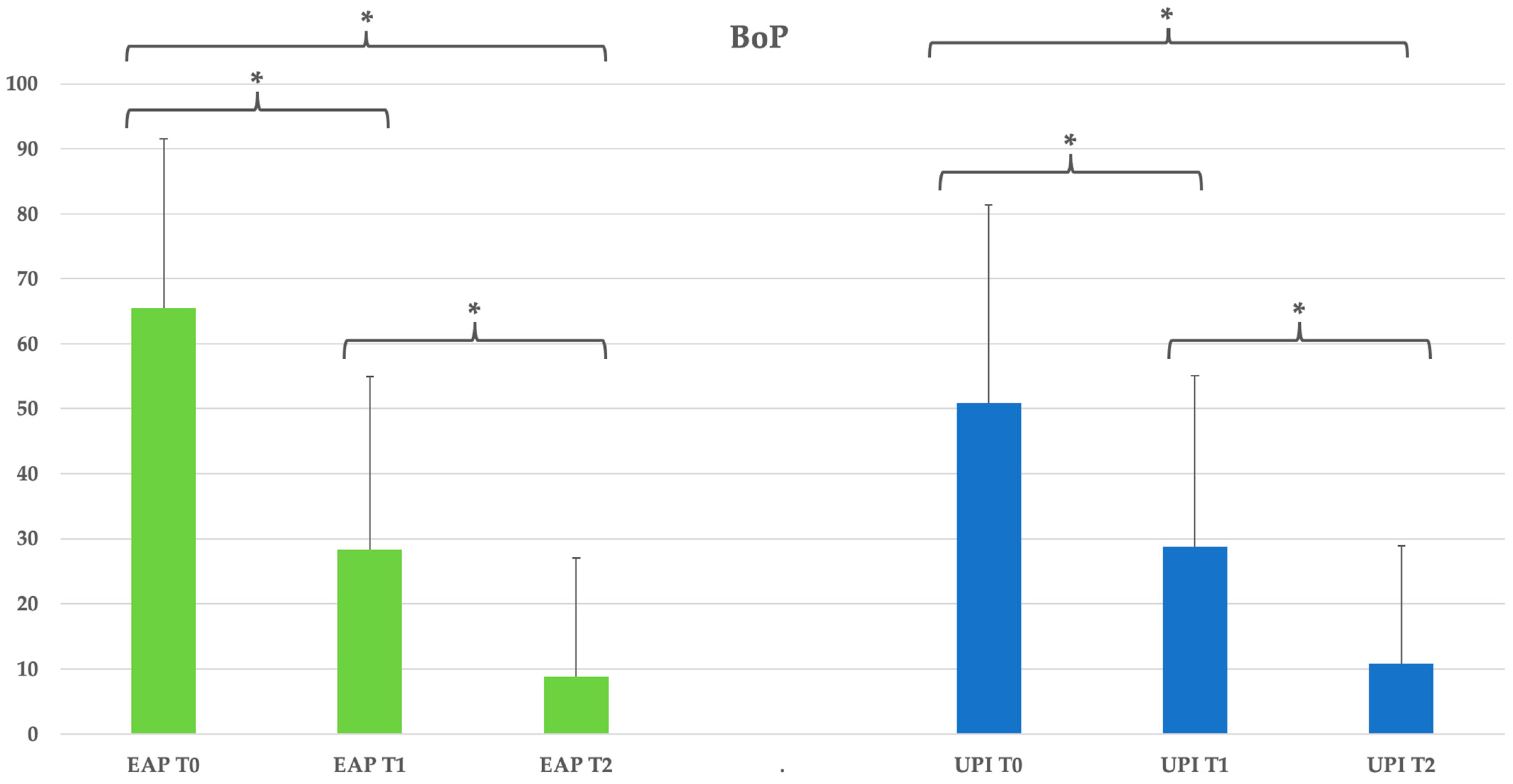
Figure 6.
Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 6.
Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 7.
EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
Figure 7.
EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The graphic in the figure shows intragroup differences: the asterisk indicates a statistically significant difference, while “ns” indicates no statistically significant difference; no intergroup difference was detected (p > 0.05).
Table 1.
Cumulative demographic characteristics of patients in Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The table presents the distribution of participants by sex and mean age and standard deviation (SD) for each treatment group.
Table 1.
Cumulative demographic characteristics of patients in Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The table presents the distribution of participants by sex and mean age and standard deviation (SD) for each treatment group.
| Patients | Sex | n (%) | Mean Age (SD) |
|---|
| Total | Males | 62 (51.67%) | 50.29 (10.76) |
| | Females | 58 (48.33%) | 49.59 (10.54) |
| EAP | Males | 29 (24.17%) | 49.24 (10.38) |
| | Females | 31 (25.83%) | 48.52 (11.98) |
| UPI | Males | 33 (27.50%) | 51.21 (11.15) |
| | Females | 27 (22.50%) | 50.81 (8.66) |
Table 2.
Demographic distribution of participants based on the prosthetic crown material used (feldspathic ceramic, zirconia, lithium disilicate) in both treatment groups. EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The table presents the number of males and females in each subgroup, along with their mean age (±standard deviation), ensuring comparability across different prosthetic materials.
Table 2.
Demographic distribution of participants based on the prosthetic crown material used (feldspathic ceramic, zirconia, lithium disilicate) in both treatment groups. EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The table presents the number of males and females in each subgroup, along with their mean age (±standard deviation), ensuring comparability across different prosthetic materials.
| Patients | Sex | n (%) | Mean Age (SD) |
|---|
| Total | Males | 62 (51.67%) | 50.29 (10.76) |
| | Females | 58 (48.33%) | 49.59 (10.54) |
| EAP-CER | Males | 9 (7.50%) | 48.00 (9.99) |
| | Females | 11 (9.17%) | 45.45 (12.23) |
| EAP-ZIR | Males | 11 (9.17%) | 52.82 (8.96) |
| | Females | 9 (7.50%) | 52.56 (10.48) |
| EAP-DISIL | Males | 9 (7.50%) | 46.11 (10.49) |
| | Females | 11 (9.17%) | 48.27 (11.38) |
| UPI-CER | Males | 12 (10.00%) | 51.33 (12.11) |
| | Females | 8 (6.67%) | 51.63 (9.81) |
| UPI-ZIR | Males | 11 (9.17%) | 50.64 (12.65) |
| | Females | 9 (7.50%) | 51.89 (5.78) |
| UPI-DISIL | Males | 9 (7.50%) | 52.00 (7.06) |
| | Females | 11 (9.17%) | 49.18 (8.73) |
Table 3.
Clinical parameters overview and statistical significance (PPD: probing pocket depth). Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
Table 3.
Clinical parameters overview and statistical significance (PPD: probing pocket depth). Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
| Group | Time | Mean | St Dev | Min | Median | Max |
|---|
| EAP | T0 | 3.70 | 0.55 | 3.00 | 4.00 | 5.00 |
| | T1 | 3.23 | 0.51 | 2.00 | 3.00 | 4.50 |
| | T2 | 2.90 | 0.43 | 2.00 | 3.00 | 4.00 |
| UPI | T0 | 3.50 | 0.54 | 3.00 | 3.25 | 4.50 |
| | T1 | 3.24 | 0.44 | 2.50 | 3.00 | 4.50 |
| | T2 | 2.98 | 0.33 | 2.00 | 3.00 | 4.00 |
Table 4.
Clinical parameters overview and statistical significance (PPD: probing pocket depth) by prosthetic material subgroups. EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
Table 4.
