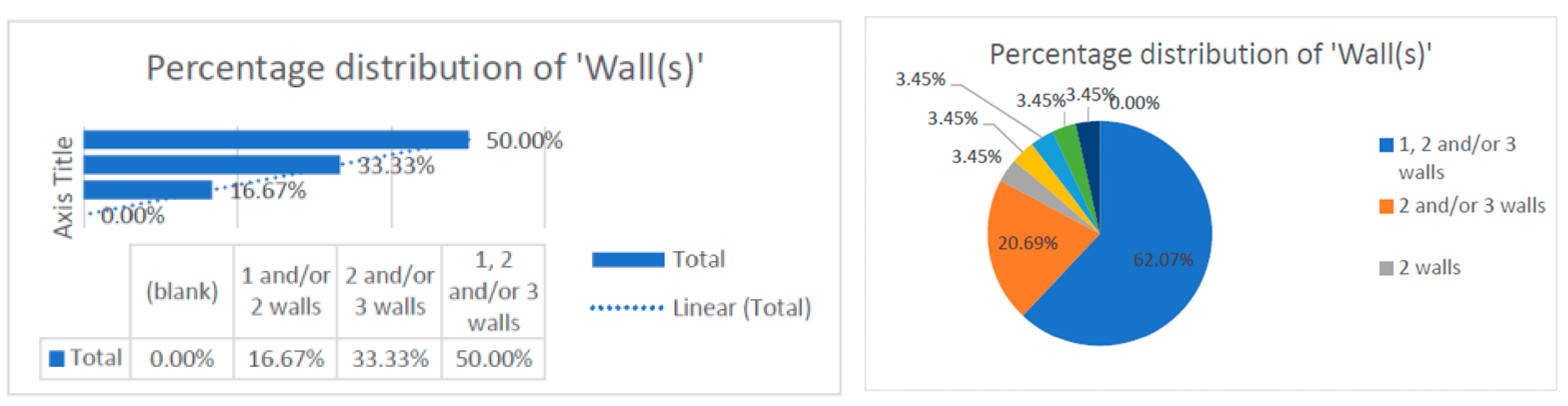Abstract
Background: Periodontitis is characterized as a change in the total periodontal tissues that includes tissue loss, as evidenced by clinical loss of attachment, and radiographically determined alveolar bone loss, periodontal pockets, and gingival bleeding. Objectives: The aim of this study was to observe and analyze recent information from the literature on the effect of enamel matrix derivative proteins on the bony defects caused by periodontitis. Methods: Through using two major online databases and search engines, the literature was manually searched for papers published until May 2024. To find relevant studies, this research utilized a combination of target keywords, and the reference lists of manuscripts that were chosen for inclusion in this study were checked and analyzed in tabular form, enabling the collection and comparison of data. Results: According to the results, the average value of the probing depth gained was 4 mm for the EMD™ alone and 4.25 mm for the EMD combined with surgical techniques such as open-flap techniques, platelet derivatives, and growth factors. In regard to clinical attachment level (CAL) gaining, average values of 3.6 mm in EMD™ alone and 3.86 mm with EMD™ combined with other techniques were observed. Conclusions: It can be concluded that the healing propensity depends on the morphological structure of the bone defect represented by the wall stage, and there is a certain coherence and correlation between the values of probing depth (PD) and clinical attachment level (CAL), whether for the use of EMD alone or its use in combination with other materials.
1. Introduction
Periodontitis is characterized as a change in the total periodontal tissues that includes tissue loss, as evidenced by clinical loss of attachment, and radiographically determined alveolar bone loss, periodontal pockets, and gingival bleeding [1,2,3,4]. The new consensus opted on a classification model for periodontitis that is further defined by a multidimensional staging and grading system that may be updated when new data become available [5]. In accordance with the Chicago criterium, 2017, periodontitis, necrotic periodontitis, and periodontitis manifestations of systemic disorders are nowadays the three recognized types of periodontal diseases that impact the deep periodontium [5]. Bone loss can be due to different factors such as malocclusion, endodontic infections, trauma induced by prostheses, orthodontic treatments, and a lack of stimulation from the absence of an antagonist tooth [6,7].
Periodontal pockets induce the destruction of supporting periodontal tissues, leading to the loosening of the teeth and thereby bone loss. Two types of periodontal pockets can be differentiated [1,2,8,9,10]:
- Suprabony (supracrestal or supra-alveolar) pockets appear when the bottom of the pocket is coronal to the crest of the alveolar bone, and the pocket wall lies coronal to the bone. This type of bone loss is always horizontal.
- Intrabony (infrabony, subcrestal, or intra-alveolar) pockets appear when the bottom of the pocket is apical to the crest of the alveolar bone. With this second type, the lateral pocket wall lies between the tooth surface and the alveolar bone. The bone loss is in most cases vertical.
The most prevalent pattern of bone loss in periodontal disease is horizontal bone loss. The height of the bone is lowered, but the bone edge remains perpendicular to the tooth surface. The interdental septa, as well as the facial and lingual plates, are all damaged, but not to the same extent, around the same tooth. Bone loss can also be caused by misplaced teeth or partial or total edentation, which prevents correct chewing and hence deprives the bone of the necessary stimulation [11].
One of the most frequently commercial products available employed in the practice for periodontology treatment is called Emdogain® (EMD) (Institute Straumann, Basel, Switzerland). Emdogain is a biological product consisting of a unique group of proteins extract of enamel matrix and contains amelogenins of various molecular weights. This gel represents a combination of freeze-dried DMA (powder) and a hydrogel (propylene glycol alginate) to complete the formulation [7,8,9,11,12,13]. This pure protein complex, freeze-dried and enriched with amelogenins, isolated from the amellar matrix collected from dental swine germs is referred to as a “enamel matrix derivative” (EMD) [13]. Amelogenins activate the proliferation and differentiation of periodontal fibroblasts and osteoblasts when absorbed on the root surface [7,9,12,13]. The regeneration of the periodontal ligament and cementum is the primary function of amelogenins in periodontal regeneration [14]. Amelogenins, present in EMD, represent an extracellular matrix protein complex that induce the formation of acellular cementa. The EMDs then promote the reconstruction of the periodontium in a complex mechanism of activation of osteo-regeneration through the bone cells while inhibiting the epithelialization of the damaged sites [5,6,7,8]. This mechanism is still relatively unknown, and studies are being conducted to understand these phenomena.
