The Whitening Efficacy of a Hydroxyapatite Toothpaste and a Blue Covarine Toothpaste: A Comparative In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Toothpastes
2.2. Experimental Protocol
2.3. Analysis
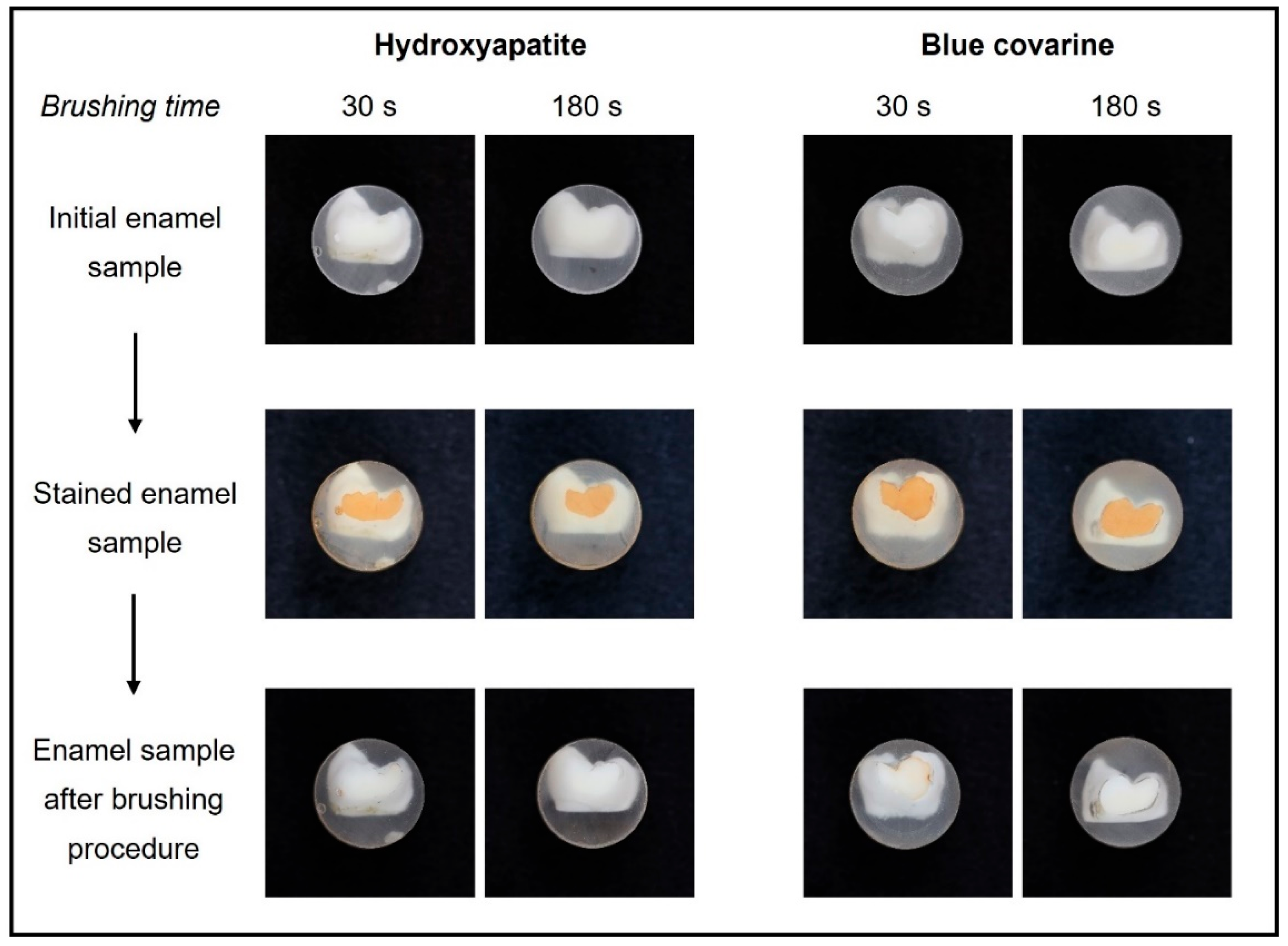
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joiner, A. Whitening toothpastes: A review of the literature. J. Dent. 2010, 38 (Suppl. S2), e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Quiñonez, C. Straight, white teeth as a social prerogative. Sociol. Health Illn. 2015, 37, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.M. Tooth whitening: What we now know. J. Evid. Based Dent. Pract. 2014, 14, 70–76. [Google Scholar] [CrossRef]
- Bhaskaran, R.; Sharma, S. Does age determine the lightness and darkness of tooth shades? A retrospective study. J. Adv. Pharm. Technol. Res. 2022, 13, S374–S377. [Google Scholar] [CrossRef] [PubMed]
- van Loveren, C. Toothpastes; Karger: Basel, Switzerland, 2013; ISBN 9783318022063. [Google Scholar]
- Sarembe, S.; Ufer, C.; Kiesow, A.; Limeback, H.; Meyer, F.; Fuhrmann, I.; Enax, J. Influence of the amount of toothpaste on cleaning efficacy: An in vitro study. Eur. J. Dent. 2023, 17, 497–503. [Google Scholar] [CrossRef]
- Limeback, H.; Meyer, F.; Enax, J. Tooth whitening with hydroxyapatite: A systematic review. Dent. J. 2023, 11, 50. [Google Scholar] [CrossRef]
- Niwa, M.; Sato, T.; Li, W.; Aoki, H.; Daisaku, T. Polishing and whitening properties of toothpaste containing hydroxyapatite. J. Mater. Sci. Mater. Med. 2001, 12, 277–281. [Google Scholar] [CrossRef]
- Dabanoglu, A.; Wood, C.; Garcia-Godoy, F.; Kunzelmann, K.H. Whitening effect and morphological evaluation of hydroxyapatite materials. Am. J. Dent. 2009, 22, 23–29. [Google Scholar]
- Shang, R.; Kaisarly, D.; Kunzelmann, K.-H. Tooth whitening with an experimental toothpaste containing hydroxyapatite nanoparticles. BMC Oral Health 2022, 22, 331. [Google Scholar] [CrossRef]