Clinical parameters overview and statistical significance (PPD: probing pocket depth) by prosthetic material subgroups. EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
| Group | Time | Mean | St Dev | Min | Median | Max |
|---|
| EAP-CER | T0 | 3.85 | 0.50 | 3.00 | 4.00 | 4.75 |
| | T1 | 3.32 | 0.52 | 2.00 | 3.00 | 4.50 |
| | T2 | 2.95 | 0.51 | 2.00 | 3.00 | 4.00 |
| EAP-ZIR | T0 | 3.70 | 0.64 | 3.00 | 3.87 | 5.00 |
| | T1 | 3.15 | 0.37 | 3.00 | 3.00 | 4.00 |
| | T2 | 2.90 | 0.45 | 2.00 | 3.00 | 4.00 |
| EAP-DISIL | T0 | 3.57 | 0.52 | 3.00 | 3.75 | 4.50 |
| | T1 | 3.25 | 0.64 | 2.00 | 3.00 | 4.00 |
| | T2 | 2.85 | 0.33 | 2.00 | 3.00 | 3.00 |
| UPI-CER | T0 | 3.44 | 0.48 | 3.00 | 3.12 | 4.00 |
| | T1 | 3.17 | 0.32 | 2.00 | 3.00 | 4.00 |
| | T2 | 3.00 | 0.28 | 2.00 | 3.00 | 3.50 |
| UPI-ZIR | T0 | 3.55 | 0.56 | 3.00 | 3.50 | 4.50 |
| | T1 | 3.34 | 0.49 | 3.00 | 3.00 | 4.50 |
| | T2 | 3.05 | 0.22 | 3.00 | 3.00 | 4.00 |
| UPI-DISIL | T0 | 3.56 | 0.56 | 3.00 | 3.62 | 4.50 |
| | T1 | 3.20 | 0.50 | 2.50 | 3.00 | 4.00 |