Periodontologists have been treating tissue abnormalities caused by periodontitis-related destruction of the tooth’s supporting tissues for many years. Periodontal disorders are complex infectious diseases with various inflammatory states [14]. The success of existing medicines in rebuilding the periodontium is thus a major challenge, and it has become a major public health concern. Although periodontal disease can be treated, irreversible tissue damage results. Various therapeutic options incorporating nonsurgical treatments, as well as conservative and regenerative surgical techniques, have been used for the treatment of intrabony defects throughout the last three decades, with varying degrees of success. The goal of regenerative periodontal therapy is to restore the lost periodontal structures such as the new formation of root cementa, periodontal ligaments, and alveolar bone. According to several studies, some periodontal lesions have been demonstrated to have bone regenerative capacity down to the remnant crest level. Several years ago, researchers, through their studies, came up with a new concept: that of tissue selection. The enamel matrix derivative, which is a combination of various proteins, can be used to illustrate this notion. The EMDs placed into the lesion trigger the induction, differentiation, and proliferation mechanisms, resulting in the regeneration of the tooth’s attachment system and, ultimately, bone defects; therefore, the null hypothesis of the present research was that there is significantly improved healing when comparing EMDs with conventional therapy. The aim of this study was to observe and analyze recent information published the literature on the effect of enamel matrix derivative proteins on the bony defects caused by periodontal disease.
2. Material and Methods
2.1. Search Method
Using the PubMed, Cochrane, and ResearchGate database, the literature was searched for papers published until May 2024. To find relevant studies, research utilized a combination of target MeSH keywords, text words, and subject headings. The authors then targeted two sets of keywords used in different combinations, as follows (Table 1):

Table 1.
Inclusion and exclusion criteria within the study framework.
- ➢
- “Emdogain intrabony defects”;
- ➢
- “Emdogain infrabony defects”.
Review article reference lists were manually searched, and in addition, the reference lists of articles that were chosen for inclusion in this study were checked and analyzed in tabular form to enable the authors to collect and compare the data.
2.2. Selection and Inclusion/Exclusion Criteria
Table 1 provides an overview of the main inclusion and exclusion criteria that were used and employed during the methodology. The studies were limited and filtered to human histological studies evaluating the effect of nonsurgical or surgical treatment for periodontal intrabony/infrabony defects, with or without the use of potentially regenerative materials.
Only articles written in English were included in this study. The regenerative material employed during the treatment procedure could include a combination of amelogenins and the following:
- barrier membranes;
- grafting materials;
- growth factors/proteins.
The authors chose the PICO framework in order to achieve the objectives of this study, since it is the most commonly used model for structuring clinical analyses and questions.
P (patient, problem)—human adult patients with no previous regenerative periodontal therapy in their medical history and no general disorders associated;
I (intervention, exposure)—periodontal regenerative therapy/bone regenerative therapy;
C (comparison)—EMD alone versus EMD plus barrier membranes/grafting materials/growth factors/proteins;
O (outcomes)—clinical attachment level, probing pocket depth, radiographic bone fill, or patient-reported outcomes at regular post-surgery check-ups.
2.3. Method of Screening
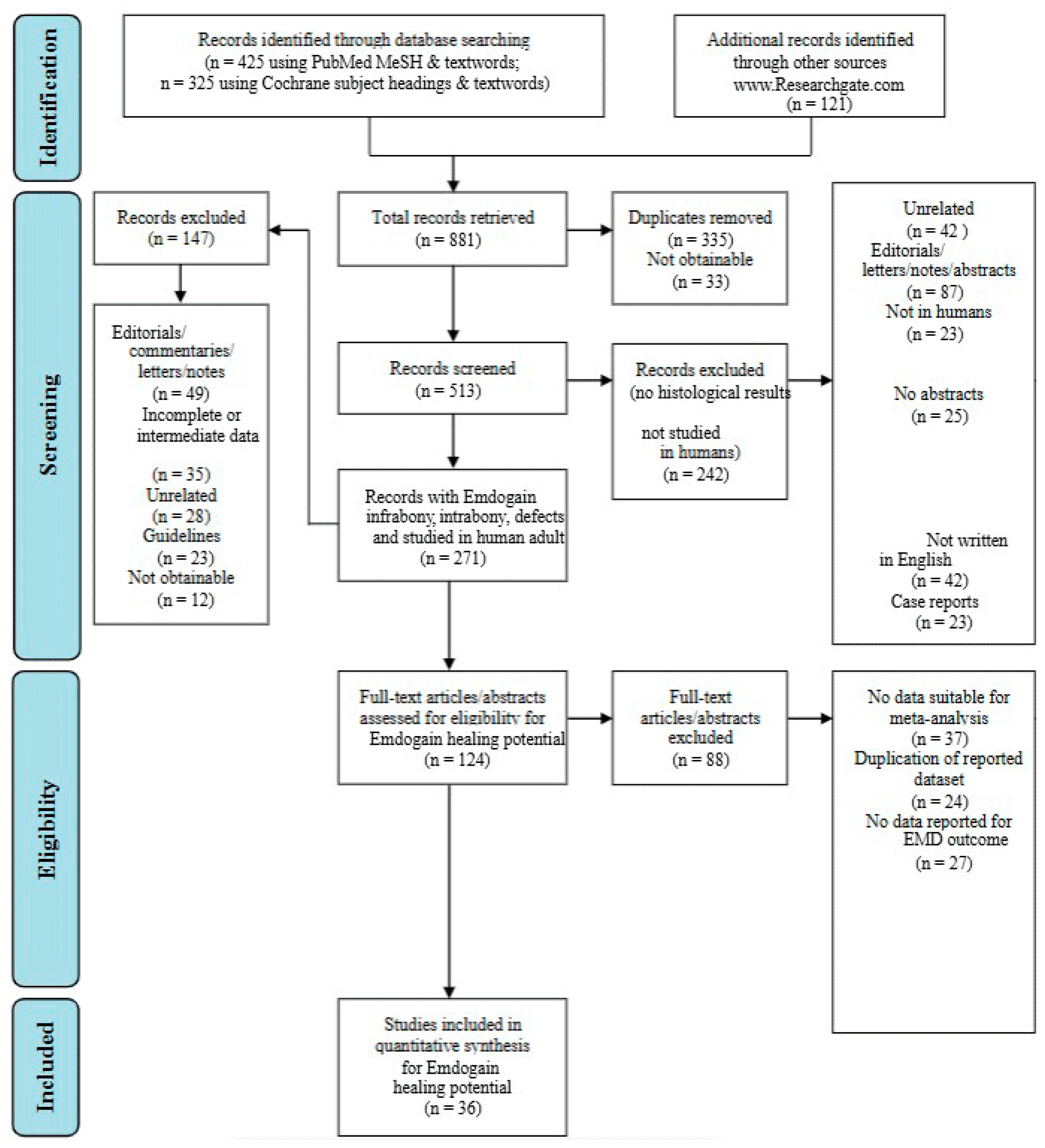
The selected studies’ titles and abstracts were independently screened. The main criterion employed to screen titles and abstracts was the following question tag: “Was the study conducted in people and did its findings present histological treatment outcomes in periodontal intrabony/infrabony lesions following the application of regenerative biomaterials?” If the screening question was answered “yes” or “uncertain”, the entire text of each item was collected. For the evaluation and quantification of the search conducted, the authors followed and complied the PRISMA statement guidelines as shown in Figure 1 [15].