- Bommer, C.; Flessa, H.P.; Xu, X.; Kunzelmann, K.H. Hydroxyapatite and self-assembling peptide matrix for non-oxidizing tooth whitening. J. Clin. Dent. 2018, 29, 57–63. [Google Scholar]
- Tao, D.; Smith, R.N.; Zhang, Q.; Sun, J.N.; Philpotts, C.J.; Ricketts, S.R.; Naeeni, M.; Joiner, A. Tooth whitening evaluation of blue covarine containing toothpastes. J. Dent. 2017, 67, S20–S24. [Google Scholar] [CrossRef]
- Joiner, A.; Philpotts, C.J.; Ashcroft, A.T.; Laucello, M.; Salvaderi, A. In vitro cleaning, abrasion and fluoride efficacy of a new silica based whitening toothpaste containing blue covarine. J. Dent. 2008, 36 (Suppl. S1), S32–S37. [Google Scholar] [CrossRef]
- Vaz, V.T.P.; Jubilato, D.P.; Oliveira, M.R.M.; Bortolatto, J.F.; Floros, M.C.; Dantas, A.A.R.; Oliveira Junior, O.B. Whitening toothpaste containing activated charcoal, blue covarine, hydrogen peroxide or microbeads: Which one is the most effective? J. Appl. Oral Sci. 2019, 27, e20180051. [Google Scholar]
- Joiner, A. A silica toothpaste containing blue covarine: A new technological breakthrough in whitening. Int. Dent. J. 2009, 59, 284–288. [Google Scholar] [CrossRef]
- Pawinska, M.; Paszynska, E.; Amaechi, B.T.; Meyer, F.; Enax, J.; Limeback, H. Clinical evidence of caries prevention by hydroxyapatite: An updated systematic review and meta-analysis. J. Dent. 2024, 151, 105429. [Google Scholar] [CrossRef]
- Hu, M.L.; Zheng, G.; Lin, H.; Yang, M.; Zhang, Y.D.; Han, J.M. Network meta-analysis on the effect of desensitizing toothpastes on dentine hypersensitivity. J. Dent. 2019, 88, 103170. [Google Scholar]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar]
- PubChem: Copper Phthalocyanine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Copper_II_-phthalocyanine (accessed on 12 July 2024).