| | T2 | 2.90 | 0.45 | 2.00 | 3.00 | 4.00 |
Table 5.
Clinical parameters overview and statistical significance (BoP: bleeding on probing). Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
Table 5.
Clinical parameters overview and statistical significance (BoP: bleeding on probing). Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
| Group | Time | Mean | St Dev | Min | Median | Max |
|---|
| EAP | T0 | 65.42 | 26.08 | 25.00 | 50.00 | 100.00 |
| | T1 | 28.33 | 26.63 | 0.00 | 25.00 | 100.00 |
| | T2 | 8.75 | 18.31 | 0.00 | 0.00 | 100.00 |
| UPI | T0 | 50.83 | 30.52 | 0.00 | 50.00 | 100.00 |
| | T1 | 28.75 | 26.37 | 0.00 | 25.00 | 100.00 |
| | T2 | 10.83 | 18.04 | 0.00 | 0.00 | 50.00 |
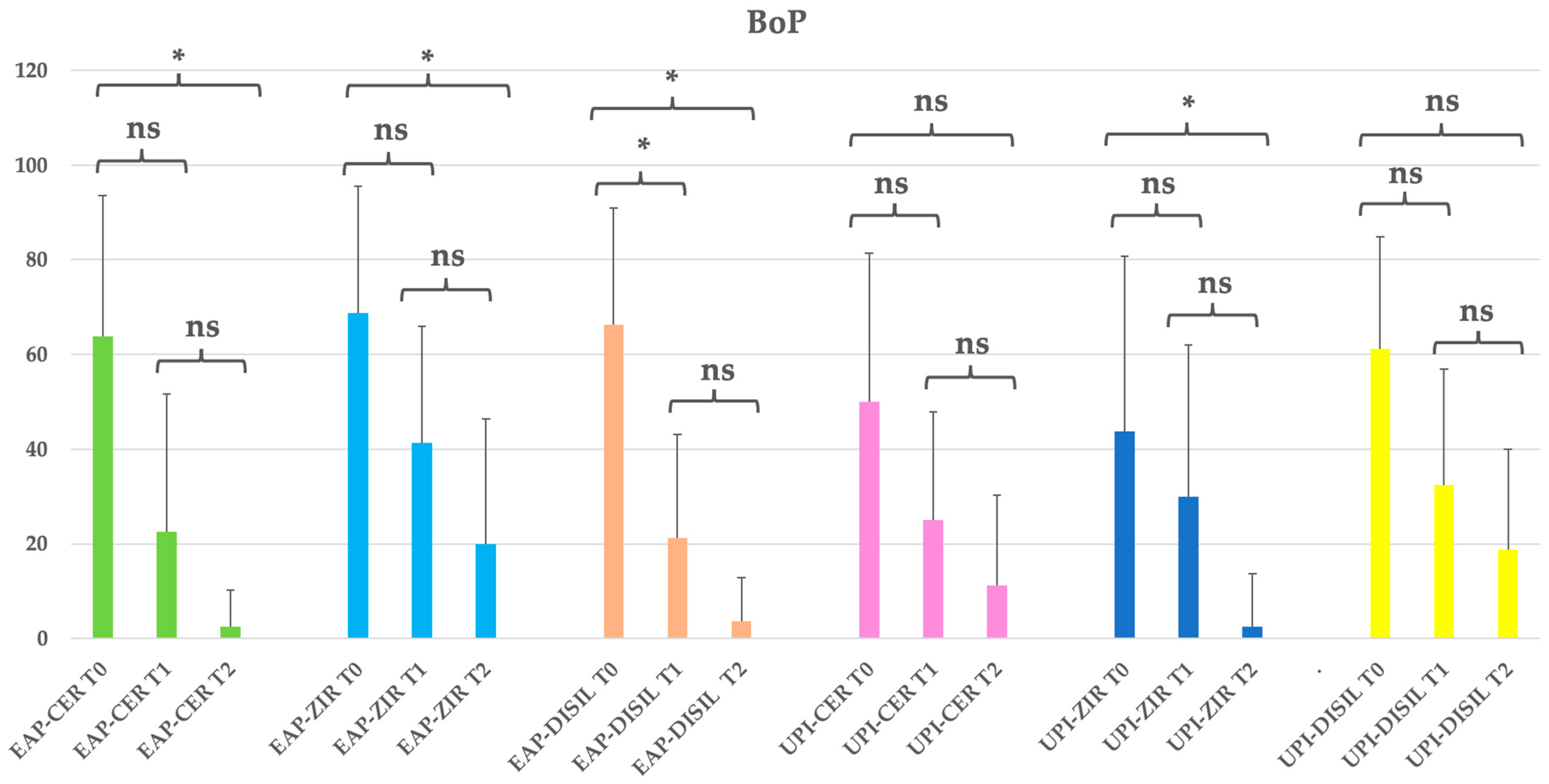
Table 6.
Clinical parameters overview and statistical significance (BoP: bleeding on probing) by prosthetic material subgroups. EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
Table 6.
Clinical parameters overview and statistical significance (BoP: bleeding on probing) by prosthetic material subgroups. EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