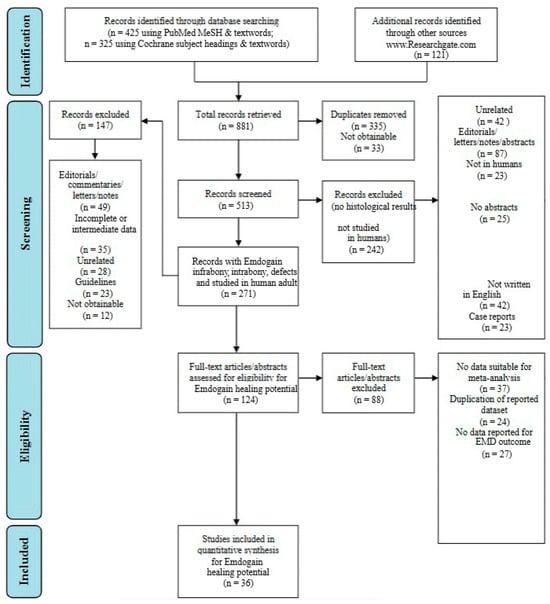
Figure 1.
Flowchart regarding the methodology conducted within the study framework [15].
The initial targeted research allowed the authors to find 881 articles in total. After filtering the research and duplicate removal, the authors were able to obtain 513 eligible papers. Following more filters applied to the search, as shown in Figure 1, the authors were able to retrieve a total of 124 papers, which were manually screened and assessed for eligibility. After eligibility was assessed, a total of 60 papers were discarded. Ultimately, a total of 36 papers were included in the present research.
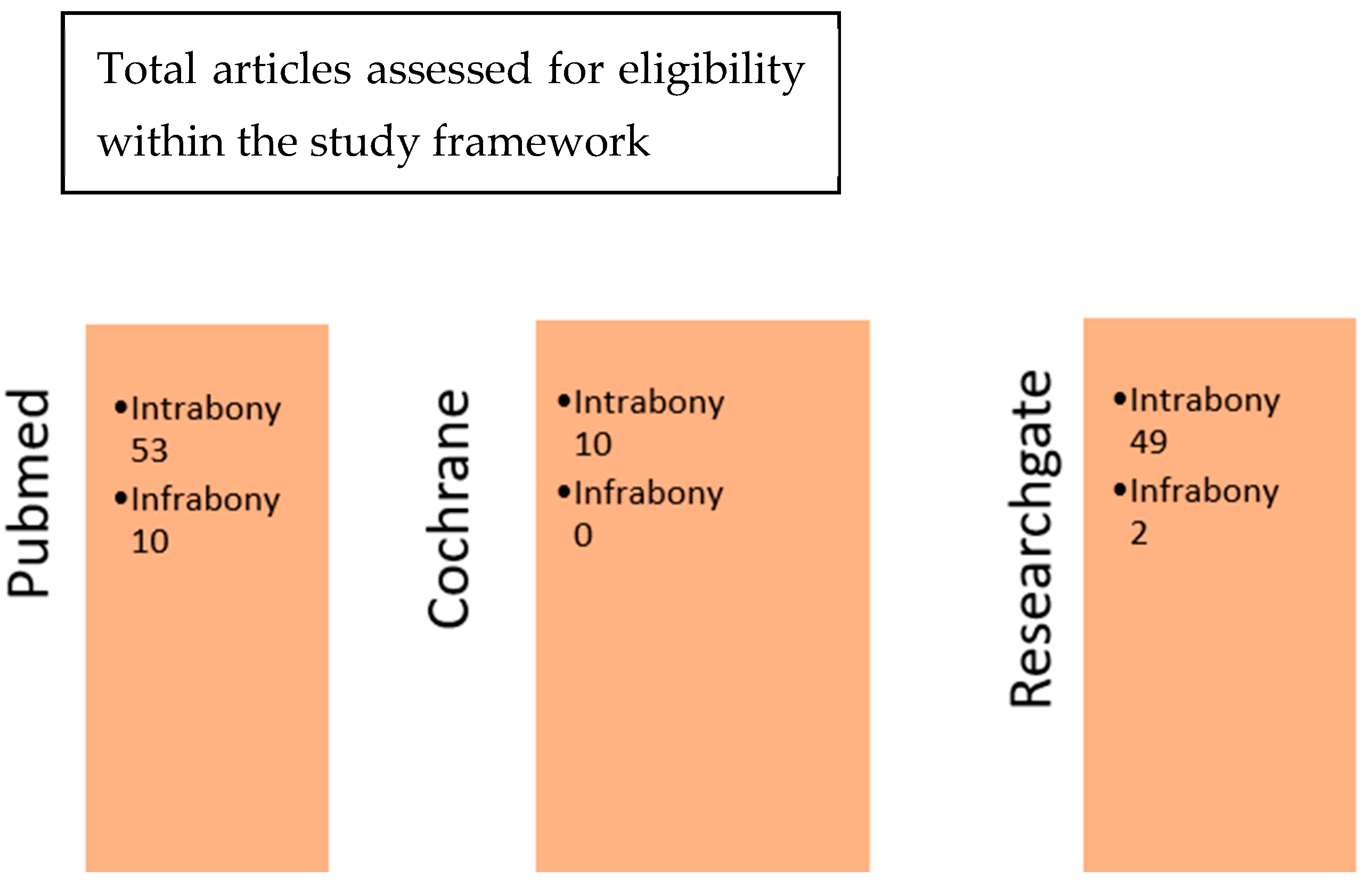
Later in this study, after refining our searches on the search engines PubMed, Cochrane, and ResearchGate, a cross-comparison according to the two sets of keywords used was performed. This cross-comparison between the respective sets of results obtained with the terms “Emdogain intrabony defects” and “Emdogain infrabony defects” allowed the authors to isolate the articles of interest for this study. By comparing the articles obtained from the three databases, the authors were able to see that some articles were either common to both or specific to both. The Cochrane database article collection contained 7 articles that were extremely specific, and they could not be found in the PubMed database (Figure 2). This specificity concerned mainly clinical research (see Table S1 in Supplementary Materials).
