Balancing the Oral Redox State: Endogenous and Exogenous Sources of Reactive Oxygen Species and the Antioxidant Role of Lamiaceae and Asteraceae
Abstract
1. Introduction
2. Materials and Methods
3. Sources of Oxidative Stress
3.1. Endogenous Sources
3.1.1. Cellular and Mitochondrial Metabolism
3.1.2. Immune Activity
3.1.3. Oral Microbiome Imbalance
3.2. Exogenous Sources
3.2.1. Alcohol Consumption
3.2.2. Smoking
3.2.3. Xenobiotics
3.2.4. Orthodontic Treatment and Dental Materials
3.2.5. High-Fat, High-Protein, and Processed Food Diets
3.2.6. The Influence of Food Preparation Methods on Oral OS
3.2.7. Radiation
4. Mechanisms by Which Free Radicals Induce OS in the Oral Cavity
4.1. Lipid Peroxidation and Cellular Membrane Destruction
4.2. Oxidation of Salivary and Enzymatic Proteins
4.3. Nucleic Acid Degradation and DNA Mutations
4.4. Activation of the Inflammatory Response and Pro-Oxidant Enzymes
4.5. Interaction Between Free Radicals and the Oral Microbiota
5. Impact of OS on Oral Health
5.1. Oral Aphthae
5.2. Dental Abscesses
5.3. Periodontitis and Gingivitis
5.4. Dental Caries
5.5. Precancerous Oral Lesions and Oral Cancer
5.6. Xerostomia (Dry Mouth)
6. Components of the Salivary Antioxidant System (Enzymatic and Non-Enzymatic)
6.1. Enzymatic Antioxidants in the Oral Cavity
6.2. Non-Enzymatic Antioxidants
7. Medicinal Plants with Antioxidant Potential from the Lamiaceae and Asteraceae Families
7.1. Bioactive Compounds with Antioxidant Roles
7.1.1. Phenolic Acids
7.1.2. Flavonoids
7.1.3. Tannins
7.1.4. Essential Oils
7.1.5. Anthocyanins
7.1.6. Terpenes
7.1.7. Alkaloids
7.2. Sources of Natural Antioxidants from Medicinal Species of the Asteraceae and Lamiaceae Families
7.2.1. Plants from the Lamiaceae Family
7.2.2. Plant Species of the Asteraceae Family with Antioxidant Constituents
8. Medicinal Plants from the Lamiaceae and Asteraceae Families in Modulating Salivary Antioxidant Defense
8.1. Lamiaceae Family
8.1.1. Lavandula angustifolia L.
8.1.2. Mentha × piperita L.
8.1.3. Ocimum basilicum L.
8.1.4. Rosmarinus officinalis L.
8.1.5. Salvia officinalis L.
8.1.6. Satureja hortensis L.
8.1.7. Thymus serpyllum L.
8.1.8. Thymus vulgaris L.
8.2. Asteraceae Family
8.2.1. Matricaria chamomilla L.
8.2.2. Cichorium intybus L.
8.2.3. Calendula officinalis L.
8.2.4. Taraxacum officinale L.
8.2.5. Arctium lappa L.
8.2.6. Achillea millefolium L.
8.2.7. Solidago virgaurea L.
9. Future Research Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OS | Oxidative stress |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| H2O2 | Hydrogen peroxide |
| NO | Nitric oxide |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| NOS | Nitric oxide synthase |
| ADH | Alcohol dehydrogenase |
| LPO | Lactoperoxidase |
| MPO | Myeloperoxidase |
| •OH | Hydroxyl radical |
| ROO• | Peroxyl radical |
| RO• | Alkoxyl radical |
| ONOO− | Peroxynitrite anion |
| O2− | Superoxide anion |
| UA | Uric acid |
| MMPs | Metalloproteinases |
| HA | Hydroxyapatite |
| MDA | Malondialdehyde |
| TAC | Total antioxidant capacity |
| OSSC | Oral squamous-cell carcinoma |
| P. gingivalis | Porphyromonas gingivalis |
| S. mutans | Streptococcus mutans |
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- El-Bahr, S.M. Biochemistry of free radicals and oxidative stress. Sci. Int. 2013, 1, 111–117. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Bondeva, T.; Wolf, G. Reactive oxygen species in diabetic nephropathy: Friend or foe? Nephrol. Dial. Transplant. 2014, 11, 1998–2003. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, L.; Hong, H. Mitochondrial Dysfunction in the Pathogenesis and Treatment of Oral Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 15483. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Brajdeș, D.C.; Bahrim, G.; Dinică, R.; Vizireanu, C. Phenolics composition and their biochemical stability confirmation by in vitro, gastrointestinal conditions simulation, for a new functional fermented beverage based on sprouted buckwheat, Rom. Biotechnol. Lett. 2013, 18, 8832–8842. [Google Scholar]
- Suvarna, R.; Rao, P.K.; Poonja, P.A.; Rai, D.; Kini, R.; Meghana, H.C. Salivary superoxide dismutase activity in smokeless tobacco consumers and non-consumers: A biochemical study. J. Cancer Res. Ther. 2023, 19, 1359–1364. [Google Scholar] [CrossRef]
- Sculley, D.V.; Langley-Evans, S.C. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin. Sci. 2003, 105, 167–172. [Google Scholar] [CrossRef]
- Mafimisebi, T.E.; Oguntade, A.E.; Ajibefun, I.A.; Mafimisebi, O.E.; Ikuemonisan, E.S. The Expanding Market for Herbal, Medicinal and Aromatic Plants In Nigeria and the International Scene. Med. Aromat. Plants 2013, 2, 144. [Google Scholar]
- Anwar, M.A.; Sayed, G.A.; Hal, D.M.; El Hafeez, M.S.A.; Shatat, A.-A.S.; Salman, A.; Eisa, N.M.; Ramadan, A.; El-Shiekh, R.A.; Hatem, S.; et al. Herbal remedies for oral and dental health: A comprehensive review of their multifaceted mechanisms including antimicrobial, anti-inflammatory, and antioxidant pathways. Inflammopharmacology 2025, 33, 1085–1160. [Google Scholar] [CrossRef]
- Moro, L. Mitochondrial Dysfunction in Aging and Cancer. J. Clin. Med. 2019, 8, 1983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.H.; Kim, H.J.; Joo, J.Y.; Lee, J.Y.; Lee, J.H.; Park, H.R. Periodontal Pathogens Promote Foam Cell Formation by Blocking Lipid Efflux. J. Dent. Res. 2021, 100, 1367–1377. [Google Scholar] [CrossRef]
- Xu, T.; Dong, Q.; Luo, Y.; Liu, Y.; Gao, L.; Pan, Y.; Zhang, D. Porphyromonas gingivalis infection promotes mitochondrial dysfunction through Drp1-dependent mitochondrial fission in endothelial cells. Int. J. Oral. Sci. 2021, 13, 28. [Google Scholar] [CrossRef]
- Vaseenon, S.; Weekate, K.; Srisuwan, T.; Chattipakorn, N.; Chattipakorn, S. Observation of Inflammation, Oxidative Stress, Mitochondrial Dynamics, and Apoptosis in Dental Pulp following a Diagnosis of Irreversible Pulpitis. Eur. Endod. J. 2023, 8, 148–155. [Google Scholar] [CrossRef]
- Dogan Buzoglu, H.; Ozcan, M.; Bozdemir, O.; Aydin Akkurt, K.S.; Zeybek, N.D.; Bayazit, Y. Evaluation of oxidative stress cycle in healthy and inflamed dental pulp tissue: A laboratory investigation. Clin. Oral Investig. 2023, 27, 5913–5923. [Google Scholar] [CrossRef]
- Vengerfeldt, V.; Mändar, R.; Saag, M.; Piir, A.; Kullisaar, T. Oxidative stress in patients with endodontic pathologies. J. Pain Res. 2017, 10, 2031–2040. [Google Scholar] [CrossRef]
- Papinska, J.A.; Durślewicz, J.; Bagavant, H.; Deshmukh, U.S. Deleting Mitochondrial Superoxide Dismutase 2 in Salivary Gland Ductal Epithelial Cells Recapitulates Non-Sjögren’s Sicca Syndrome. Int. J. Mol. Sci. 2024, 25, 5983. [Google Scholar] [CrossRef]
- Katsiougiannis, S.; Stergiopoulos, A.; Moustaka, K.; Havaki, S.; Samiotaki, M.; Stamatakis, G.; Tenta, R.; Skopouli, F.N. Salivary gland epithelial cell in Sjögren’s syndrome: Metabolic shift and altered mitochondrial morphology toward an innate immune cell function. J. Autoimmun. 2023, 136, 103014. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, C.; Zhang, J.; Lai, R.; Zhang, X.; Guo, Z. Mitochondrial Displacement Loop Region SNPs Modify Sjögren’s Syndrome Development by Regulating Cytokines Expression in Female Patients. Front. Genet. 2022, 13, 847521. [Google Scholar] [CrossRef] [PubMed]
- De Benedittis, G.; Latini, A.; Colafrancesco, S.; Priori, R.; Perricone, C.; Novelli, L.; Borgiani, P.; Ciccacci, C. Alteration of Mitochondrial DNA Copy Number and Increased Expression Levels of Mitochondrial Dynamics-Related Genes in Sjögren’s Syndrome. Biomedicines 2022, 10, 2699. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Breaking bad: Manipulation of the host response by Porphyromonas gingivalis. Eur. J. Immunol. 2014, 44, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 18, 1269581. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Saluja, H.M.; Sachdeva, S.; Mani, A. Role of reactive oxygen species and antioxidants in periodontal disease. J. Cell. Biotechnol. 2021, 7, 125–140. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–6661. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, X.; Zhou, X.; Ning, J. Oxidative stress response: A critical factor affecting the ecological competitiveness of Streptococcus mutans. J. Oral Microbiol. 2023, 15, 2292539. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szulc, A.; Zalewska, A. Does Oxidative Stress Induced by Alcohol Consumption Affect Salivary Glands and the Composition of Saliva? Front. Physiol. 2017, 8, 22. [Google Scholar]
- Waszkiewicz, N.; Jelski, W.; Zalewska, A.; Szulc, A.; Szmitkowski, M.; Zwierz, K.; Szajda, S.D. Salivary alcohol dehydrogenase in non-smoking and smoking alcohol-dependent persons. Alcohol 2014, 48, 611–616. [Google Scholar] [CrossRef]
- Contreras-Zentella, M.L.; Villalobos-García, D.; Hernández-Muñoz, R. Ethanol Metabolism in the Liver, the Induction of Oxidant Stress, and the Antioxidant Defense System. Antioxidants 2022, 11, 1258. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Chojnowska, S.; Zalewska, A.; Szulc, A.; Zwierz, K.; Szajda, S.D. Salivary hexosaminidase in smoking alcoholics with bad periodontal and dental states. Drug Alcohol Depend. 2013, 129, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N.; Szajda, S.D.; Jankowska, A.; Kępka, A.; Dobryniewski, J.; Szulc, A.; Zwierz, K. The effect of the binge drinking session on the activity of salivary, serum, urinary beta hexosaminidase: Preliminary data. Alcohol Alcohol. 2008, 43, 446–450. [Google Scholar] [CrossRef]
- Watjen, W.; Beyersmann, D. Cadmium-induced apoptosis in C6 glioma cells: Influence of oxidative stress. Biometals 2004, 17, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.I. The influence of smoking on host responses in periodontal infections. Periodontology 2000 2007, 43, 267–277. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Fiaschi, A.I.; Cozzolino, A.; Ruggiero, G.; Giorgi, G. Glutathione, ascorbic acid and antioxidant enzymes in the tumor tissue and blood of patients with oral squamous cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2005, 9, 361–367. [Google Scholar]
- Bain, C.A.; Weng, D.; Meltzer, A.; Kohles, S.S.; Stach, R.M. A meta-analysis evaluating the risk for implant failure in patients who smoke. Compend. Contin. Educ. Dent. 2002, 20, 628–640. [Google Scholar]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative Processes and Xenobiotic Metabolism in Plants: Mechanisms of Defense and Potential Therapeutic Implications. J. Xenobiot. 2024, 14, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress-The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Vogeley, C.; Sondermann, N.C.; Woeste, S.; Momin, A.A.; Gilardino, V.; Hartung, F.; Heinen, M.; Maaß, S.K.; Mescher, M.; Pollet, M.; et al. Unraveling the differential impact of PAHs and dioxin-like compounds on AKR1C3 reveals the EGFR extracellular domain as a critical determinant of the AHR response. Environ. Int. 2022, 158, 106989. [Google Scholar] [CrossRef] [PubMed]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef]