| Group | Time | Mean | St Dev | Min | Median | Max |
|---|
| EAP-CER | T0 | 63.75 | 29.77 | 25.00 | 50.00 | 100.00 |
| | T1 | 22.50 | 29.13 | 0.00 | 0.00 | 75.00 |
| | T2 | 2.50 | 7.69 | 0.00 | 0.00 | 25.00 |
| EAP-ZIR | T0 | 68.75 | 26.75 | 25.00 | 25.00 | 100.00 |
| | T1 | 41.25 | 24.70 | 0.00 | 0.00 | 100.00 |
| | T2 | 20.00 | 26.40 | 0.00 | 0.00 | 100.00 |
| EAP-DISIL | T0 | 66.25 | 24.70 | 25.00 | 25.00 | 100.00 |
| | T1 | 21.25 | 21.88 | 0.00 | 0.00 | 50.00 |
| | T2 | 3.75 | 9.16 | 0.00 | 0.00 | 25.00 |
| UPI-CER | T0 | 50.00 | 31.41 | 0.00 | 50.00 | 100.00 |
| | T1 | 25.00 | 22.94 | 0.00 | 25.00 | 50.00 |
| | T2 | 11.25 | 18.98 | 0.00 | 0.00 | 50.00 |
| UPI-ZIR | T0 | 43.75 | 37.06 | 0.00 | 50.00 | 100.00 |
| | T1 | 30.00 | 32.04 | 0.00 | 25.00 | 100.00 |
| | T2 | 2.50 | 11.18 | 0.00 | 0.00 | 50.00 |
| UPI-DISIL | T0 | 61.25 | 23.61 | 0.00 | 50.00 | 100.00 |
| | T1 | 32.50 | 24.47 | 0.00 | 50.00 | 50.00 |
| | T2 | 18.75 | 21.27 | 0.00 | 12.50 | 50.00 |
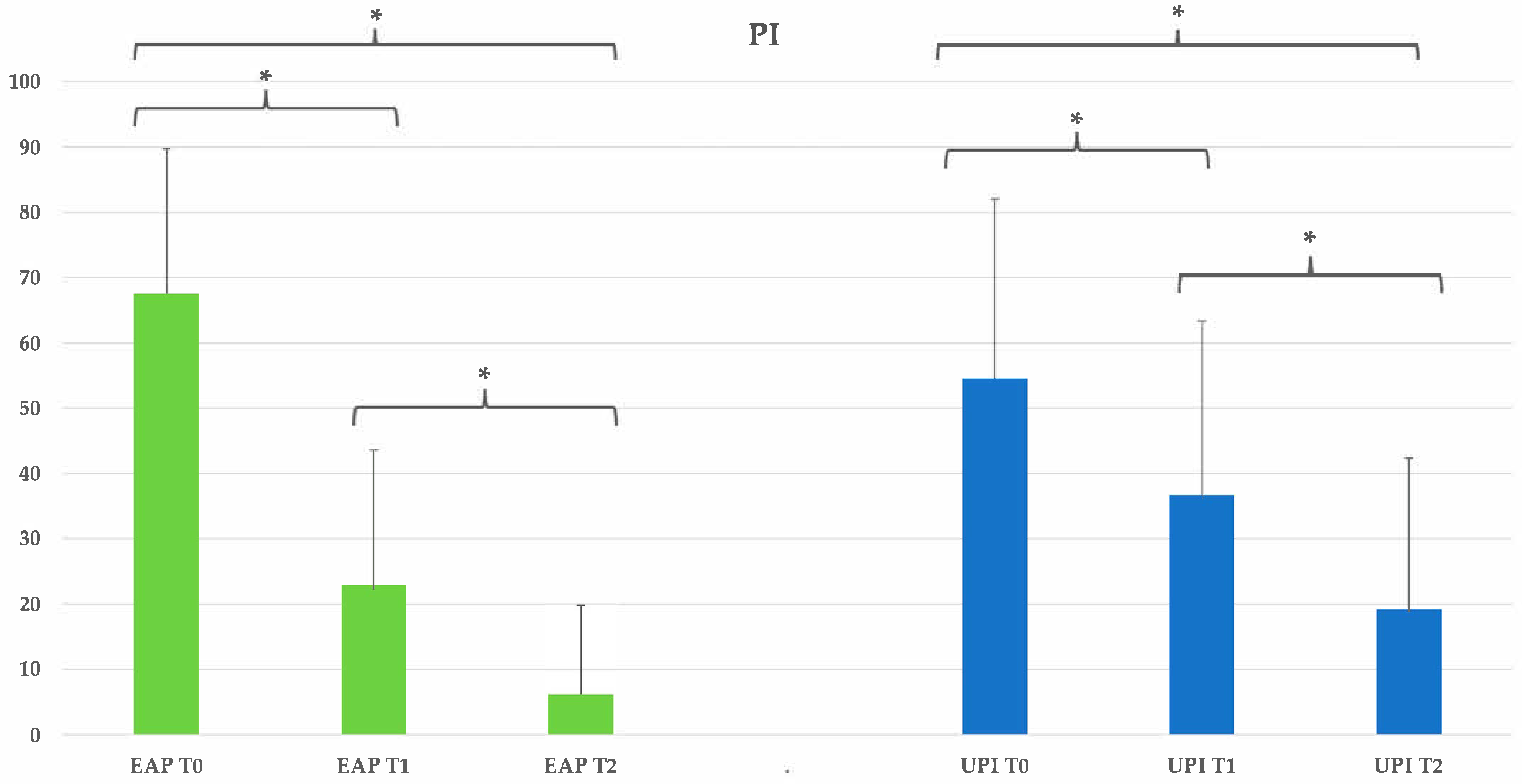
Table 7.