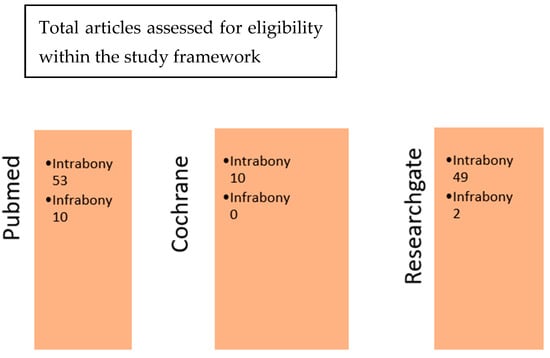
Figure 2.
Cross-comparison of the three database search engines.
2.4. Data Extraction and Analysis
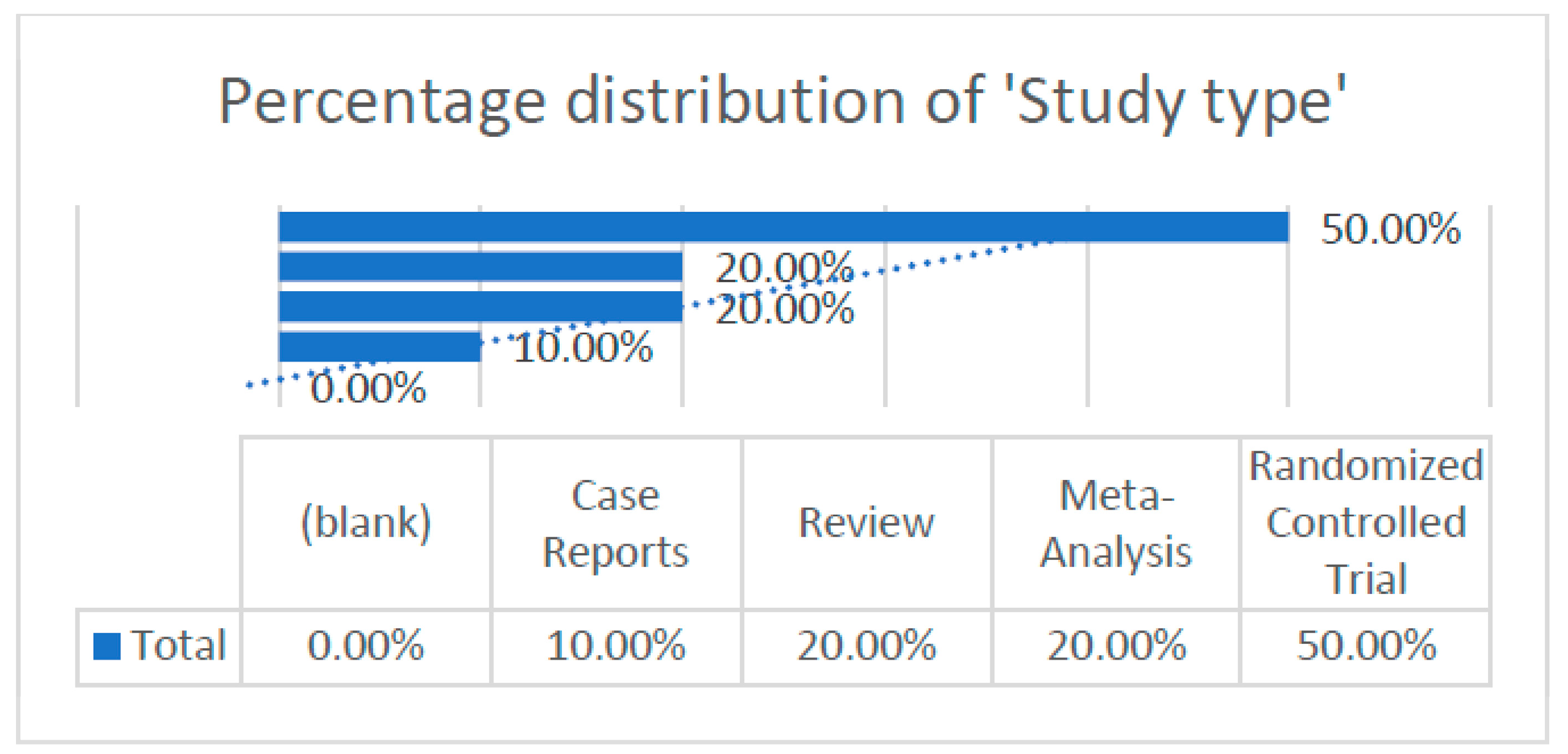
According to the articles obtained, we were able to extract and analyze different data, which are illustrated in the following graph (Figure 3).