- Zhao, Z. Hydroxyl radical generations form the physiologically relevant Fenton-like reactions. Free. Radic. Biol. Med. 2023, 208, 510–515. [Google Scholar] [CrossRef]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef]
- Amin, W.; Farah, A.; Mahmoud, A. Quantitative analysis of trace elements in sound and carious enamel of primary and permanent dentitions. Br. J. Med. Med. Res. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Mavropoulos, E. Studies on the mechanisms of lead immobilization by hydroxyapatite. Environ. Sci. Technol. 2002, 36, 1625–1629. [Google Scholar] [CrossRef]
- Qamar, Z. Influence of trace elements on dental enamel properties: A review. J. Pak. Med. Assoc. 2017, 67, 116–120. [Google Scholar]
- Dorta, D.J.; Leite, S.; DeMarco, K.C.; Prado, I.M.; Rodrigues, T.; Mingatto, F.E.; Uyemura, S.A.; Santos, A.C.; Curti, C. A proposed sequence of events for cadmium-induced mitochondrial impairment. J. Inorg. Biochem. 2003, 97, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef]
- Chandrajith, R.; Diyabalanage, S.; Dissanayake, C.B. Geogenic fluoride and arsenic in groundwater of Sri Lanka and its implications to community health. Groundw. Sustain. Dev. 2020, 10, 100359. [Google Scholar] [CrossRef]
- Yang, H. Associations of urinary total arsenic and arsenic species and periodontitis. Int. Dent. J. 2024, 74, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M. Mercury in dental amalgam: A risk analysis. Neurotoxicology 2020, 81, 382–386. [Google Scholar] [CrossRef]
- Joy, A.; Quresi, A. Mecury in dental amalgam, online retail and the Minamata convention on mercury. Environ. Sci. Technol. 2020, 54, 14139–14142. [Google Scholar] [CrossRef]
- Kaneez, F.S.; Masood, A.; Tushar, D. Oxidative Stress Gated by Fenton and Haber–Weiss Reactions and Its Association with Alzheimer’s Disease. Adv. Biomed. Res. 2014, 3, 83. [Google Scholar]
- Primožič, J.; Poljšak, B.; Jamnik, P.; Kovač, V.; Čanadi Jurešić, G.; Spalj, S. Risk Assessment of Oxidative Stress Induced by Metal Ions Released from Fixed Orthodontic Appliances during Treatment and Indications for Supportive Antioxidant Therapy: A Narrative Review. Antioxidants 2021, 10, 1359. [Google Scholar] [CrossRef]
- Tomova, Z.; Tomov, D.; Vlahova, A. The impact of dental metal restorations on the oral oxidative stress level. J. Clin. Exp. Dent. 2023, 15, e205–e209. [Google Scholar] [CrossRef]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed. Laser Surg. 2013, 31, 95–104. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- George, G.N.; Singh, S.P.; Hoover, J.; Pickering, I.J. The chemical forms of mercury in aged and fresh dental amalgam surfaces. Chem. Res. Toxicol. 2009, 22, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Huda, A.; Taghreed, Z.; Ali, Y. Trace elements and oxidative strss markers in saliva of subjects with amalgam fillings. J. Bangh. Coll. Dent. 2012, 24, 63–66. [Google Scholar]
- Daokar, S.; Siddiqui, S.; AlJeaidi, Z.A.; Mustafa, M.; Mapari, P.S.; Nadeem, F. Assessment of Oxidative Stress Induced by Various Restorative Materials: An In Vivo Biochemical Study. J. Int. Oral Health 2016, 8, 670–674. [Google Scholar]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Zalewska, A.; Knaś, M.; Żendzian-Piotrowska, M.; Waszkiewicz, N.; Szulimowska, J.; Prokopiuk, S.; Car, H. Antioxidant profile of salivary glands in high fat diet-induced insulin resistance rats. Oral Dis. 2014, 20, 560–566. [Google Scholar] [CrossRef]
- Mejean, C.; Morzel, M.; Neyraud, E.; Issanchou, S.; Martin, C.; Bozonnet, S. Salivary composition is associated with liking and usual nutrient intake. PLoS ONE 2015, 10, e137473. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S.; Edman, J.S.; Tomlin, K.F.; Perkins, C.E. Effect of 6-month adherence to a very low carbohydrate diet program. Am. J. Med. 2002, 113, 30–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, Y.; Wang, X. Origin and Fate of Acrolein in Foods. Foods 2022, 11, 2083. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, S.-I.; Bae, W.-J.; Min, K.-S.; Shin, E.-S.; Oh, G.-S.; Pae, H.-O.; Kim, E.-C. Heat Stress Activates Interleukin-8 and the Antioxidant System via Nrf2 Pathways in Human Dental Pulp Cells. J. Endod. 2009, 35, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, D.; Brehwens, K.; Halle, M.; Marczyk, M.; Sollazzo, A.; Polanska, J.; Munck-Wikland, E.; Wojcik, A.; Haghdoost, S. Influence of genetic background and oxidative stress response on risk of mandibular osteoradionecrosis after radiotherapy of head and neck cancer. Head Neck 2016, 38, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Lambade, P.N.; Lambade, D.; Goel, M. Osteoradionecrosis of the mandible: A review. Oral Maxillofac. Surg. 2013, 17, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Yeh, H.Y.; Chao, C.T.; Chiang, C.K. Superoxide dismutase 2 (SOD2) in vascular calcification: A focus on vascular smooth muscle cells, calcification pathogenesis, and therapeutic strategies. Oxid. Med. Cell Longev. 2021, 2021, 6675548. [Google Scholar] [CrossRef]
- Kurtul, N.; Gökpınar, E. Salivary lipid peroxidation and total sialic acid levels in smokers and smokeless tobacco users as Maraş powder. Mediat. Inflamm. 2012, 2012, 619293. [Google Scholar] [CrossRef]
- Rai, B.; Kharb, S.; Jain, R.; Anand, S.C. Salivary lipid peroxidation product malonaldehyde in various dental diseases. World J. Med. Sci. 2006, 1, 100–101. [Google Scholar]
- Gokul, S.; Patil, V.S.; Jailkhani, R.; Hallikeri, K.; Kattappagari, K.K. Oxidant-antioxidant status in blood and tumor tissue of oral squamous cell carcinoma patients. Oral Dis. 2010, 16, 29–33. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Celec, P. Oxidative Stress and Antioxidants in the Diagnosis and Therapy of Periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar]
- Davies, M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Aspee, A.; Lissi, E.A. Acid phosphatase reaction with peroxyl radicals: Inactivation mechanism and behavior of the partially modified ensemble. Arch. Biochem. Biophys. 2000, 379, 245–251. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Arenas, A.; Lissi, E.; Silva, E. The role of protein-derived free radicals as intermediaries of oxidative processes. Biomol. Concepts 2014, 5, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Houee-Levin, C.; Bobrowski, K. The use of the methods of radiolysis to explore the mechanisms of free radical modifications in proteins. J. Proteom. 2013, 92, 51–62. [Google Scholar] [CrossRef]
- Dinç, G.; Fentoğlu, Ö.; Doğru, A.; İlhan, İ.; Kırzıoğlu, F.Y.; Orhan, H. The evaluation of salivary oxidative stress in patients with familial mediterranean fever and chronic periodontitis. J. Periodontol. 2018, 89, 1112–1120. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T.; Domazou, A.; Steinmann, D.; Bounds, P.L.; Koppenol, W.H. Reduction of protein radicals by GSH and ascorbate: Potential biological significance. Amino Acids 2010, 39, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Gebicki, J.M. Intracellular GSH and ascorbate inhibit radical-induced protein chain peroxidation in HL-60 cells. Free Radic. Biol. Med. 2012, 52, 420–426. [Google Scholar] [CrossRef]
- Ide, H.; Shoulkamy, M.I.; Nakano, T.; Miyamoto-Matsubara, M.; Salem, A.M. Repair and biochemical effects of DNA-protein cross-links. Mutat. Res. 2011, 711, 113–122. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.R.; Chapple, I.L.; Matthews, J.B. Neutrophil superoxide release and plasma C-reactive protein levels pre- and post-periodontal therapy. J. Clin. Periodontol. 2016, 43, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Daisuke, E.; Takaaki, T.; Naofumi, T.; Toshihiro, S.; Tetsuji, A.; Reiko, Y.; Tatsuo, Y.; Tatsuo, W. Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis. Arch. Oral Biol. 2008, 53, 324–329. [Google Scholar]
- Chapple, I.L.C.; Brock, G.R.; Milward, M.R.; Ling, N.; Matthews, J.B. Compromised GCF total antioxidant capacity in periodontitis: Cause or effect? J. Clin. Periodontol. 2007, 34, 103–110. [Google Scholar] [CrossRef]
- Kanzaki, H.; Wada, S.; Narimiya, T.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front. Physiol. 2017, 8, 351. [Google Scholar] [CrossRef]
- Stanisic, D.; Obradovic, R.; Vujovic, S.; Jovanovic, M.; Zivkovic, V. The connection of periodontal disease and diabetes mellitus: The role of matrix metalloproteinases and oxidative stress. Serbian J. Exp. Clin. Res. 2019, 1019, 1–10. [Google Scholar] [CrossRef]
- Souza, J.A.; Rossa, C.J.; Garlet, G.P.; Nogueira, A.V.; Cirelli, J.A. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J. Appl. Oral. Sci. 2012, 20, 128–138. [Google Scholar] [CrossRef]
- Oben, K.Z.; Alhakeem, S.S.; McKenna, M.K.; Brandon, J.A.; Mani, R.; Noothi, S.K.; Jinpeng, L.; Akunuru, S.; Dhar, S.K.; Singh, I.P. Oxidative stress-induced JNK/AP-1 signaling is a major pathway involved in selective apoptosis of myelodysplastic syndrome cells by Withaferin-A. Oncotarget 2017, 8, 77436–77452. [Google Scholar] [CrossRef]
- Melino, M.; Hii, C.S.; McColl, S.R.; Ferrante, A. The effect of the JNK inhibitor, JIP peptide, on human T lymphocyte proliferation and cytokine production. J. Immunol. 2008, 181, 7300–7306. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Zhang, Y.; Ding, Y. NLRP3 inflammasome activity and periodontal disease pathogenesis–A comprehensive review. J. Oral Microbiol. 2023, 15, 2151234. [Google Scholar]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; González-Torres, P.; Pittis, A.A.; Bejarano, L.A.; Cozzuto, L.; Andreu-Somavilla, N.; Alloza-Trabado, M.; Valentín, A.; Ksiezopolska, E.; Company, C.; et al. Citizen science charts two major “stomatotypes” in the oral microbiome of adolescents and reveals links with habits and drinking water composition. Microbiome 2018, 6, 218. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef]
- Džunková, M.; Martinez-Martinez, D.; Gardlík, R.; Behuliak, M.; Janšáková, K.; Jiménez, N.; Vázquez-Castellanos, J.F.; Martí, J.M.; D’auria, G.; Bandara, H.M.; et al. Oxidative stress in the oral cavity is driven by individual-specific bacterial communities. Biofilms Microbiomes 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Hyde, A.; Parisot, J.; McNichol, A.; Bonev, A. Nisin-induced changes in Bacillus morphology suggest a paradigm of antibiotic action. Proc. Natl. Acad. Sci. USA 2007, 103, 19896–19901. [Google Scholar] [CrossRef]
- Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’Ambrosio, F. The Interaction between the Oral Micro-biome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862–1878. [Google Scholar] [CrossRef]
- Rocha, F.G.; Moye, Z.D.; Ottenberg, G.; Tang, P.; Campopiano, D.J.; Gibson, F.C., 3rd; Davey, M.E. Porphyromonas gingivalis Sphingolipid Synthesis Limits the Host Inflammatory Response. J. Dent. Res. 2020, 99, 568–576. [Google Scholar] [CrossRef]
- Di Spirito, F. Oral-Systemic Health and Disorders: Latest Prospects on Oral Antisepsis. Appl. Sci. 2022, 12, 8185. [Google Scholar] [CrossRef]
- Kołodziej, U.; Maciejczyk, M.; Miąsko, A.; Matczuk, J.; Knaś, M.; Żukowski, P.; Zalewska, A. Oxidative modification in the salivary glands of high fat-diet induced insulin resistant rats. Front. Physiol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Gasmi, B.A.; Noor, S.; Menzel, A.; Gasmi, A. Oral Aphthous: Pathophysiology, Clinical Aspects and Medical Treatment. Arch. Razi. Inst. 2021, 76, 1155–1163. [Google Scholar]
- Avci, E.; Akarslan, Z.Z.; Erten, H.; Coskun-Cevher, S. Oxidative stress and cellular immunity in patients with recurrent aphthous ulcers. Braz. J. Med. Biol. Res. 2014, 47, 355–360. [Google Scholar] [CrossRef]
- Ghasemi, S.; Farokhpour, F.; Mortezagholi, B. Systematic review and meta-analysis of oxidative stress and antioxidant markers in recurrent aphthous stomatitis. PLoS ONE 2023, 18, 960. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ríos, P.; Pussinen, P.J.; Vernal, R.; Hernández, M. Oxidative Stress in the Local and Systemic Events of Apical Periodontitis. Front. Physiol. 2017, 8, 869. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Estornut, C.; Rinaldi, G.; Carceller, M.C.; Estornut, S.; Pérez-Leal, M. Systemic and local effect of oxidative stress on recurrent aphthous stomatitis: Systematic review. J. Mol. Med. 2024, 102, 453–463. [Google Scholar] [CrossRef]
- Waddington, R.J.; Moseley, R.; Embery, G. Periodontal disease mechanisms—Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef]
- Canakci, C.F.; Cicek, Y.; Canakci, V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry 2005, 70, 619–628. [Google Scholar] [CrossRef]
- Mancini, A.; Raimondo, S.; Persano, M.; Silvestrini, A. Oxidative Stress and Inflammation in Osteoporosis. Antioxidants 2023, 12, 642. [Google Scholar]
- Han, Y.; Huang, Y.; Gao, P.; Yang, Q.; Jia, L.; Zheng, Y.; Li, W. Leptin aggravates periodontitis by promoting M1 polarization via NLRP3. J. Dent. Res. 2022, 101, 675–685. [Google Scholar] [CrossRef]
- Liu, X.; Hou, Y.; Yang, M.; Xin, X.; Deng, Y.; Fu, R.; Xiang, X.; Cao, N.; Liu, X.; Yu, W. N-Acetyl-L-Cysteine-derived carbonized polymer dots with ROS scavenging via keap1-nrf2 pathway regulate alveolar bone homeostasis in periodontitis. Adv. Healthc. Mater. 2023, 6, e2300890. [Google Scholar] [CrossRef]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Kajfasz, J.K.; Rivera-Ramos, I.; Scott-Anne, K.; Gregoire, S.; Abranches, J.; Lemos, J.A. Transcription of oxidative stress genes is directly activated by SpxA1 and, to a lesser extent, by SpxA2 in Streptococcus mutans. J. Clin. 2015, 197, 2160–2170. [Google Scholar] [CrossRef]
- Buczko, P.; Zalewska, A.; Szarmach, I. Saliva and oxidative stress in oral cavity and in some systemic disorders. J. Physiol. Pharmacol. 2015, 66, 3–9. [Google Scholar] [PubMed]
- Rahman, M.T.; Hossain, A.; Pin, C.H.; Yahya, N.A. Zinc and metallothionein in the development and progression of dental caries. Biol. Trace. Elem. Res. 2018, 187, 51–58. [Google Scholar] [CrossRef]
- de Sousa Né, Y.G.; Frazão, D.R.; Bittencourt, L.O.; Fagundes, N.C.F.; Marañón-Vásquez, G.; Crespo-Lopez, M.E.; Maia, L.C.; Lima, R.R. Are Dental Caries Associated with Oxidative Stress in Saliva in Children and Adolescents? A Systematic Review. Metabolites 2022, 12, 858. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potential malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Petti, S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003, 39, 770–780. [Google Scholar] [CrossRef]
- Sardaro, N.; Della Vella, F.; Incalza, M.A.; Di Stasio, D.; Lucchese, A.; Contaldo, M.; Laudadio, C.; Petruzzi, M. Oxidative stress and oral mucosal diseases: An overview. In Vivo 2019, 33, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Żukowski, P.; Maciejczyk, M.; Matczuk, J.; Kurek, K.; Waszkiel, D.; Żendzian Piotrowska, M.; Zalewska, A. Effect of N-acetylcysteine on antioxidant defense, oxidative modification, and salivary gland function in a rat model of insulin resistance. Oxid. Med. Cell Longev. 2018, 2018, 6581970. [Google Scholar] [CrossRef]
- Onopiuk, B.M.; Dąbrowska, Z.N.; Rogalska, J.; Brzóska, M.M.; Dąbrowski, A.; Bijowski, K.; Onopiuk, P.; Mroczko, B.; Orywal, K.; Dąbrowska, E. The beneficial impact of the black chokeberry extract against oxidative stress in the sublingual salivary gland of rats intoxicated with cadmium. Oxid. Med. Cell Longev. 2021, 2021, 6622245. [Google Scholar] [CrossRef]
- Alnuaimi, O.; Mammdoh, J.; Al Allaf, L. The Role of Selenium in Mitigating the Adverse Effect of Cyclophosphamide on the Rat Submandibular Salivary Glands. Egypt J. Vet. Sci. 2022, 53, 505–516. [Google Scholar] [CrossRef]
- Ungureanu, L.B.; Grădinaru, I.; Ghiciuc, C.M.; Amălinei, C.; Gelețu, G.L.; Petrovici, C.G.; Stănescu, R.Ș. Atrophy and Inflammatory Changes in Salivary Glands Induced by Oxidative Stress after Exposure to Drugs and Other Chemical Substances: A Systematic Review. Medicina 2023, 59, 1692. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 5, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Ferreiro, M.S.; Gallardo, I.; Newman, H.N.; Bullon, P. The antioxidant capacity of saliva. J. Clin. Periodontol. 2002, 29, 189–194. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Perry, J.J.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef]
- Kang, W.; Jia, Z.; Tang, D.; Zhang, Z.; Gao, H.; He, K.; Feng, Q. Fusobacterium nucleatum facilitates apoptosis, ROS generation, and inflammatory cytokine production by activating AKT/MAPK and NF-kappaB signaling pathways in human gingival fibroblasts. Oxid. Med. Cell Longev. 2019, 2019, 1681972. [Google Scholar]
- Rath-Deschner, B.; Nogueira, A.V.B.; Memmert, S.; Nokhbehsaim, M.; Cirelli, J.A.; Eick, S.; Miosge, N.; Kirschneck, C.; Kesting, M.; Deschner, J.; et al. Regulation of anti-apoptotic SOD2 and BIRC3 in periodontal cells and tissues. Int. J. Mol. Sci. 2021, 22, 20591. [Google Scholar] [CrossRef]
- Lewandowski, L.; Kepinska, M.; Milnerowicz, H. Alterations in concentration/activity of superoxide dismutases in context of obesity and selected single nucleotide polymorphisms in genes: SOD1, SOD2, SOD3. Int. J. Mol. Sci. 2020, 21, 45069. [Google Scholar] [CrossRef]
- Wei, Q.; Qian, S.; Li, N.H.; Hongle, C.; Zhao, W.C. Superoxide dismutase 2 scavenges ROS to promote osteogenic differentiation of human periodontal ligament stem cells by regulating Smad3 in alveolar bone-defective rat. J. Periodontol. 2024, 95, 469–482. [Google Scholar]
- Ding, D.; Li, N.; Ge, Y.; Wu, H.; Yu, J.; Qiu, W.; Fang, F. Current status of superoxide dismutase 2 on oral disease progression by supervision of ROS. Biomed. Pharmacother. 2024, 175, 116605. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zha, L.; Li, B.; Zhang, L.; Yu, T.; Li, L. Correlation between superoxide dismutase 1 and 2 polymorphisms and susceptibility to oral squamous cell carcinoma. Exp. Ther. Med. 2014, 7, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liang, J.; He, Q.; Wan, Q.; Hou, J.; Lian, K.; Wang, A. The serum biomarker chemerin promotes tumorigenesis and metastasis in oral squamous cell carcinoma. Clin. Sci. 2019, 133, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory response mechanisms of the dentine-pulp complex and the periapical tissues. Int. J. Mol. Sci. 2021, 22, 31480. [Google Scholar] [CrossRef]
- Subapriya, R.; Kumaraguruparan, R.; Ramachandran, C.R.; Nagini, S. Oxidant-antioxidant status in patients with oral squamous cell carcinomas at different intraoral sites. Clin. Biochem. 2002, 35, 489–493. [Google Scholar] [CrossRef]
- Bagul, D.N. Serum levels of antioxidant in patients with oral squamous cell carcinoma: A preliminary study. IOSR J. Dent. Med. Sci. 2013, 11, 28–32. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Wroczyński, P.; Samolczyk-Wanyura, D. Comparison of antioxidant enzymes activity and the concentration of uric acid in the saliva of patients with oral cavity cancer, odontogenic cysts and healthy subjects. J. Oral Pathol. Med. 2011, 40, 726–730. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Stancill, J.S.; Corbett, J.A. Hydrogen peroxide detoxification through the peroxiredoxin/thioredoxin antioxidant system: A look at the pancreatic β-cell oxidant defense. Vitam. Horm. 2023, 121, 45–66. [Google Scholar]
- Vlasits, J.; Jakopitsch, C.; Bernroitner, M.; Zamocky, M.; Furtmüller, P.G.; Obinger, C. Mechanisms of catalase activity of heme peroxidases. Arch. Biochem. Biophys. 2010, 500, 74–81. [Google Scholar] [CrossRef]
- Brunelli, L.; Yermilov, J.S.; Beckman, C. Modulation of catalase peroxidatic and catalatic activity by nitric oxide. Free Radic. Biol. Med. 2001, 30, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Fraile, M.; González-Jubete, A.; González, L.O.; Vizoso, F.J. Mesenchymal (Stem) Stromal Cells Based as New Therapeutic Alternative in Inflammatory Bowel Disease: Basic Mechanisms, Experimental and Clinical Evidence, and Challenges. Int. J. Mol. Sci. 2022, 23, 8905. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, E.; Doğan, S. Glutathione Peroxidase in Health and Diseases. Glutathione System and Oxidative Stress in Health and Disease. IntechOpen 2020, 49, 1–16. [Google Scholar]
- Maciejczyk, M.; Zalewska, A.; Ładny, J.R. Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly. Oxid. Med. Cell Longev. 2019, 2019, 4393460. [Google Scholar] [CrossRef]
- Schlorke, D.; Flemmig, J.; Gau, J.; Furtmüller, P.G.; Obinger, C.; Arnhold, J. New insights into thiocyanate oxidation by human myeloperoxidase. J. Inorg. Biochem. 2016, 162, 117–126. [Google Scholar] [CrossRef]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. Int. J. Mol. Sci. 2019, 20, 1443. [Google Scholar] [CrossRef]
- Nijakowski, K.; Jankowski, J.; Gruszczyński, D.; Surdacka, A. Salivary Alterations of Myeloperoxidase in Patients with Systemic Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12078. [Google Scholar] [CrossRef]