Clinical parameters overview and statistical significance (PI:plaque index). Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
Table 7.
Clinical parameters overview and statistical significance (PI:plaque index). Group EAP (erythritol-based air-polishing) and Group UPI (ultrasonic instrumentation with PEEK inserts). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
| Group | Time | Mean | St Dev | Min | Median | Max |
|---|
| EAP | T0 | 67.50 | 22.22 | 25.00 | 50.00 | 100.00 |
| | T1 | 22.92 | 20.73 | 0.00 | 25.00 | 50.00 |
| | T2 | 6.25 | 13.52 | 0.00 | 0.00 | 50.00 |
| UPI | T0 | 54.58 | 27.42 | 0.00 | 50.00 | 100.00 |
| | T1 | 36.67 | 26.63 | 0.00 | 50.00 | 100.00 |
| | T2 | 19.17 | 23.18 | 0.00 | 0.00 | 75.00 |
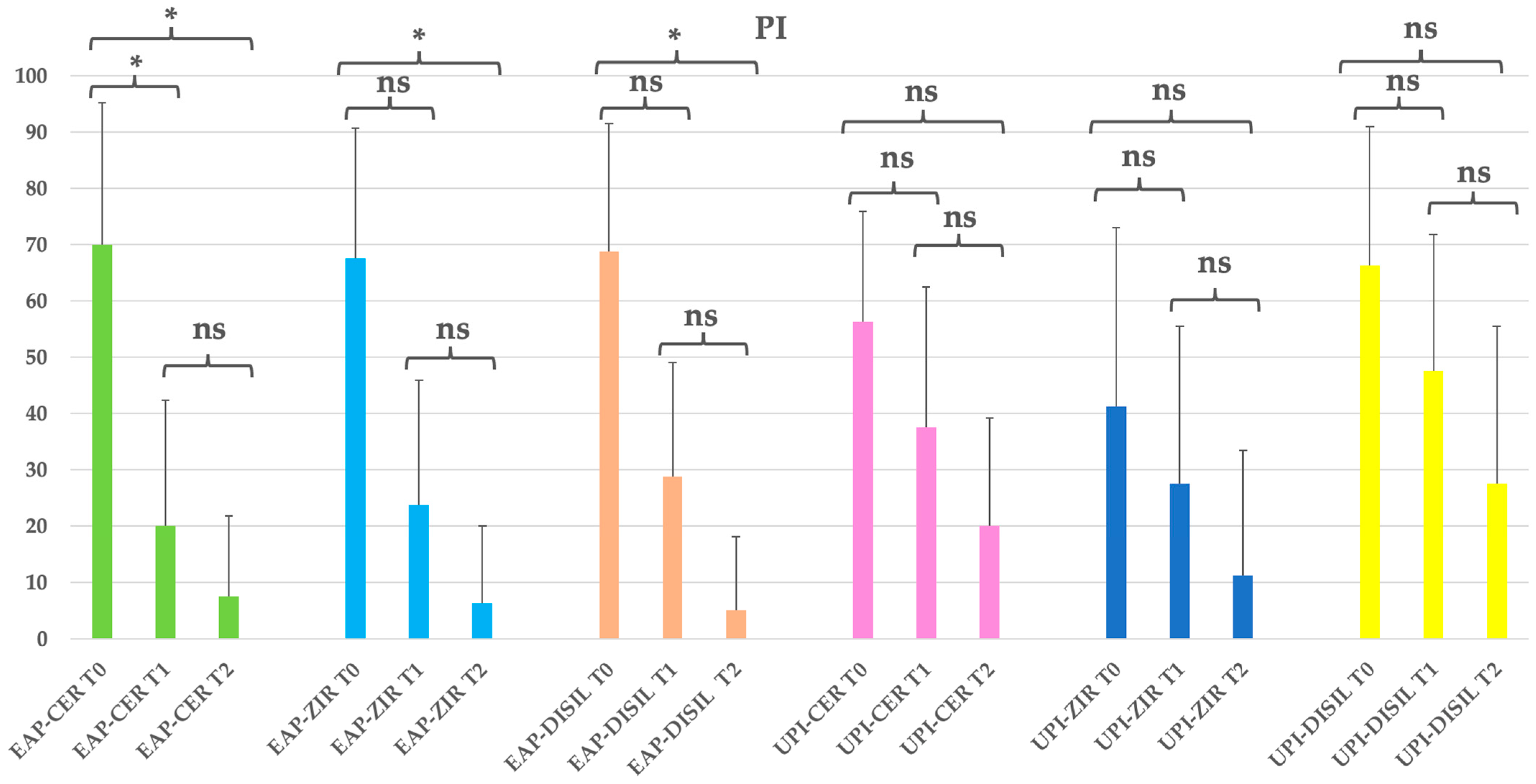
Table 8.
Clinical parameters overview and statistical significance (PI: plaque index) by Prosthetic material Subgroups. EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
Table 8.
Clinical parameters overview and statistical significance (PI: plaque index) by Prosthetic material Subgroups. EAP-CER (erythritol-based air-polishing group with feldspathic ceramic crown), EAP-ZIR (erythritol-based air-polishing group with zirconia crown), EAP-DISIL (erythritol-based air-polishing group with lithium disilicate crown), UPI-CER (ultrasonic instrumentation with PEEK inserts group with feldspathic ceramic