- Stookey, G.K.; Burkhard, T.A.; Schemehorn, B.R. In vitro removal of stain with dentifrices. J. Dent. Res. 1982, 61, 1236–1239. [Google Scholar]
- Wang, C.; Lucas, R.; Smith, A.J.; Cooper, P.R. An in vitro screening assay for dental stain cleaning. BMC Oral Health 2017, 17, 37. [Google Scholar] [CrossRef]
- Wuelknitz, P. Cleaning power and abrasivity of European toothpastes. Adv. Dent. Res. 1997, 11, 576–579. [Google Scholar]
- Lath, D.L.; Johnson, C.; Smith, R.N.; Brook, A.H. Measurement of stain removal in vitro: A comparison of two instrumental methods. Int. J. Dent. Hyg. 2006, 4, 129–132. [Google Scholar] [CrossRef]
- Pratten, J.; Smith, A.W.; Wilson, M. Response of single species biofilms and microcosm dental plaques to pulsing with chlorhexidine. J. Antimicrob. Chemother. 1998, 42, 453–459. [Google Scholar]
- Collins, L.Z.; Naeeni, M.; Platten, S.M. Instant tooth whitening from a silica toothpaste containing blue covarine. J. Dent. 2008, 36 (Suppl. S1), S21–S25. [Google Scholar] [CrossRef]
- Joiner, A.; Philpotts, C.J.; Alonso, C.; Ashcroft, A.T.; Sygrove, N.J. A novel optical approach to achieving tooth whitening. J. Dent. 2008, 36 (Suppl. S1), S8–S14. [Google Scholar] [CrossRef]
- Tao, D.; Sun, J.N.; Wang, X.; Zhang, Q.; Naeeni, M.A.; Philpotts, C.J.; Joiner, A. In vitro and clinical evaluation of optical tooth whitening toothpastes. J. Dent. 2017, 67, S25–S28. [Google Scholar] [CrossRef]
- Schlafer, S.; Poulsen, P.N.; Johansen, J.; Trap, L.; Leite, F.R.M. The whitening effect of single brushing with blue-covarine containing toothpaste—A randomized controlled trial. J. Dent. 2021, 105, 103559. [Google Scholar] [CrossRef]
- Meireles, S.S.; Sousa, J.P.d.; Lins, R.B.E.; Sampaio, F.C. Efficacy of whitening toothpaste containing blue covarine: A double-blind controlled randomized clinical trial. J. Esthet. Restor. Dent. 2021, 33, 341–350. [Google Scholar] [CrossRef]
- Dantas, A.A.R.; Bortolatto, J.F.; Roncolato, Á.; Merchan, H.; Floros, M.C.; Kuga, M.C.; Oliveira Junior, O.B.d. Can a bleaching toothpaste containing Blue Covarine demonstrate the same bleaching as conventional techniques? An in vitro, randomized and blinded study. J. Appl. Oral Sci. 2015, 23, 609–613. [Google Scholar] [CrossRef]