- Sande López, L.; García-Mato, E.; de Coo, A.; Cruz, R.; Antequera, D.; Diz, P.; Carro, E.; Rivas, B. Salivary Lactoferrin Levels and Polymorphisms in Down Syndrome Individuals with Periodontitis. J. Clin. Med. 2025, 14, 1815. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defense against microbial and viral attack. Cell Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef]
- Jaiswal, A.; Madaan, S.; Acharya, N.; Kumar, S.; Talwar, D.; Dewani, D. Salivary Uric Acid: A Noninvasive Wonder for Clinicians? Cureus 2021, 13, e19649. [Google Scholar] [CrossRef] [PubMed]
- Muchandi, S.; Walimbe, H.; Bijle, M.; Nankar, M.; Chaturvedi, S.; Karekar, P. Comparative evaluation and correlation of salivary total antioxidant capacity and salivary pH in caries-free and severe early childhood caries children. J. Contemp. Dent. Pract. 2015, 16, 234–237. [Google Scholar] [PubMed]
- Vernerová, A.; Kujovská Krčmová, L.; Melichar, B.; Švec, F. Non-invasive determination of uric acid in human saliva in the diagnosis of serious disorders. Clin. Chem. Lab. Med. 2020, 59, 797–812. [Google Scholar] [CrossRef]
- Narang, D.; Jain, A. Estimation of salivary uric acid in dental caries: A biochemical study. J. Adv. Med. Dent. Scie. Res. 2019, 7, 106–108. [Google Scholar]
- Lushchak, V.I. Oxidative stress and mechanisms of protection against it in bacteria. Biochemistry 2001, 66, 476–489. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Vijayavel, K.; Gopalakrishnan, S.; Thilagam, H.; Balasubramanian, M.P. Dietary ascorbic acid and α-tocopherol mitigates oxidative stress induced by copper in the thornfish Terapon jarbua. Sci. Total Environ. 2006, 372, 157–163. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chen, H.S.; Chen, S.L.; Ho, Y.P.; Ho, K.Y.; Wu, Y.M.; Hung, C.C. Lipid peroxidation: A possible role in the induction and progression of chronic periodontitis. J. Periodont. Res. 2005, 40, 378–384. [Google Scholar] [CrossRef]
- Dalai, C.; Ignat-Romanul, I.; Rosca, E.; Muresan, M.; Micle, O.; Bodog, F. Correlation between histopathological aspects of periodontitis and biochemical changes of oxidative stress. Rom. J. Morphol. Embryol. 2013, 54, 817–822. [Google Scholar]
- Oztürk, L.K.L.; Furuncuoglu, H.; Atala, M.H.; Uluköylü, O.; Akyüz, S.; Yarat, A. Association between dental-oral health in young adults and salivary glutathione, lipid peroxidation and sialic acid levels and carbonic anhydrase activity. Braz. J. Med. Biol. Res. 2008, 41, 956–959. [Google Scholar] [CrossRef]
- De Araujo, F.F.; Marcon, R.M.; Cristante, A.F.; Filho, T. Glutathione effect on functional and histological recovery after spinal cord injury in rats. Clinics 2024, 23, 100359. [Google Scholar] [CrossRef] [PubMed]
- Kükürt, A.; Gelen, V. Understanding Vitamin C: Comprehensive Examination of Its Biological Significance and Antioxidant Properties. Ascorbic Acid—Biochemistry and Functions; IntechOpen: London, UK, 2024; pp. 1–14. [Google Scholar]
- Sen Gupta, P.; Karmakar, S.; Biswas, I.; Ghosal, J.; Banerjee, A.; Roy, S.; Mandal, D.P.; Bhattacharjee, S. Vitamin E alleviates chlorpyrifos induced glutathione depletion, lipid peroxidation and iron accumulation to inhibit ferroptosis in hepatocytes and mitigate toxicity in zebrafish. Chemosphere 2024, 359, 142252. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current Use of Fenton Reaction in Drugs and Food. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.; Dinică, R.M.; Bahrim, G.E.; Vizireanu, C.; Baroiu, L.; Iancu, A.V.; Drăgănescu, M. New Insights into the Antioxidant Compounds of Achenes and Sprouted Buckwheat Cultivated in the Republic of Moldova. Appl. Sci. 2021, 11, 10230. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H.; Truscott, T.G. The interaction of dietary carotenoids with radical species. Arch. Biochem. Biophys. 2001, 385, 13–19. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Upasana, B.; Jyotirekha, G.H. Chapter 6—Plant polyphenols as potent antioxidants: Highlighting the mechanism of antioxidant activity and synthesis/development of some polyphenol conjugates. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–266. [Google Scholar]
- Lupoae, M.; Lupoae, P.; Borda, D.; Cristea, V.; Bocioc, E. Allelopathic potential of the Ranunculus rionii L. and Ceratophyllum demersum L. extracts against microbial and microalgal cultures. Environ. Eng. Manag. 2016, 15, 473–480. [Google Scholar] [CrossRef]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef]
- Ozgová, S.; Hermánek, J.; Gut, I. Different antioxidant effects of polyphenols on lipid peroxidation and hydroxyl radicals in the NADPH-, Fe-ascorbate- and Fe-microsomal systems. Biochem. Pharmacol. 2003, 66, 1127–1137. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Edo, G.I.; Nwachukwu, S.C.; Ali, A.B.M.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. A review on the composition, extraction and applications of phenolic compounds. Ecol. Front. 2025, 45, 7–23. [Google Scholar] [CrossRef]
- Rogozinska, M.; Biesaga, M. Decomposition of Flavonols in the Presence of Saliva. Appl. Sci. 2020, 10, 7511. [Google Scholar] [CrossRef]
- Schestakow, A.; Meyer-Probst, C.T.; Hannig, C.; Hannig, M. Prevention of dental biofilm formation with polyphenols: A systematic review. Planta Med. 2023, 89, 1026–1033. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; Chen, F.; Chai, Y. Polyphenols in Oral Health: Homeostasis Maintenance, Disease Prevention, and Therapeutic Applications. Nutrients 2023, 15, 4384. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, H.; Shompa, S.A.; Islam, M.M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, M.N.R.; Fatema, N.; et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon 2024, 10, e27533. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Pandey, M.; Gupta, S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009, 85, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Chu, P.M.; Tuan, V.P.; Te, J.S.L.; Lee, I.T. The promising role of antioxidant phytochemicals in the prevention and treatment of periodontal disease via the inhibition of oxidative stress pathways: Updated insights. Antioxidants 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Nagy, M.; Mučaji, P.; Grančai, D. Pharmacognosy Biogenesis of Natural Substances; Osveta: Martin, Slovakia, 2011. [Google Scholar]
- Jain, P.L.B.; Patel, S.R.; Desai, M.A. Patchouli oil: An overview on extraction method, composition and biological activities. J. Essent. Oil Res. 2022, 34, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant activities of essential oils and their major components in scavenging free radicals, inhibiting lipid oxidation and reducing cellular oxidative stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl radical reactions in water solution: A gym for proton-coupled electron-transfer theories. Food Chem. 2016, 22, 7924–7934. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, Ş. Essential oil composition and antioxidant activities of eight cultivars of lavender and lavandin from western Anatolia. Ind. Crop. Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Lajayer, B.A.; Hadian, J.; Astatkie, T. Applications of essential oils and plant extracts in different industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Locatelli, M.; Pereira, J.A.M.; Oliveira, H.; Arlorio, M.; Fernandes, I.; Perestrelo, R.; Freitas, V.; Bordiga, M. Behind the scenes of anthocyanins—From the health benefits to potential applications in food, pharmaceutical and cosmetic fields. Nutrients 2022, 14, 5133. [Google Scholar] [CrossRef]
- González-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Hu, J.P.; Takahashi, N.; Yamada, T. Coptidis Rhizoma inhibits growth and proteases of oral bacteria. Oral Dis. 2000, 6, 297–302. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharmacol. Res. 2017, 123, 92–102. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Paul, M.; Ma, J.K.-C. Plant-made pharmaceuticals: Leading products and production platforms. Biotechnol. Appl. Biochem. 2011, 58, 58–67. [Google Scholar] [CrossRef]
- Caprari, C.; Fantasma, F.; Monaco, P.; Divino, F.; Iorizzi, M.; Ranalli, G.; Fasano, F.; Saviano, G. Chemical profiles, in vitro antioxidant and antifungal activity of four different Lavandula angustifolia L. EOs. Molecules 2023, 28, 392. [Google Scholar] [CrossRef] [PubMed]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef]
- Nedeltcheva-Antonova, D.; Gechovska, K.; Bozhanov, S.; Antonov, L. Exploring the Chemical Composition of Bulgarian Lavender Absolute (Lavandula angustifolia Mill.) by GC/MS and GC-FID. Plants 2022, 11, 3150. [Google Scholar] [CrossRef]
- Turrini, F.; Beruto, M.; Mela, L.; Curir, P.; Triglia, G.; Boggia, R.; Zunin, P.; Monroy, F. Ultrasound-Assisted Extraction of Lavender (Lavandula angustifolia Miller, Cultivar Rosa) Solid By-Products Remaining after the Distillation of the Essential Oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. Supercritical Carbon Dioxide (scCO2) Extraction of Phenolic Compounds from Lavender (Lavandula angustifolia) Flowers: A Box-Behnken Experimental Optimization. Molecules 2019, 24, 3354. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Nat. Prod. Res. 2017, 31, 2575–2580. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Da Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.D.S.; Donadio, M.; Nunes, F.B.; De Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Ciências 2015, 87, 1397–1408. [Google Scholar] [CrossRef]
- Yadikar, N.; Bobakulov, K.; Li, G.; Aisa, H.A. Seven new phenolic compounds from Lavandula angustifolia. Phytochem. Lett. 2018, 23, 149–154. [Google Scholar] [CrossRef]
- Héral, B.; Stierlin, É.; Fernandez, X.; Michel, T. Phytochemicals from the genus Lavandula: A review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- Dong, G.; Bai, X.; Aimila, A.; Aisa, H.; Maiwulanjiang, M. Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC. Molecules 2020, 25, 3166. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Koşar, M.; Başer, K.H.C.; Hiltunen, R. Phenolic Profile and Antioxidant Evaluation of Mentha x piperita L. (Peppermint) Extracts. Nat. Prod. Commun. 2009, 4, 1934578X0900400419. [Google Scholar] [CrossRef]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.S.; Battistin, A.; Pauletti, G.; Rota, L.; Serafini, L.A. Antioxidant properties of essential oils from Mentha species evidenced by electrochemical methods. Rev. Bras. Plantas Med. 2009, 11, 372–382. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Ma, A.; Bao, Y.; Wang, M.; Sun, Z. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Taylan, O.; Cebi, N.; Sagdic, O. Rapid screening of Mentha spicata essential oil and L-menthol in Mentha piperita essential oil by ATR-ftir spectroscopy coupled with multivariate analyses. Foods 2021, 10, 202. [Google Scholar] [CrossRef]