crown), UPI-ZIR (ultrasonic instrumentation with PEEK inserts group with zirconia crown), UPI-DISIL (ultrasonic instrumentation with PEEK inserts group with lithium disilicate crown). The table presents the mean, standard deviation (St Dev), minimum (Min), median, and maximum (Max) values for each clinical parameter assessed at different time points (T0, T1, T2) in both treatment groups.
| Group | Time | Mean | St Dev | Min | Median | Max |
|---|
| EAP-CER | T0 | 70.00 | 25.13 | 25.00 | 62.50 | 100.00 |
| | T1 | 20.00 | 22.36 | 0.00 | 12.50 | 50.00 |
| | T2 | 7.50 | 14.28 | 0.00 | 0.00 | 50.00 |
| EAP-ZIR | T0 | 67.50 | 23.08 | 50.00 | 50.00 | 100.00 |
| | T1 | 23.75 | 22.18 | 0.00 | 25.00 | 50.00 |
| | T2 | 6.25 | 13.75 | 0.00 | 0.00 | 50.00 |
| EAP-DISIL | T0 | 68.75 | 22.76 | 50.00 | 50.00 | 100.00 |
| | T1 | 28.75 | 20.32 | 0.00 | 25.00 | 50.00 |
| | T2 | 5.00 | 13.08 | 0.00 | 0.00 | 50.00 |
| UPI-CER | T0 | 56.25 | 19.66 | 25.00 | 50.00 | 100.00 |
| | T1 | 37.50 | 25.00 | 0.00 | 50.00 | 100.00 |
| | T2 | 20.00 | 19.19 | 0.00 | 25.00 | 50.00 |
| UPI-ZIR | T0 | 41.25 | 31.70 | 0.00 | 50.00 | 100.00 |
| | T1 | 27.50 | 27.98 | 0.00 | 25.00 | 75.00 |
| | T2 | 11.25 | 22.18 | 0.00 | 0.00 | 75.00 |
| UPI-DISIL | T0 | 66.25 | 24.70 | 25.00 | 50.00 | 100.00 |
| | T1 | 47.50 | 24.20 | 0.00 | 50.00 | 100.00 |
| | T2 | 27.50 | 27.98 | 0.00 | 25.00 | 75.00 |