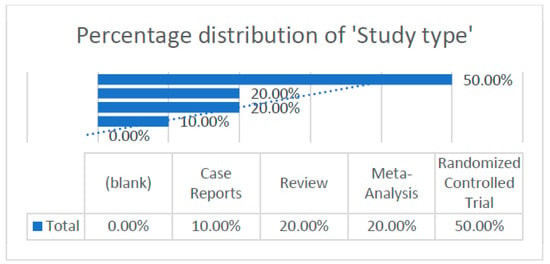
Figure 3.
Percentage distribution of study type within the research framework.
- -
- Distribution of study types.
2.5. Risk of Bias and Quality Control
A quality assessment of the included studies was performed using the Revman Cochrane™ approach. The risk of bias tool identifies the domains specified in the Cochrane risk of bias instruments for systematic reviews. All the authors clearly defined both their study objective and the population (number, characteristics, and eligibility) on which they were going to carry out the research. Moreover, to assess the methodological quality of the included studies, the data from each article were independently evaluated by the authors using a structured manual form. The evaluation focused on key categories such as study design, primary outcomes related to oxidative stress and periodontal health, sample size, and study results.
3. Results
Summary Table of Articles from the Search Engine Databases
After analyzing the articles collected [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] according to the keyword combinations intrabony/infrabony bone defects, the authors compiled a summary of the data in the following tables, which allowed a statistical analysis and discussion of the data inherent to Emdogain on several types of bone defects.
This data collection [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] revealed that the number of treated defect walls per study included one, two, and/or three walls. The data were correlated with the type of study conducted, which was randomized. Most of the studies evaluated distribution of two and/or three walls (83.33%), and some studies conducted research on one-wall defects only (16.67%). This observation can be corelated with the technical difficulty of regenerating bone defects in only one wall and can be a reason for the reduced distribution of one-wall defects (16.67%) (Figure 4).
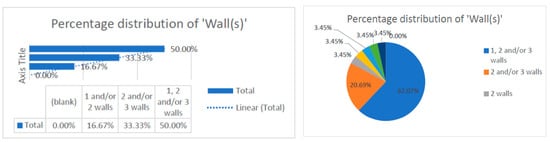
Figure 4.
Distribution of the numbers of wall defects within the research.
- -
- Distribution of the numbers of walls
- -
- Distribution of change in probing depth (PD).
Although it is statistically difficult to compare the values between methods due to the diversity of techniques, i.e., Emdogain combined with a membrane or with a growth factor, as shown in Table 2, Figure 5 and Figure 6, the different PD values and the techniques used revealed that the recorded average value of the probing depth gain is about 4 mm for the EMD alone and 4.25 mm for the EMD combined with different other techniques [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62].

Table 2.
Summary table for “Emdogain infrabony/intrabony defects”.
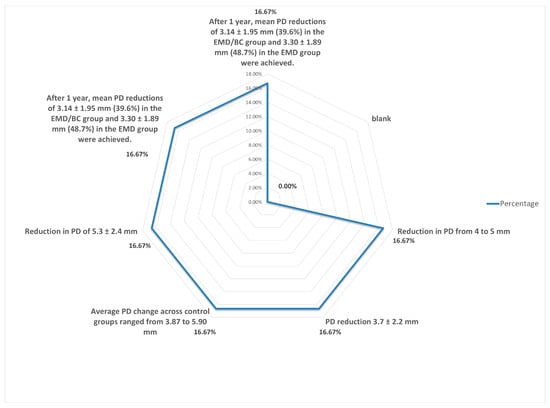
Figure 5.
Distribution in change in probing depth.
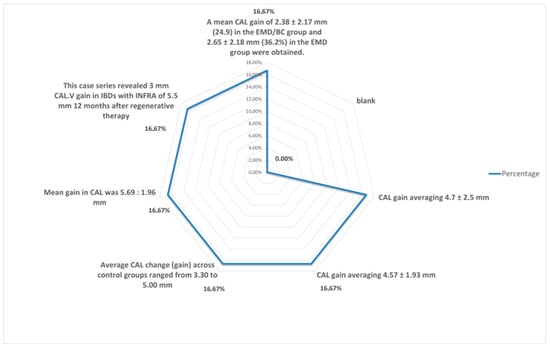
Figure 6.
Percentage distribution of changes in probing depth.
- -
- Distribution of change in clinical attachment level (CAL).
When comparing data regarding the CAL, average values of 3.6 mm for the gain in CAL with EMD alone and 3.86 mm with EMD combined were calculated. As seen in Figure 7 data regarding CAL varies, as the techniques combined with Emdogain were numerous [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62].
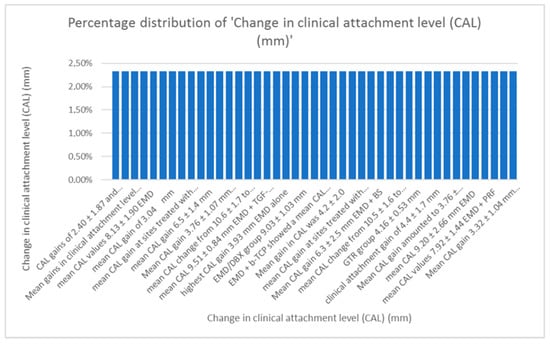
Figure 7.
Distribution of changes in clinical attachment level.