- Mahendran, G.; Rahman, L. Ethnomedicinal, phytochemical and pharmacological updates on peppermint (Mentha piperita)—A review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef] [PubMed]
- Abdi, G.; Shokrpour, M.; Karami, L.; Salami, S.A. Prolonged Water Deficit Stress and Methyl Jasmonate-Mediated Changes in Metabolite Profile, Flavonoid Concentrations and Antioxidant Activity in Peppermint (Mentha × piperita L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 70–80. [Google Scholar] [CrossRef]
- Arrahmouni, R.; Ouazzani, C.; Er-Ramly, A.; Moustaghfir, A.; Dami, A.; Ballouch, L. Chemical composition of Moroccan commercial essential oils of mint: Mentha spicata, Mentha piperita, and Mentha pulegium. Trop. J. Nat. Prod. Res. 2023, 7, 2708–2712. [Google Scholar]
- Al-Mijalli, S.H.; Elsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of volatile compounds of Mentha piperita and Lavandula multifida and investigation of their antibacterial, antioxidant, and antidiabetic properties. Evid. Based Complement. Altern. Med. 2022, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.R.; Akhtar, S.; Sestili, P.; Ismail, T.; Neugart, S.; Qamar, M.; Esatbeyoglu, T. Toxicity, Antioxidant Activity, and Phytochemicals of Basil (Ocimum basilicum L.) Leaves Cultivated in Southern Punjab, Pakistan. Foods 2022, 11, 1239. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Diversitatea, potențialul fitochimic și medicinal al genului Ocimum L. (Lamiaceae). Phytochem. Rev. 2020, 19, 907–953. [Google Scholar] [CrossRef]
- Hosamane, M.; Acharya, A.B.; Vij, C.; Trivedi, D.; Setty, S.B.; Thakur, S.L. Evaluation of holy basil mouthwash as an adjunctive plaque control agent in a four-day plaque regrowth model. J. Clin. Exp. Dent. 2014, 6, e491–e496. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, anti-oxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Benyoucef, F.; Dib, M.E.A.; Arrar, Z.; Costa, J.; Muselli, A. Synergistic Antioxidant Activity and Chemical Composition of Essential Oils from Thymus fontanesii, Artemisia herba-alba and Rosmarinus officinalis. J. Appl. Biotechnol. Rep. 2018, 5, 151–156. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.; Sayyad, A.; Stosic-Grujicic, N.; Stojanovic, S.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and Antioxidant properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L., Laminaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Seo, J.W.; Ham, D.Y.; Choi, H.J.; Kim, M.J.; Na, J.K.; Kim, S.K.; Seong, E.S. Antioxidant Activity and Phenolic Compound of Rosemary Under Artificial LED Lights. Agronomy 2025, 15, 636. [Google Scholar] [CrossRef]
- Bejenaru, L.E.; Segneanu, A.-E.; Bejenaru, C.; Biţă, A.; Tuţulescu, F.; Radu, A.; Ciocîlteu, M.V.; Mogoşanu, G.D. Seasonal Variations in Chemical Composition and Antibacterial and Antioxidant Activities of Rosmarinus officinalis L. Essential Oil from Southwestern Romania. Appl. Sci. 2025, 15, 681. [Google Scholar] [CrossRef]
- Chatterjee, K.; Tamta, B.; Mukopadayay, S. A review on “pharmacological, phytochemical, and medicinal properties of Rosmarinus officinalis (Rosemary)”. Int. J. Health Sci. 2022, 6, 3491–3500. [Google Scholar] [CrossRef]
- Dent, M.; Kovačević, D.B.; Bosiljkov, T.; Dragović-Uzelac, V. Polyphenolic Composition and Antioxidant Capacity of Indigenous Wild Dalmatian Sage (Salvia officinalis L.). Croat. Chem. Acta 2017, 90, 451–460. [Google Scholar] [CrossRef]
- Maleš, I.; Dragović-Uzelac, V.; Jerković, I.; Zorić, Z.; Pedisić, S.; Repajić, M.; Garofulić, I.E.; Dobrinčić, A. Non-Volatile and Volatile Bioactives of Salvia officinalis L., Thymus serpyllum L. and Laurus nobilis L. Extracts with Potential Use in the Development of Functional Beverages. Antioxidants 2022, 11, 1140. [Google Scholar] [CrossRef] [PubMed]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef]
- Francik, S.; Francik, R.; Sadowska, U.; Bystrowska, B.; Zawiślak, A.; Knapczyk, A.; Nzeyimana, A. Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials 2020, 13, 5811. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Ferrante, C.; Menghini, L.; Angelini, P.; Flores, G.A.; Muscatello, B.; Braca, A.; De Leo, M. Hydrosols from Rosmarinus officinalis, Salvia officinalis, and Cupressus sempervirens: Phytochemical Analysis and Bioactivity Evaluation. Plants 2022, 11, 349. [Google Scholar] [CrossRef]
- Bojor, O. Guide of Medicinal and Aromatic Plants from A to Z; Fiat Lux: Bucharest, Romania, 2003; pp. 94–95. [Google Scholar]
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical Profile and Biological Activities of Satureja hortensis L.: A Review of the Last Decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Masoum, S.; Mehrara, B.; Hosseini, H. Application of linear multivariate calibration techniques to identify the peaks responsible for the antioxidant activity of Satureja hortensis L. and Oliveria decumbens Vent. essential oils by gas chromatography–mass spectrometry. J. Chromatogr. B. 2015, 1001, 75–81. [Google Scholar] [CrossRef]
- Jafari, F.; Ghavidel, F.; Zarshenas, M.M. A Critical Overview on the Pharmacological and Clinical Aspects of Popular Satureja Species. J. Acupunct. Meridian Stud. 2016, 9, 118–127. [Google Scholar] [CrossRef]
- Sharifzadeh, A.; Khosravi, A.R.; Ahmadian, S. Chemical composition and antifungal activity of Satureja hortensis L. essential oil against planktonic and biofilm growth of Candida albicans isolates from buccal lesions of HIV+ individuals. Microb. Pathogen 2016, 96, 1–9. [Google Scholar] [CrossRef]
- Mašković, J.M.; Jakovljević, V.; Živković, V.; Mitić, M.; Kurćubić, L.V.; Mitić, J.; Mašković, P.Z. Optimization of Ultrasound-Assisted Extraction of Phenolics from Satureja hortensis L. and Antioxidant Activity: Response Surface Methodology Approach. Processes 2024, 12, 2042. [Google Scholar] [CrossRef]
- Plánder, S.; Gontaru, L.; Blazics, B.; Veres, K.; Kéry, Á.; Kareth, S.; Simándi, B. Major antioxidant constituents from Satureja hortensis L. extracts obtained with different solvents. Eur. J. Lipid Sci. Technol. 2012, 114, 772–779. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M. Pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2016, 54, 375–412. [Google Scholar] [CrossRef]
- Khlebnikova, D.A.; Efanova, E.M.; Danilova, N.A.; Shcherbakova, Y.V.; Rivera Sidorova, I. Flavonoid Accumulation in an Aseptic Culture of Summer Savory (Satureja hortensis L.). Plants 2022, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Movahhedkhah, S.; Rasouli, B.; Seidavi, A.; Mazzei, D.; Laudadio, V.; Tufarelli, V. Summer Savory (Satureja hortensis L.) Extract as Natural Feed Additive in Broilers: Effects on Growth, Plasma Constituents, Immune Response, and Ileal Microflora. Animals 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Raudone, L.; Zymone, K.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V.; Janulis, V. Phenological Changes in Triterpenic and Phenolic Composition of Thymus L. Species. Ind. Crops Prod. 2017, 109, 445–451. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization of Aroma-Active and Phenolic Profiles of Wild Thyme (Thymus serpyllum) by GC-MS-Olfactometry and LC-ESI-MS/MS. J. Food Sci. Technol. 2016, 53, 1957–1965. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Pressurized-Liquid Extraction as an Efficient Method for Valorization of Thymus serpyllum Herbal Dust towards Sustainable Production of Antioxidants. Molecules 2021, 26, 2548. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef]
- Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Hidalgo-García, L.; Molina-Tijeras, J.A.; García, F.; Pischel, I.; Romero, M.; Duarte, J.; Diez-Echave, P.; Rodríguez-Cabezas, M.E.; et al. The Antioxidant Activity of Thymus serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice. Antioxidants 2022, 11, 1073. [Google Scholar] [CrossRef]
- Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Kljakić, A.C.; Pojić, M.; Mandić, A.; Stupar, A.; Santos, F.; Duarte, A.R.C.; Mišan, A. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules 2022, 27, 1508. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Health-Promoting Effects of Thymus Phenolic-Rich Extracts: Antioxidant, Anti-Inflammatory and Antitumoral Properties. Antioxidants 2020, 9, 814. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Félix, L.M.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Polyphenol composition and biological activity of Thymus citriodorus and Thymus vulgaris: Comparison with endemic Iberian Thymus species. Food Chem. 2020, 331, 127362. [Google Scholar] [CrossRef] [PubMed]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef]
- Pandur, E.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas aeruginosa LPS-Activated THP-1 Macrophages. Antioxidants 2022, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, K.; Dimov, M.; Valev, T.; Iliev, I.; Damyanova, S.; Oprea, O.B.; Stoyanova, A. Chemical Composition and Antioxidant Activities of Three Bulgarian Garden Thyme Essential Oils. Appl. Sci. 2024, 14, 10261. [Google Scholar] [CrossRef]
- Wesolowska, A.; Jadczak, D. Comparison of the chemical composition of essential oils isolated from two thyme (Thymus vulgaris L.) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 829–835. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef]
- Stoilova, I.; Bail, S.; Buchbauer, G.; Krastanov, A.; Stoyanova, A.; Schmidt, E.; Jirovetz, L. Chemical composition, olfactory evaluation and antioxidant effects of an essential oil of Thymus vulgaris L. from Germany. Nat. Prod. Commun. 2008, 3, 1047–1050. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- El-Assri, E.-M.; Eloutassi, N.; El Barnossi, A.; Bakkari, F.; Hmamou, A.; Bouia, A. Wild chamomile (Matricaria recutita L.) from the Taounate Province, Morocco: Extraction and valorisation of the antibacterial activity of its essential oils. Trop. J. Nat. Prod. Res. 2021, 5, 883–888. [Google Scholar]
- El-Hefny, M.; Abo Elgat, W.A.A.; Al-Huqail, A.A.; Ali, H.M. Essential and recovery oils from Matricaria chamomilla flowers as environmentally friendly fungicides against four fungi isolated from cultural heritage objects. Processes 2019, 7, 809. [Google Scholar] [CrossRef]
- Maynard, R.C.; Ogundipe, S.O.; Ferrarezi, R.S.; Suh, J.H.; Lombardini, L. Apigenin Accumulation in Matricaria chamomilla and Petroselinum crispum Produced in a Vertical Hydroponic System. HortScience 2025, 60, 17–22. [Google Scholar] [CrossRef]