- Pawinska, M.; Paszynska, E.; Limeback, H.; Amaechi, B.T.; Fabritius, H.-O.; Ganss, B.; O’Hagan-Wong, K.; Schulze zur Wiesche, E.; Meyer, F.; Enax, J. Hydroxyapatite as an active ingredient in oral care: An international symposium report. Bioinspired Biomim. Nanobiomater. 2024, 13, 1–14. [Google Scholar] [CrossRef]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.; Jeroncic, A. Fluoride toothpastes of different concentrations for preventing dental caries. cochrane Database Syst. Rev. 2019, 3, Cd007868. [Google Scholar] [CrossRef]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [PubMed]
- Lelli, M.; Marchetti, M.; Foltran, I.; Roveri, N.; Putignano, A.; Procaccini, M.; Orsini, G.; Mangani, F. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: A comparative in vivo study. Front. Physiol. 2014, 5, 333. [Google Scholar]
- Jin, J.; Xu, X.; Lai, G.; Kunzelmann, K.H. Efficacy of tooth whitening with different calcium phosphate-based formulations. Eur. J. Oral Sci. 2013, 121, 382–388. [Google Scholar] [PubMed]
- Harks, I.; Jockel-Schneider, Y.; Schlagenhauf, U.; May, T.W.; Gravemeier, M.; Prior, K.; Petersilka, G.; Ehmke, B. Impact of the daily use of a microcrystal hydroxyapatite dentifrice on de novo plaque formation and clinical/microbiological parameters of periodontal health. A randomized trial. PLoS ONE 2016, 11, e0160142. [Google Scholar]
- Tellefsen, G.; Liljeborg, A.; Johannsen, A.; Johannsen, G. The role of the toothbrush in the abrasion process. Int. J. Dent. Hyg. 2011, 9, 284–290. [Google Scholar] [CrossRef]
- Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; Fuhrmann, I.; Fabritius, H.-O. Toothpaste abrasion and abrasive particle content: Correlating high-resolution profilometric analysis with relative dentin abrasivity (RDA). Dent. J. 2023, 11, 79. [Google Scholar] [CrossRef]
| Toothpaste with Whitening Claims | Number of Whitening Toothpastes | Ingredient Percentage (%) |
|---|---|---|
| Blue covarine a | 250 | 17.3 |
| Hydroxyapatite b | 106 | 7.3 |
| Hydrogen peroxide c | 39 | 2.7 |
| Total number | 1443 | --- |