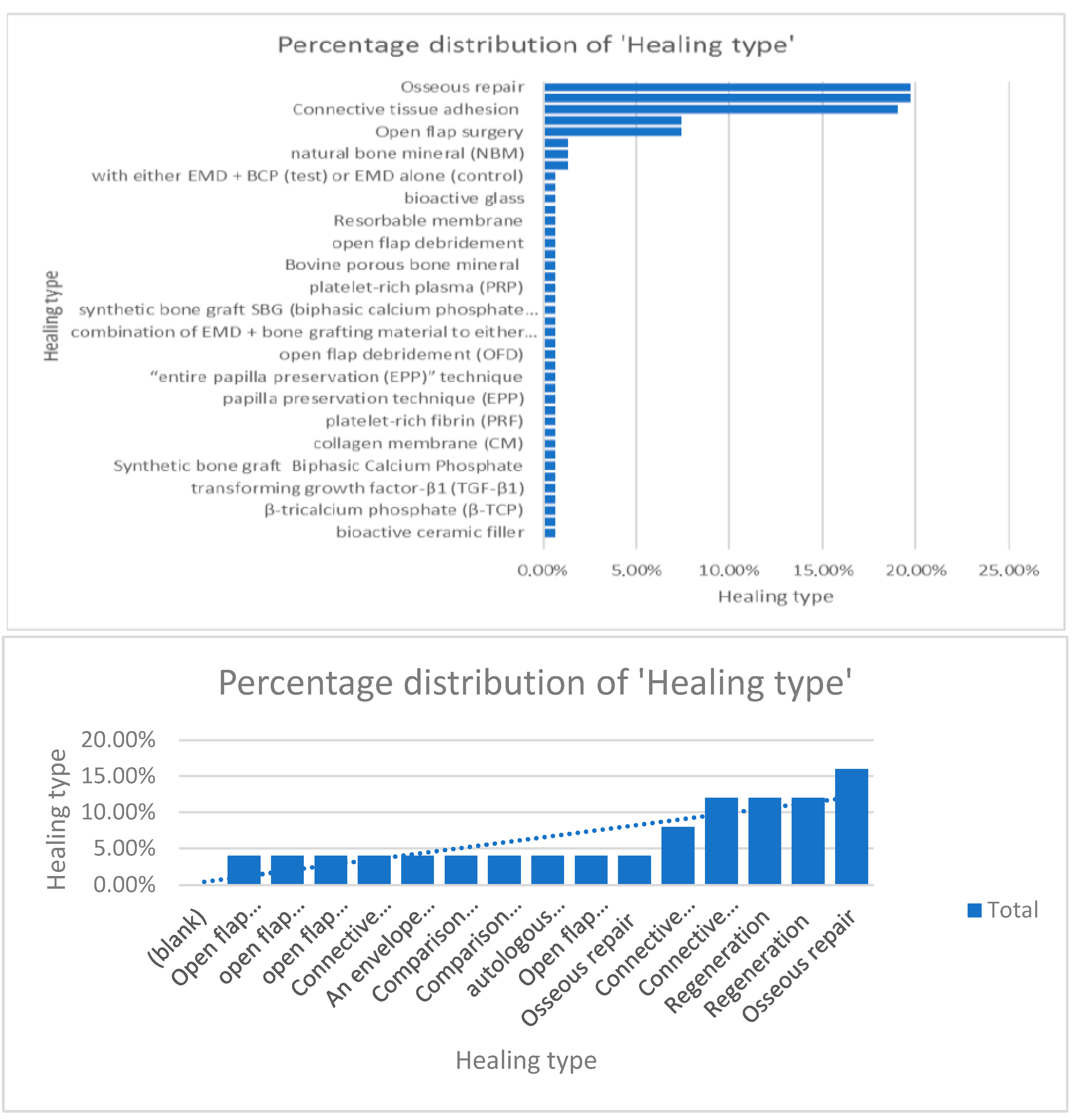
When comparing information regarding the percentage of distribution regarding the surgical protocol followed and healing type, the authors concluded that osseous repair, connective tissue adhesion, and open-flap surgery were the most used techniques [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] (Figure 8).
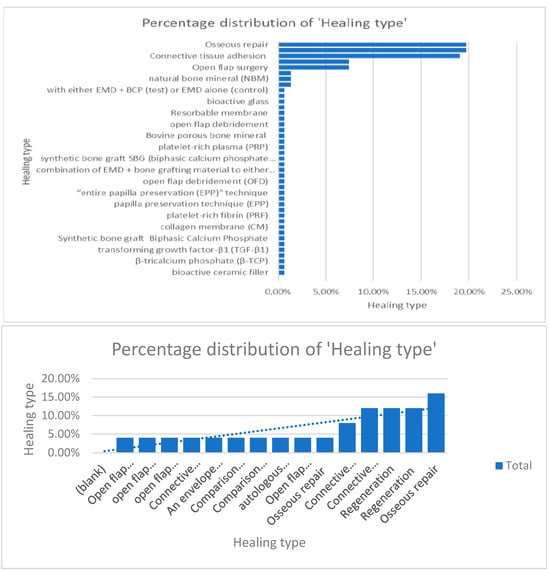
Figure 8.
Percentage distribution of the surgical techniques used.