- Ghoniem, A.A.; El-Hai, K.M.A.; El-Khateeb, A.Y.; Eldadamony, N.M.; Mahmoud, S.F.; Elsayed, A. Enhancing the potentiality of Trichoderma harzianum against pythium pathogen of beans using chamomile (Matricaria chamomilla, L.) flower extract. Molecules 2021, 26, 1178. [Google Scholar] [CrossRef] [PubMed]
- Petrulova, V.; Vilkova, M.; Kovalikova, Z.; Sajko, M.; Repcak, M. Ethylene Induction of non-enzymatic metabolic antioxidants in Matricaria chamomilla. Molecules 2020, 25, 5720. [Google Scholar] [CrossRef]
- Orav, A.; Raal, A.; Arak, E. Content and composition of the essential oil of Chamomilla recutita (L.) Rauschert from some European countries. Nat. Prod. Res. 2010, 24, 48–55. [Google Scholar] [CrossRef]
- Piri, E.; Sourestani, M.M.; Khaleghi, E.; Mottaghipisheh, J.; Zomborszki, Z.P.; Hohmann, J.; Csupor, D. Chemo-diversity and antiradical potential of twelve Matricaria chamomilla L. populations from Iran: Proof of ecological effects. Molecules 2019, 24, 1315. [Google Scholar] [CrossRef]
- Elsemelawy, S.A. Antidiabetic and antioxidative activity of chamomile (Matricaria chamomilla L.) powder on diabetic rats. J. Stud. Searches Specif. Educ. 2017, 3, 97–112. [Google Scholar] [CrossRef]
- Pouille, C.L.; Ouaza, S.; Roels, E.; Behra, J.; Tourret, M.; Molinié, R.; Fontaine, J.-X.; Mathiron, D.; Gagneul, D.; Taminiau, B. Chicory: Understanding the effects and effects of this functional food. Nutrients 2022, 14, 957. [Google Scholar] [CrossRef]
- Lupanova, I.A. Study of Cichorium intybus L. Herb extract hepatoprotective activity in vitro and in vivo. Probl. Biol. Med. Pharm. Chem. 2022, 25, 20–30. [Google Scholar] [CrossRef]
- Saeed, M.; Abd El-Hack, M.E.; Alagawany, M.; Arain, M.A.; Muhammad Arif, M.A.; Mirza, M.A.; Naveed, M.; Chao Sun, C.S.; Muhammad Sarwar, M.S.; Maryam Sayab, M.S. Plant Cichorium intybus (Cichory): Chemical composition, pharmacology, nutritional and health applications. J. Int. Pharmacol. 2017, 13, 351–360. [Google Scholar] [CrossRef]
- Shin, H.; Kim, J.; Heo, H.; Lee, J.; Kim, Y. Comparison between antioxidant activities and functional components of roasted chicory root extracts produced using different ethanol concentrations. J. Korean Soc. Food Sci. Nutr. 2024, 53, 272–280. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical composition and nutritional benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative feed for animals. Sci. World J. 2017, 2017, 7343928. [Google Scholar]
- Puhlmann, M.L.; de Vos, W.M. Back to the roots: Review of the use of fiber-rich taproots Cichorium intybus L. Adv. Nutr. 2020, 11, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Rahimullah, T.G. Phytochemical and antibacterial screening of Cichorium intybus seeds used in traditional medicine systems of Pakistan. Int. J. Basic Med. Pharmacol. 2019, 8, 2049. [Google Scholar]
- Upadhayay, V.K.; Pandey, D.; Singh, S.P.; Gohat, T. Chicory (Cichorium intybus): An ethnomedicinal plant with broad-spectrum medicinal properties. In Plant Secondary Metabolites for Human Health; Discovery Publishing House Pvt. Ltd.: New Delhi, India, 2021; Chapter 12; pp. 150–169. [Google Scholar]
- Ashwlayan, V.D.; Kumar, A.; Verma, M.; Garg, V.K.; Gupta, S. Therapeutic Potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6, 149–155. [Google Scholar] [CrossRef]
- Chroho, M.; Drioiche, A.; Saidi, S.; Zair, T.; Bouissane, L. Total phenolic and flavonoids contents and in vitro evaluation of antioxidant activity of several Calendula officinalis (Marigold) extracts. J. Biol. Res. 2021, 94, 9680. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef]
- Yalgi, V.S.; Bhat, K.G. Compare and evaluate the antibacterial efficacy of sodium hypochlorite and Calendula officinalis against Streptococcus mutans as a root canal irrigating solution: An in vivo study. J. Int. Oral Health 2020, 12, 74–79. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Nayagam, A.A.; Natarajan, R. Wound healing potentials of herbal ointment containing Calendula officinalis Linn. on the alteration of immunological markers and biochemical parameters in excision wounded animals. Clin. Phytosci. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- El-Sayed, M.K.; Hommos, A.M.; Kotry, G.S.; Labib, G.S. The effect of a calendula-based topical formula versus oxidized regenerated cellulose on palatal wound healing: A randomized controlled clinical trial. Alex. Dent. J. 2021, 46, 45–53. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic fractions from dandelion leaves and petals as modulators of the lipid profile and antioxidant status in an in vivo study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef]
- Jedrejek, D.; Lis, B.; Rolnik, A.; Stochmal, A.; Olas, B. Comparative phytochemical, cytotoxicity, antioxidant and hemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019, 126, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M.J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods. 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Lis, B.; Jedrejek, D.; Rywaniak, J.; Soluch, A.; Stochmal, A.; Olas, B. Flavonoid Preparations from Taraxacum officinale L. Fruits-A Phytochemical, Antioxidant and Hemostasis Studies. Molecules 2020, 25, 5402. [Google Scholar] [CrossRef]
- Sareedenchai, V.; Zidorn, C. Flavonoids as chemosystematic markers in the tribe Cichorieae of the Asteraceae. Biochem. Syst. Ecol. 2010, 38, 935–957. [Google Scholar] [CrossRef]
- Carlotto, J.; Da Silva, L.M.; Dartora, N.; Maria-Ferreira, D.; Sabry, D.D.A.; Filho, A.P.S.; Werner, M.F.d.P.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M.; et al. Identification of a dicaffeoylquinic acid isomer from Arctium lappa with a potent anti-ulcer activity. Talanta 2015, 135, 50–57. [Google Scholar] [CrossRef]
- Carlotto, J.; de Souza, L.M.; Baggio, C.H.; Werner, M.F.D.P.; Maria-Ferreira, D.; Sassaki, G.L.; Iacomini, M.; Cipriani, T.R. Polysaccharides from Arctium lappa L.: Chemical structure and biological activity. Int. J. Biol. Macromol. 2016, 91, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; Alsharif, S.M.; Xiao, J.; Musharraf, S.G.; Zhao, C.; Saeed, A.; Gao, R.; Said, N.S.; Di Minno, A.; Daglia, M.; et al. Arctium lappa (Burdock): Insights from ethnopharmacology potential, chemical constituents, clinical studies, pharmacological utility and nanomedicine. Biomed Pharmacother. 2023, 158, 114104. [Google Scholar] [CrossRef]
- De Almeida, A.B.A.; Sánchez-Hidalgo, M.; Martín, A.R.; Luiz-Ferreira, A.; Trigo, J.R.; Vilegas, W.; Dos Santos, L.C.; Souza-Brito, A.R.M.; De La Lastra, C.A. Anti-inflammatory intestinal activity of Arctium lappa L. (Asteraceae) in TNBS colitis model. J. Ethnopharmacol. 2013, 146, 300–310. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of five new classes of chlorogenic acids in burdock (Arctium lappa L.) roots by liquid chromatography/tandem mass spectrometry. Food Funct. 2011, 2, 63–71. [Google Scholar] [CrossRef]
- Mouhid, L.; Gómez de Cedrón, M.; Quijada-Freire, A.; Fernández-Marcos, P.J.; Reglero, G.; Fornari, T.; Ramírez de Molina, A. Yarrow supercritical extract ameliorates the metabolic stress in a model of obesity induced by high-fat diet. Nutrients 2019, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Peixoto, V.; Teixeira, A.; Sousa, D.; Barros, L.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Achillea millefolium L. hydroethanolic extract inhibits growth of human tumor cell lines by interfering with cell cycle and inducing apoptosis. Food Chem. Toxicol. 2018, 118, 635–644. [Google Scholar] [CrossRef]
- Vidović, S.; Vasić, A.; Vladić, J.; Jokić, S.; Aladić, K.; Gavarić, A.; Nastić, N. Carbon dioxide supercritical fluid extracts from yarrow and rose hip herbal dust as valuable sources of aromatic and lipophilic compounds. Sustain. Chem. Pharm. 2021, 22, 100494. [Google Scholar] [CrossRef]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A review of its ethnomedicinal uses, phytochemistry, and pharmacological activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of polyphenol profile and antimutagenic and antioxidant activities in two species used as sources of Solidaginis herba—Goldenrod. Chem. Biodivers. 2018, 15, e1800023. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Solidago virgaurea L., Herba; European Medicines Agency: London, UK, 2008. [Google Scholar]
- Thiem, B.; Wesołowska, M.; Skrzypczak, L.; Budzianowski, J. Phenolic compounds in two Solidago L. species from in vitro culture. Acta Pol. Pharm. 2001, 58, 277–281. [Google Scholar]
- Dobjanschi, L.; Paltinean, R.; Vlase, L.; Babota, M.; Fritea, L.; Tamas, M. Comparative phytochemical research of Solidago genus: S. graminifolia. Note I. Flavonoids. Acta Biol. Marisiensis 2018, 1, 18–26. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, R.; Singh, D. Plant polyphenols, terpenes, and terpenoids in oral health: A comprehensive review. J. Med. Plants Res. 2024, 18, 123–135. [Google Scholar]
- Miastkowska, M.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Kucharska, M.; Kilanowicz, A.; Cudzik, D.; Cudzik, K. Enhanced biological activity of a novel preparation of Lavandula angustifolia essential oil. Molecules 2021, 26, 2458. [Google Scholar] [CrossRef]
- Radu, C.M.; Radu, C.C.; Bochiș, S.A.; Arbănași, E.M.; Lucan, A.I.; Murvai, V.R.; Zaha, D.C. Revisiting the therapeutic effects of essential oils on the oral microbiome. Pharmacy 2023, 11, 33. [Google Scholar] [CrossRef]
- Betlej, I.; Andres, B.; Cebulak, T.; Kapusta, I.; Balawejder, M.; Żurek, N.; Jaworski, S.; Lange, A.; Kutwin, M.; Pisulewska, E. Phytochemical composition and antimicrobial properties of new Lavandula angustifolia ecotypes. Molecules 2024, 29, 1740. [Google Scholar] [CrossRef] [PubMed]
- Altaei, D.T. Topical lavender oil for the treatment of recurrent aphthous ulceration. Am. J. Dent. 2012, 25, 39–43. [Google Scholar] [PubMed]
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic compounds and biological activity of selected Mentha species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential oil and extracts, their biological activities, and perspectives on the development of new medicinal and cosmetic products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Wang, Y.; Li, Y.; Li, Q.; Zhang, L. The distinctive role of menthol in pain and analgesia: Mechanisms, practices, and advances. Front. Mol. Neurosci. 2022, 15, 1006908. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Rani, N.; Singla, R.K.; Narwal, S.; Tanushree; Kumar, N.; Rahman, M.M. Medicinal plants used as an alternative to treat gingivitis and periodontitis. Evid. Based Complement. Alternat. Med. 2022, 2022, 2327641. [Google Scholar] [CrossRef]