| Hydroxyapatite toothpaste (Bioniq® Repair Whitening Toothpaste, Dr. Kurt Wolff GmbH & Co. KG, Germany) | Blue covarine toothpaste (Signal White Now Toothpaste, Unilever, The Netherlands) |
| Aqua, Hydroxyapatite (20%), Glycerin, Hydrated Silica, Sorbitol, Sodium Myristoyl Sar-cosinate, Tetrapotassium Pyrophosphate, Sodium Methyl Cocoyl Taurate, Aroma, Cellulose Gum, Propanediol, Silica, Zinc PCA, Sodium Saccharin, Sodium Chloride, Phenoxyethanol, Menthol, Mentha Oiperita Oil, Anethole, Limonene, Pinene, Be-ta-caryophyllene, Terpineol, Carvone. | Hydrogenated Starch Hydrolysate, Aqua, Hydrated Silica, Sodium Lauryl Sulfate, Aroma, Cellulose Gum, Sodium Fluoride, Sodium Saccharin, PVM/MA Copolymer, Glycerin, Trisodium Phosphate, Limonene, CI 74160, CI 74260. (Contains sodium fluoride [1450 ppm fluoride]; CI 74160: Blue covarine, i.e., copper phthalocyanine (C32H16CuN8)). |
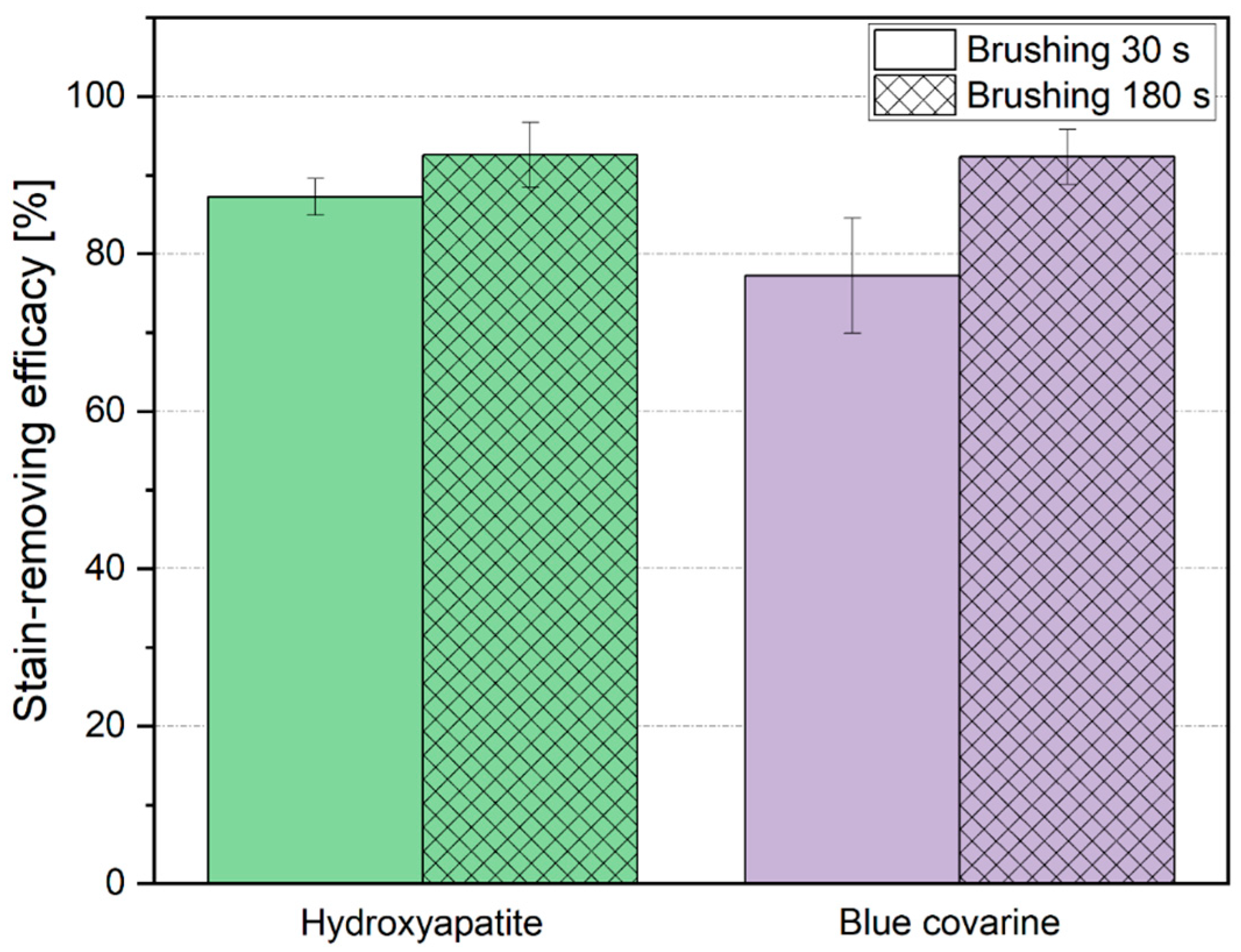
| Mean Stain-Removing Efficacy in % (±Standard Deviation) | ||
|---|---|---|
| Toothpaste | 30 s Brushing | 180 s Brushing |
| Hydroxyapatite toothpaste | 87.3 ± 2.1 | 92.6 ± 4.1 |
| Blue covarine toothpaste | 77.2 ± 7.3 | 92.4 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enax, J.; Fandrich, P.; Schulze zur Wiesche, E.; Amaechi, B.T. The Whitening Efficacy of a Hydroxyapatite Toothpaste and a Blue Covarine Toothpaste: A Comparative In Vitro Study. Dent. J. 2025, 13, 143. https://doi.org/10.3390/dj13040143
Enax J, Fandrich P, Schulze zur Wiesche E, Amaechi BT. The Whitening Efficacy of a Hydroxyapatite Toothpaste and a Blue Covarine Toothpaste: A Comparative In Vitro Study. Dentistry Journal. 2025; 13(4):143. https://doi.org/10.3390/dj13040143
Chicago/Turabian StyleEnax, Joachim, Pascal Fandrich, Erik Schulze zur Wiesche, and Bennett T. Amaechi. 2025. "The Whitening Efficacy of a Hydroxyapatite Toothpaste and a Blue Covarine Toothpaste: A Comparative In Vitro Study" Dentistry Journal 13, no. 4: 143. https://doi.org/10.3390/dj13040143
APA StyleEnax, J., Fandrich, P., Schulze zur Wiesche, E., & Amaechi, B. T. (2025). The Whitening Efficacy of a Hydroxyapatite Toothpaste and a Blue Covarine Toothpaste: A Comparative In Vitro Study. Dentistry Journal, 13(4), 143. https://doi.org/10.3390/dj13040143