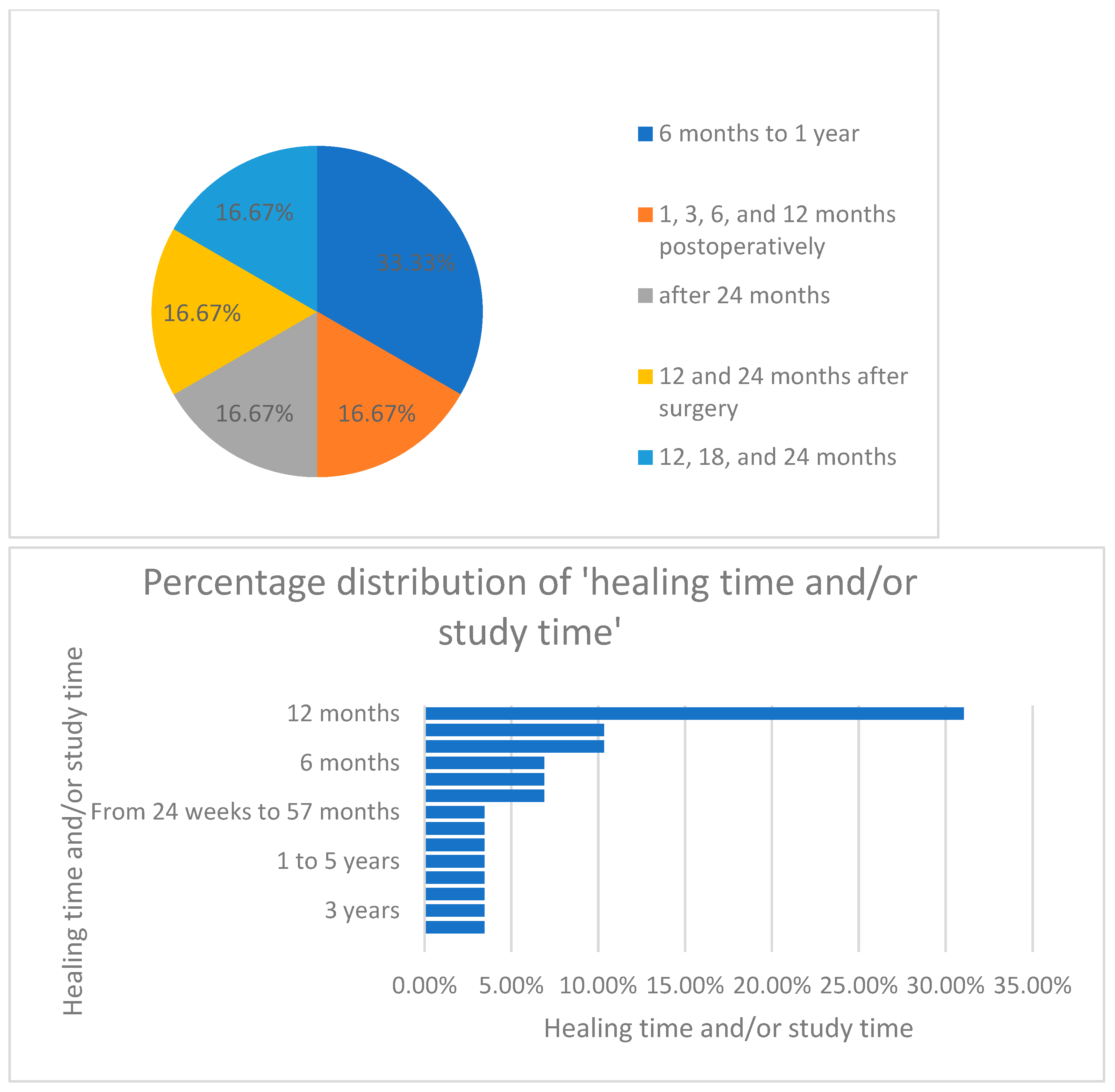
Furthermore, when analyzing the distribution of healing time and study period, as seen in Figure 9, the authors concluded that most of the studies (33%) were conducted over a period of 12 months.
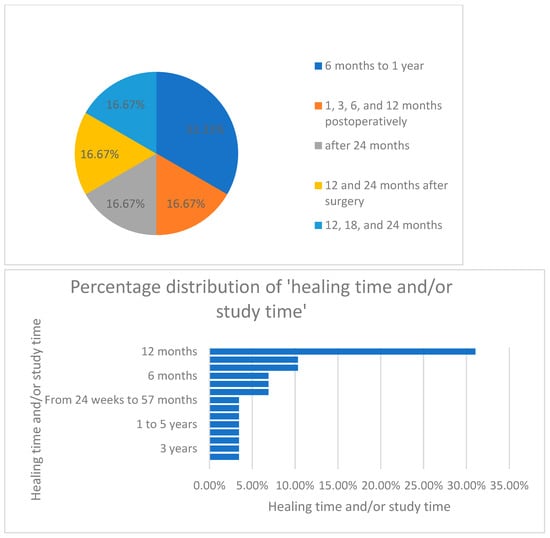
Figure 9.
Percentage distribution of the healing time/study period.
4. Discussion
Using a range of techniques and materials, periodontal regeneration in human intrabony defects can be accomplished to varying degrees, according to the findings of the current systematic study. The major goals of periodontal treatment are to eliminate infection and resolve chronic inflammation in order to stop its progression and prevent it from recurring. A lack of bleeding on probing and minimal probing pocket depths (less than 4 mm) are the clinical signs of this. Following the application of various bone grafts and analogs, guided tissue regeneration, biological agents, and other combinations, periodontal regeneration has been observed. Recent pioneering research showed that enamel matrix proteins might act as essential regenerative proteins capable of supporting periodontal regeneration, including the development of new cementa, functionally orientated periodontal ligament fibers, and new alveolar bone. There has been a good number of articles about the biological basis and therapeutic application of EMD.
Several interesting facts about clinical research have emerged. Sculean et al. [61] in his detailed observation of several studies has shown that, unexpectedly, the amount of periodontal regeneration, or new cementa with inserting fibers that are functionally orientated and new bone, was quite consistent across all treatments. Except for alloplastic substances and biological agents employed as monotherapies, which demonstrated little periodontal regeneration despite a wide range in intrabony defect depth prior to therapy, those findings were confirmed. A further interesting finding is that, in the majority of situations, the amount of newly formed cementa was either greater than or equal to the amount of newly formed bone and that wound stability and the provision of space are crucial for fully realizing the periodontum’s innate capacity for regeneration [61].
In the same way, clinical in vivo research revealed that even extremely extensive periodontal defects transplanted with a bone substitute by guided tissue regeneration exhibited complete regeneration given enough time for tissue remodeling and maturation. Therefore, the diminished bone growth seen in a number of biopsies may simply be the product of a short healing period [62].
A crucial factor that may lead to bias during the histopathological analysis and that must be taken into account for a fair interpretation of the healing outcome, and also for an equitable comparison between treatment modalities, is the variation in morphological characteristics and dimensions of naturally developing periodontal defects [63,64,65,66]. In fact, the vascular and cellular resources of the periodontal ligaments, alveolar bone, and gingiva that surround the defect appear to have a significant impact on the repair of deep three-walled intraosseous lesions and deep dehiscence or gingival recession defects. Contrarily, it is clear that in two- or one-walled intraosseous defects, the distribution and contribution of tissue resources are drastically changed and diminished [67,68,69]. In fact, the proportions of the defects seem to be a significant factor in predicting healing achievements in clinical settings, both after conventional surgical therapy. Where wide defects responded with less bone gain compared to narrow defects, and after periodontal regenerative surgery, better clinical outcomes, in other words, larger clinical attachment level (CAL) gain and bone fill, were achieved in deep, narrow intrabony defects compared to wide, shallow defects [63,64,65,66,67,68,69].