- da Silva Moura, E.d.S.; D’Antonino Faroni, L.R.; Fernandes Heleno, F.F.; Aparecida Zinato Rodrigues, A.A.Z.; Figueiredo Prates, L.H.; Lopes Ribeiro de Queiroz, M.E. Optimal extraction of Ocimum basilicum essential oil by association of ultrasound and hydro-distillation and its potential as a biopesticide against a major stored grains pest. Molecules 2020, 25, 2781. [Google Scholar] [CrossRef] [PubMed]
- Şahin, H.; Turumtay, E.A.; Yildiz, O.; Kolayli, S. Grayanotoxin-III detection and anti-oxidant activity of mad honey. Int. J. Food Prop. 2015, 18, 2665–2674. [Google Scholar] [CrossRef]
- Mohamed, F.H.; Abd Elaziz, N.Y. Impact of Ocimum basilicum leaves powder on immune response of chicken vaccinated against Newcastle disease virus. Egypt. J. Agric. Res. 2020, 98, 270–287. [Google Scholar]
- Tewari, D.; Pandey, H.K.; Sah, A.N.; Meena, H.; Chander, V.; Singh, R.; Singh, P. Phytochemical, anti-oxidant and antidepressant evaluation of Ocimum basilicum enuiflorum kilimandscharicum grown in India. J. Biol. Active Prod. Nat. 2015, 5, 120–131. [Google Scholar]
- Güez, C.M.; Souza, R.O.d.; Fischer, P.; Leão, M.F.d.M.; Duarte, J.A.; Boligon, A.A.; Athayde, M.L.; Zuravski, L.; Oliveira, L.F.S.D.; Machado, M.M. Evaluation of basil extract (Ocimum basilicum) on oxidative, antigenotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Brazil J. Pharm. Sci. 2017, 53, e15098. [Google Scholar]
- Refaey, M.S.; Abosalem, E.F.; Yasser El-Basyouni, R.; Elsheriri, S.E.; Elbehary, S.H.; Fayed, M.A.A. Exploring the therapeutic potential of medicinal plants and their active principles in dental care: A comprehensive review. Heliyon 2024, 10, e37641. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; de Jesus, D.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) extract decreases the biofilms viability of oral health interest. Braz. Dent. Sci. 2017, 20, 64–69. [Google Scholar] [CrossRef]
- Günther, M.; Karygianni, L.; Argyropoulou, A.; Anderson, A.C.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. The antimicrobial effect of Rosmarinus officinalis extracts on oral initial adhesion ex vivo. Clin. Oral Investig. 2022, 26, 4369–4380. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.-E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef]
- Okasha, M.I.; Mostafa, M.H.; El-Araby, S.M. Evaluation of antibacterial effect of Rosmarinus officinalis extract on Streptococcus mutans in children. Al-Azhar Dent. J. Girls 2022, 9, 161–165. [Google Scholar] [CrossRef]
- Valones, M.A.A.; Silva, I.C.G.; Gueiros, L.A.M.; Leao, J.C.; Caldas, A.F., Jr.; Carvalho, A.A.T. Clinical assessment of rosemary-based toothpaste (Rosmarinus officinalis Linn.): A randomized controlled double-blind study. Braz. Dent. J. 2019, 30, 146–151. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, C.; Ma, S.; Gao, Y.; Wang, R. Protective Effect and Mechanism of Action of Rosmarinic Acid on Radiation-Induced Parotid Gland Injury in Rats. Dose Response 2020, 18, 1559325820907782. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Scumaci, D.; Catalano, A.; Sinicropi, M.S.; Tundis, R.; Alcaro, S.; Borges, F. An update on recent studies focusing on the antioxidant properties of Salvia species. Antioxidants 2023, 12, 2106. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Beheshti-Rouy, M.; Azarsina, M.; Rezaie-Soufi, L.; Alikhani, M.Y.; Roshanaie, G.; Komaki, S. The antibacterial effect of sage extract (Salvia officinalis) mouthwash against Streptococcus mutans in dental plaque: A randomized clinical trial. Iran J. Microbiol. 2015, 7, 173–177. [Google Scholar] [PubMed]
- Sookto, T.; Srithavaj, T.; Thaweboon, S.; Thaweboon, B.; Shrestha, B. In vitro effects of Salvia officinalis L. essential oil on Candida albicans. Asian Pac. J. Trop. Biomed. 2013, 3, 376–380. [Google Scholar] [CrossRef]
- Santos, T.d.S.A.; Meccatti, V.M.; Pereira, T.C.; Marcucci, M.C.; Hasna, A.A.; Valera, M.C.; de Oliveira, L.D.; Carvalho, C.A.T. Antibacterial effect of combinations of Salvia officinalis and Glycyrrhiza glabra, hydroalcoholic extracts against Enterococcus spp. Coatings 2023, 13, 1579. [Google Scholar] [CrossRef]
- Hagh, L.G.; Arefian, A.; Farajzade, A.; Dibazar, S.; Samiea, N. The antibacterial activity of Satureja hortensis extract and essential oil against oral bacteria. Dent. Res. J. 2019, 16, 153–159. [Google Scholar]
- Mohtashami, S.; Rowshan, V.; Tabrizi, L.; Babalar, M.; Ghani, A. Summer savory (Satureja hortensis L.) essential oil constituent oscillation at different storage conditions. Ind. Crops Prod. 2018, 111, 226–231. [Google Scholar] [CrossRef]
- Ejaz, A.; Waliat, S.; Arshad, M.S.; Khalid, W.; Khalid, M.Z.; Rasul Suleria, H.A.; Luca, M.I.; Mironeasa, C.; Batariuc, A.; Ungureanu-Iuga, M.; et al. A comprehensive review of summer savory (Satureja hortensis L.): Promising ingredient for production of functional foods. Front Pharmacol. 2023, 24, 1198970. [Google Scholar] [CrossRef]
- Adiguzel, A.; Ozer, H.; Kilic, H.; Cetin, B. Screening of antimicrobial activity of essential oil and methanol extract of Satureja hortensis on foodborne bacteria and fungi. Czech J. Food Sci. 2007, 25, 81. [Google Scholar] [CrossRef]
- Sabzghabaee, A.M.; Davoodi, N.; Ebadian, B.; Aslani, A.; Ghannadi, A. Clinical evaluation of the essential oil of Satureja hortensis for the treatment of denture stomatitis. Dent. Res. J. 2012, 9, 198–202. [Google Scholar]
- Jalil, B.; Pischel, I.; Feistel, B.; Suarez, C.; Blainski, A.; Spreemann, R.; Roth-Ehrang, R.; Heinrich, M. Wild thyme (Thymus serpyllum L.): A review of the current evidence of nutritional and preventive health benefits. Front. Nutr. 2024, 11, 1380962. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid. Based Complement. Alternat. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Balanč, B.; Petrović, P.; Pravilović, R.; Djordjević, V. Pharmacological potential of Thymus serpyllum L. (wild thyme) extracts and essential oil: A review. J. Eng. Proces Manag. 2021, 13, 32–41. [Google Scholar] [CrossRef]
- Fani, M.; Kohanteb, J. In vitro antimicrobial activity of Thymus vulgaris essential oil against major oral pathogens. J. Evid. Based Complement. Altern. Med. 2017, 22, 660–666. [Google Scholar] [CrossRef]
- Altındal, D.; Deveci, K.C.; Öner Talmaç, A.G.; Talmaç, A.C.; Çalışır, M. Effects of thyme on halitosis in gingivitis patients: Can thyme mouthwash prevent halitosis—A randomized trial. Int. J. Dent. Hyg. 2023, 21, 426–432. [Google Scholar] [CrossRef]
- Kameri, A.; Dragidella, A.; Haziri, A.; Hashani, Z.; Kurteshi, K.; Kurti, A. Antifungal and genotoxic effects of Thymus serpyllum as a root canal irrigant. Clin. Exp. Dent. Res. 2024, 10, e837. [Google Scholar] [CrossRef]
- Dasila, K.; Singh, M. Bioactive compounds and biological activities of Elaegnus latifolia L.: An underutilized fruit of North-East Himalaya, India. S. Afr. J. Bot. 2022, 145, 177–185. [Google Scholar] [CrossRef]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef]
- Salehi, B.; Prakash Mishra, A.; Shukla, I.; Sharifi-Rad, M.; del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasri Nasrabadi, N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Soto-Mendívil, E.A.; Moreno-Rodríguez, J.F.; Estarrón-Espinosa, M.; García-Fajardo, J.A.; Obledo-Vázquez, E.N. Chemical composition and fungicidal activity of the essential oil Thymus vulgaris against Alternaria citri. e-Gnosis 2006, 4, 1–7. [Google Scholar]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.; Horhat, F. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56. [Google Scholar]
- Sas, I. Thymus vulgaris extract formulated as cyclodextrin complexes: Synthesis, characterization, antioxidant activity and in vitro cytotoxicity assessment. Farmacia 2019, 67, 442–451. [Google Scholar]
- Botelho, M.; Nogueira, N.A.P.; Bastos, G.; Fonseca, S.; Lemos, T.; Matos, F.; Montenegro, D.; Heukelbach, J.; Rao, V.S.; Brito, G. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz. J. Med. Biol. Res. 2007, 40, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Waheed, M.; Hussain, M.B.; Saeed, F.; Afzaal, M.; Ahmed, A.; Irfan, R.; Akram, N.; Ahmed, F.; Hailu, G.G. Phytochemical Profiling and Therapeutic Potential of Thyme (Thymus spp.): A Medicinal Herb. Food Sci. Nutr. 2024, 12, 9893–9912. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plant extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Salehi, B.; Lopez-Jornet, P.; López, E.P.-F.; Calina, D.; Sharifi-Rad, M.; Ramírez-Alarcón, K.; Forman, K.; Fernández, M.; Martorell, M.; Setzer, W.N.; et al. Plant-Derived Bioactives in Oral Mucosal Lesions: A Key Emphasis to Curcumin, Lycopene, Chamomile, Aloe vera, Green Tea and Coffee Properties. Biomolecules 2019, 9, 106. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 transitional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crop. Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- de Lucena, R.N.; Lins, R.D.; Ramos, I.N.; Cavalcanti, A.L.; Gomes, R.C.; Maciel, M.D. Estudo clínico comparativo do efeito anti-inflamatório da Matricaria recutita e da clorexidina em pacientes com gengivite crônica. Braz. J. Med. Biol. Res. 2009, 11, 31–36. [Google Scholar]
- Singh, Y.P.; Sachdeva, O.P.; Aggarwal, S.K.; Chugh, K.; Lal, H. Blood glutathione levels in head and neck malignancies. Indian J. Clin. Biochem. 2008, 23, 290–292. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Ciocia, A.M.; Netti, A.; Viapiano, F.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Inchingolo, A.D.; Dipalma, G.; et al. Benefits of natural antioxidants on oral health. Antioxidants 2023, 12, 1309. [Google Scholar] [CrossRef]
- Pytko-Polończyk, J.; Stawarz-Janeczek, M.; Kryczyk-Poprawa, A.; Muszyńska, B. Antioxidant-rich natural raw materials in the prevention and treatment of selected oral cavity and periodontal diseases. Antioxidants 2021, 10, 1848. [Google Scholar] [CrossRef]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evid. Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef]
- Vella, F.M.; Pignone, D.; Laratta, B. The Mediterranean species Calendula officinalis and Foeniculum vulgare as valuable sources of bioactive compounds. Molecules 2024, 29, 3594. [Google Scholar] [CrossRef] [PubMed]
- Ak, G.; Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Cakır, O.; Bensari, S.; Yılmaz, M.A.; Gallo, M.; Montesano, D. A comparative bio-evaluation and chemical profiles of Calendula officinalis L. extracts prepared via different extraction techniques. Appl. Sci. 2020, 10, 5920. [Google Scholar] [CrossRef]
- Rigane, G.; Younes, S.B.; Ghazghazi, H.; Salem, R.B. Investigation into the biological activities and chemical composition of Calendula officinalis L. growing in Tunisia. Int. Food Res. J. 2013, 20, 3001. [Google Scholar]
- Heijnen, C.G.; Haenen, G.R.; Oostveen, R.M.; Stalpers, E.M.; Bast, A. Protection of flavonoids against lipid peroxidation: The structure activity relationship revisited. Free Radic. Res. 2002, 36, 575–581. [Google Scholar] [CrossRef]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.; Nomizo, A.; Gerlach, R.F.; Fonseca, M.J. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metallo-proteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar] [CrossRef]
- Asadi-Yousefabad, S.-L.; Tanideh, N.; Jamshidzadeh, A.; Sepehrimanesh, M.; Hosseinzadeh, M.; Koohi-Hosseinabadi, O.; Najibi, A.; Raam, M.; Daneshi, S. Healing acceleration of acetic acid-induced colitis by marigold (Calendula officinalis) in male rats. Saudi J. Gastroenterol. 2016, 22, 50–56. [Google Scholar] [CrossRef]
- Verma, P.K.; Raina, R.; Sultana, M.; Singh, M.; Kumar, P. Total antioxidant and oxidant status of plasma and renal tissue of cisplatin-induced nephrotoxic rats: Protection by floral extracts of Calendula officinalis Linn. Ren. Fail. 2016, 38, 142–150. [Google Scholar] [CrossRef]
- Jędrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem Biol Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Haghi, G.; Hatami, A.; Mehran, M. UPLC and HPLC of caffeoyl esters in wild and cultivated Arctium lappa L. Food Chem. 2013, 138, 321–326. [Google Scholar] [CrossRef]
- Gentil, M.; Pereira, J.V.; Sousa, Y.T.; Pietro, R.; Neto, M.D.; Vansan, L.P.; de Castro França, S. In vitro evaluation of the antibacterial activity of Arctium lappa as a phytotherapeutic agent used in intracanal dressings. Phytother. Res. 2006, 20, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Groppo, F.C.; Bergamaschi, C.D.C.; Cogo, K.; Franz-Montan, M.; Motta, R.H.; de Andrade, E.D. Use of phytotherapy in dentistry. Phytother. Res. 2008, 22, 993–998. [Google Scholar] [CrossRef]
- Tonea, A.; Badea, M.; Oana, L.; Sava, S.; Vodnar, D. Antibacterial and antifungal activity of endodontic intracanal medications. Clujul Med. 2017, 90, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Jaimand, K.; Rezaee, M.B.; Mozaffarian, V. Chemical constituents of the leaf and flower oils from Achillea millefolium ssp. elbursensis Hub.Mor. from Iran rich in chamazulene. J. Essent. Oil Res. 2006, 18, 293–295. [Google Scholar] [CrossRef]
- Miranzadeh, S.; Adib-Hajbaghery, M.; Soleymanpoor, L.; Ehsani, M. Effect of adding the herb Achillea millefolium on mouthwash on chemotherapy-induced oral mucositis in cancer patients: A double-blind randomized controlled trial. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2015, 19, 207–213. [Google Scholar] [CrossRef]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea Millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Tyszkiewicz, E.W. Assessment Report on Solidago virgaurea L., Herba; Evaluation of Medicines for Human Use; EMEA/HMPC/285759/2007; European Medicines Agency: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Laurençon, L.; Sarrazin, E.; Chevalier, M.; Prêcheur, I.; Herbette, G.; Fernandez, X. Inhibition of Candida albicans yeast-hyphal conversion by triterpenoid saponins from the aerial parts of Solidago virgaurea alpestris. Phytochemistry 2013, 86, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Prêcheur, I.; Rolland, Y.; Hasseine, L.; Orange, F.; Morisot, A.; Landreau, A. Solidago virgaurea L. plant extract targeted against Candida albicans to reduce oral microbial biomass: A double-blind randomized trial on healthy adults. Antibiotics 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Crestez, A.M.; Nechita, A.; Daineanu, M.P.; Busila, C.; Tatu, A.L.; Ionescu, M.A.; Martinez, J.D.; Debita, M. Oral Cavity Microbiome Impact on Respiratory Infections Among Children. Pediatr. Health Med Ther. 2024, 15, 311–323. [Google Scholar] [CrossRef]
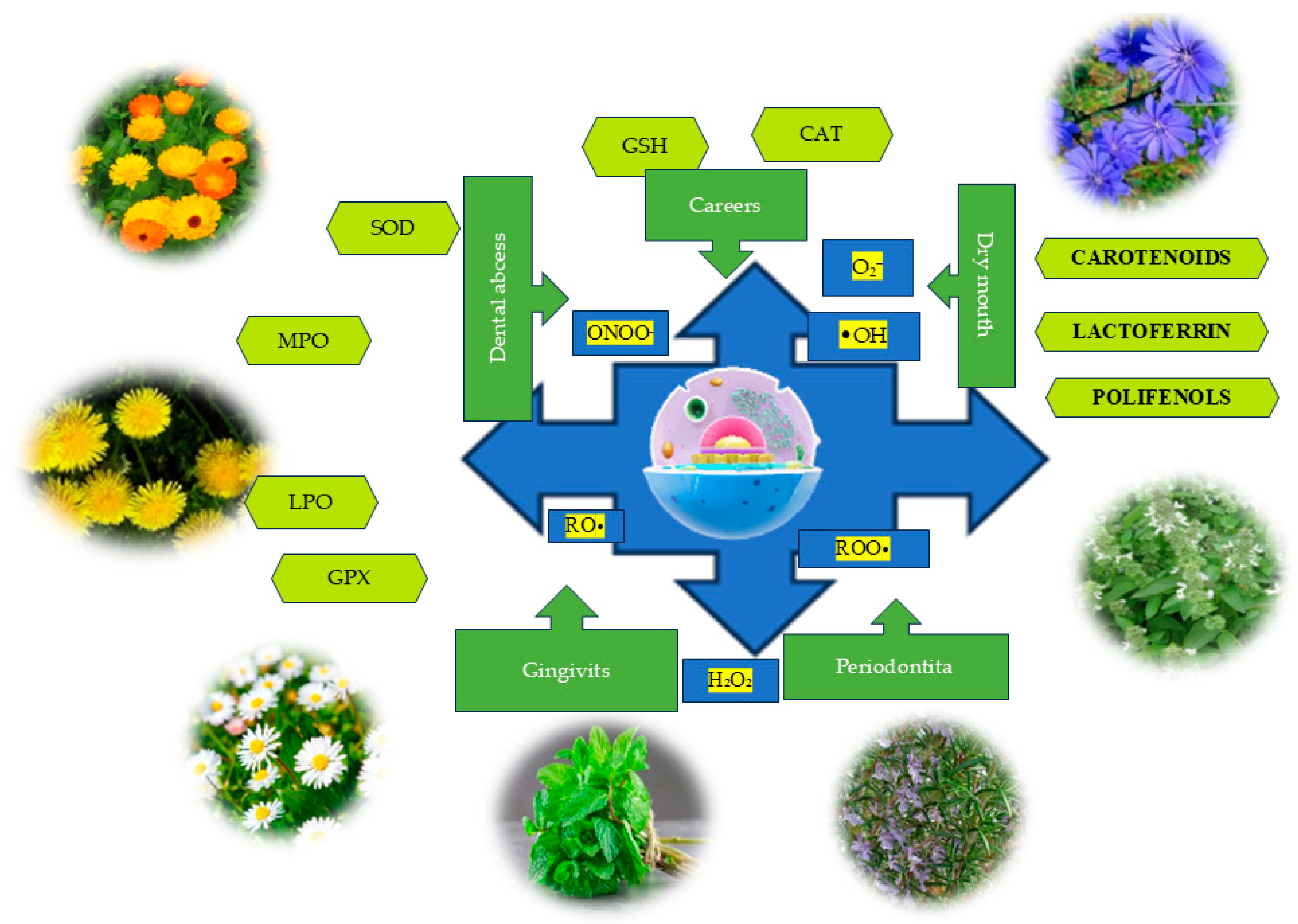

| Scientific Name | Plant Product Used | Antioxidant Compounds | References |
|---|---|---|---|
| Lavandula angustifolia L. (lavender) | Lavandulae flores | Phenolic, apigenin, kaempferol, caftaric acid, essential oil, anthocyanins, phytosterols, minerals, tannins, linalool, borneol, linalyl acetate, terpene compounds (oxygenated monoterpenes), sesquiterpenes, triterpenoids, coumarins, and aliphatic compounds (hydrocarbons, ketones, esters, etc.). | [219,220,221,222,223,224,225,226,227,228,229] |
| Menthax piperita L. (peppermint) | Menthae Folium | Flavonoids and methoxylated flavones, lipophilic tannins, polyphenolic substances, essential oils, amino acids such as L-serine, threonine, 4-aminobutyric acid, 5-oxo-L-proline, and unsaturated fatty acids. | [230,231,232,233,234,235,236,237,238] |
| Ocimum basilicum L. (basil) | Basilici herba | Polyphenols (phenolic acids, flavonoids), alkaloids, tannins, essential oil rich in linalool, estragole, methyl chavicol, eugenol, ocimene, anethole, and tannoids. | [239,240,241,242,243] |
| Rosmarinus officinalis L. (rosemary) | Rosmarini folium | Phenolic compounds: flavonoids (mainly flavones, but also flavonols and flavanones), phenolic acids, diterpenoids (carnosic acid, carnosol, and rosmanol derivatives), triterpenoid (betulinic acid), lignans (medioresinol derivatives), and volatile molecules (mainly terpenes, alcohols, esters, aldehydes, and ketones). | [244,245,246,247,248,249,250,251,252,253] |
| Salvia officinalis L. (sage) | Salviae folium | Phenolic compounds, essential oil, tannins (rosmarinic acid), flavonoids, and vitamins (ascorbic acid, vitamin B1). | [254,255,256,257,258] |
| Satureja hortensis L. (summer savory) | Saturejae herba | Polyphenols, volatile oil, triterpenic acids, tannins, mucilage, resins, mineral salts, gamma-terpinene, carvacrol, cymene, alpha-terpinene, alpha-pinene, myrcene, beta-caryophyllene, borneol, caryophyllene oxide, alpha-terpineol, thymol, camphene and camphor, nitrogenous substances, cellulose, essential oils, ursolic acid, oleanolic acid, mucilages, resins, sisterine, and vitamins B1 and C. | [259,260,261,262,263,264,265,266,267,268] |
| Thymus serpyllum L. (thyme) | Serpylli herba | Volatile oils (borneol, thymol, carvacrol), caffeic and rosmarinic acids, tannins and thymol, polyphenols, phenolic acids, caffeic acids, anthocyanins, flavonoids, inulin, carotenes, ascorbic acid, vitamin A, vitamin C, and vitamin E. | [255,269,270,271,272,273,274] |
| Thymus vulgaris L. (garden thyme) | Thymi herba | Phenolic compounds, vitamins (A, C, and E), essential oils, thymol, p-cymene, γ-terpinene, borneol, carvacrol, β-linalool, cis-sabinene hydrate, eucalyptol, α-terpinene, carvacrol methyl ether, and thymol methyl ether. | [275,276,277,278,279,280,281,282] |
| Scientific Name | Plant Product Used | Antioxidant Compounds | References |
|---|---|---|---|
| Matricaria chamomilla L. (chamomile) | Matricaria flower | Apigenin, quercetin, luteolin, terpenoids, phenolic compounds, phenolic acids, flavonoids, coumarins, and essential oil. | [284,285,286,287,288,289,290,291] |
| Cichorium intybus L. (chicory) | Cichorium flower | Phenolic acids (chlorogenic acids, isochlorogenic acids, neochlorogenic acids, caffeic acid, and cichoric acid), flavonoids and polyphenols, and vitamins A and C. | [292,293,294,295,296,297,298,299] |
| Calendula officinalis L. (pot marigold) | Calendulae flos cum receptaculis | Carotenoids, flavonoids, polyphenols, volatile oil, saponosides, carotenoids, flavonoids, flavonoid glycosides, vitamin C, malic acid, protein substances, and esters. | [300,301,302,303,304,305,306] |
| Taraxacum officinale L. (dandelion) | Taraxaci flos | Phenolic compounds and flavonoids, hydroxycinnamic acid, cichoric acid, caftaric acid, chlorogenic acids, free aglycones and flavonoid glycosides, flavones such as luteolin, chrysoeriol, and apigenin, and various flavonols, such as quercetin and its O-glucoside and rutinoside glycosides. | [307,308,309,310,311,312] |
| Arctium lappa L. (greater burdock) | Radix bardane Bardane folium | Phenolic acids—primarily isomers of dicaffeoylquinic acid and their derivatives, caffeic acids, tannins, anthocyanins, flavonoids, polyphenols, inulin, and carotenes. Ascorbic acid, arctiin and arctigenin from the lignan group, and onopordopicrin from the sesquiterpene lactone group, as well as flavonoids—luteolin, quercetin, quercitrin, and rutin—and phenolic acids. | [313,314,315,316,317,318,319] |
| Achillea millefolium L. (yarrow) | Millefolii flores Millefolii herba | Volatile oil containing sesquiterpenes (mainly azulene and achillein lactone), tannins, flavonoids, and acids. Achilleic acid, formic acid, acetic acid, aconitic acid, ascorbic acid, folic acid, propionic acid, valerianic acid, palmitic acid, stearic acid, myristic acid, linoleic acid, onecinic acid, and flavones. | [320,321,322] |
| Solidago virgaurea L. (goldenrod) | Solidaginis herba | Volatile oil, polyphenolcarboxylic acid derivatives, quercetol, rutin, anthocyanins, flavonoids (quercetin, kaempferol), and terpenes (essential oil). | [323,324,325,326,327] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, C.N.; Mariana, L.; Budacu, C.C.; Mitea, G.; Radu, M.D.; Dumitru, A.O.; Lupoae, A.; Tatu, A.; Topor, G. Balancing the Oral Redox State: Endogenous and Exogenous Sources of Reactive Oxygen Species and the Antioxidant Role of Lamiaceae and Asteraceae. Dent. J. 2025, 13, 222. https://doi.org/10.3390/dj13050222
Dumitru CN, Mariana L, Budacu CC, Mitea G, Radu MD, Dumitru AO, Lupoae A, Tatu A, Topor G. Balancing the Oral Redox State: Endogenous and Exogenous Sources of Reactive Oxygen Species and the Antioxidant Role of Lamiaceae and Asteraceae. Dentistry Journal. 2025; 13(5):222. https://doi.org/10.3390/dj13050222
Chicago/Turabian StyleDumitru, Caterina Nela, Lupoae Mariana, Cristian Constantin Budacu, Gabriela Mitea, Marius Daniel Radu, Alina Oana Dumitru, Andreea Lupoae, Alin Tatu, and Gabi Topor. 2025. "Balancing the Oral Redox State: Endogenous and Exogenous Sources of Reactive Oxygen Species and the Antioxidant Role of Lamiaceae and Asteraceae" Dentistry Journal 13, no. 5: 222. https://doi.org/10.3390/dj13050222
APA StyleDumitru, C. N., Mariana, L., Budacu, C. C., Mitea, G., Radu, M. D., Dumitru, A. O., Lupoae, A., Tatu, A., & Topor, G. (2025). Balancing the Oral Redox State: Endogenous and Exogenous Sources of Reactive Oxygen Species and the Antioxidant Role of Lamiaceae and Asteraceae. Dentistry Journal, 13(5), 222. https://doi.org/10.3390/dj13050222