In line with our research, Miron et al. [21,38,57] pointed out the effects on early wound healing. All sites were re-evaluated using a visual analog scale to determine the level of post-treatment discomfort after a median of 4 weeks. The EMD administered had a beneficial impact on intraosseous defects, as shown by an evaluation of postoperative regeneration, healing, and morbidity. Regarding the clinical outcomes following intrabony defects using EMD alone, Miron et al. [21,38,57] highlighted in his clinical research and other several studies that EMD significantly improved CAL gains and pocket depths. These results were mainly achieved via an open-flap debridement (OFD) surgical technique.
When comparing the values, the authors were able to collect and statistically calculate those combinations including the guided tissue generation technique (GTR), barrier membranes, or grafting materials, and EMD combined with these different techniques and biomaterials acted in synergy and had a significant positive impact on bone regeneration, especially in horizontal bone loss. Vertical or angular defects affect the bone in an oblique manner, causing a hollowed-out depression alongside the root. The defect’s base is perpendicular to the surrounding bone, frequently resulting from the presence of an infrabony periodontal pocket; therefore, treatment success is more difficult.
Despite the fact that the enamel matrix derivative has been around for more than 20 years as a periodontal tissue regenerator [56,59,60], it is also astonishing that it is still one of the few biomaterials that can histologically show genuine periodontal regeneration with the production of new cementa, periodontal ligaments, and alveolar bone that is still readily available for clinical usage [63,64,65,66,67,68,69]. Future research is needed in order to validate the results of the present research, since the main limitation of the present study is the quite-low number of articles included in the methodology. Specific enamel matrix proteins have several biological functions, and more studies are being conducted to characterize how these activities affect the behavior of cells and tissues. From a clinical standpoint, it is also increasingly crucial to continue researching the use of EMDs to see if bone regeneration outcomes may be further enhanced by minor adjustments to EMD support systems or by minimally invasive surgical techniques. EMD continues to be one of the benchmarks for biologic-assisted periodontal regeneration.
5. Conclusions
The use of EMD alone or its use in combination with other materials (membranes, growth factors, etc.) and techniques offered reduced pocket depth, a gain in clinical attachment, and bone repairment. Through the present research concerning the use of EMD in relation to bone defects, the authors conclude that the healing propensity depends on the morphological structure of the bone defect represented by the wall stage and that there is a certain coherence and correlation between the values of probing depth (PD) and clinical attachment lost (CAL).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/dj13030092/s1, Table S1: PRISMA guideline checklist; original tables regarding the methodology conducted.
Author Contributions
Conceptualization, A.V. and E.B.; data curation, S.-I.P.; formal analysis, B.R.S.; investigation, A.V.; methodology, A.B.; project administration E.B.; resources, B.R.S. and A.B.; software, E.B.; supervision, S.-I.P.; validation, A.V.; visualization, A.B.; writing—original draft, E.B. and A.V.; writing—review and editing, S.-I.P. and B.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Targu Mures, Research Grant number 163/1/10.01.2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Clin. Periodontol. 2018, 45, 9–16. [Google Scholar] [CrossRef]
- Socransky, S.S.; Kaffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Newman, M.G.; Carranza, F.A. Carranza’s Clinical Periodontology, 12th ed.; Elsevier/Saunders: St. Louis, MO, USA, 2015. [Google Scholar]
- Jayakumar, A.; Rohini, S.; Naveen, A.; Haritha, A.; Reddy, K. Horizontal alveolar bone loss: A periodontal orphan. J. Indian Soc. Periodontol. 2010, 14, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Goldman, H.M.; Cohen, D.W. The Infrabony Pocket: Classification and Treatment. J. Periodontol. 1958, 29, 272–291. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S.; et al. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Saccucci, M.; Di Carlo, G.; Zeza, B.; Ambrosca, M.; Paolantonio, M.; Sammartino, G.; Mongardini, C.; Polimeni, A. Clinical Evaluation of the Regenerative Potential of EMD and NanoHA in Periodontal Infrabony Defects: A 2-Year Follow-Up. BioMed Res. Int. 2014, 2014, 492725. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G.; Acunzo, R.; Barnett, A.; Pagni, G. The soft tissue wall technique for the regenerative treatment of non-contained infrabony defects: A case series. Int. J. Periodontics Restor. Dent. 2013, 33, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Röllke, L.; Schacher, B.; Wohlfeil, M.; Dannewitz, B.; Kaltschmitt, J.; Krieger, J.K.; Krigar, D.M.; Reitmeir, P.; Kim, T. Enamel Matrix Derivative in Propylene Glycol Alginate for Treatment of Infrabony Defects With or Without Systemic Doxycycline: 12- and 24-Month Results. J. Periodontol. 2014, 85, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Mueller, V.T.; Welch, K.; Bratu, D.C.; Wang, H.-L. Early and late studies of EMD use in periodontal intrabony defects. J. Periodont. Res. 2013, 48, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.; Ferrantino, L.; Bernardini, L.; Lencioni, M.; Masiero, S. Minimally Invasive Surgical Technique in Periodontal Regeneration: A Randomized Controlled Clinical Trial Pilot Study. Int. J. Periodontics Restor. Dent. 2016, 36, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S68–S77. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Karanxha, L.; Panda, S.; Bucchi, C.; Nadathur Doraiswamy, J.; Sankari, M.; Ramamoorthi, S.; Varghese, S.; Taschieri, S. Autologous platelet concen-trates for treating periodontal infrabony defects. Cochrane Database Syst. Rev. 2018, 11, CD011423. [Google Scholar] [CrossRef]
- Ragghianti Zangrando, M.S.; Chambrone, D.; Pasin, I.M.; Conde, M.C.; Pannuti, C.M.; de Lima, L.A.P.A. Two-year randomized clinical trial of enamel matrix derivative treated infrabony defects: Radiographic analysis. BMC Oral Health 2014, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Losada, M.; González, R.; Garcia, À.P.; Santos, A.; Nart, J. Treatment of Non-Contained Infrabony Defects With Enamel Matrix Derivative Alone or in Combination With Biphasic Calcium Phosphate Bone Graft: A 12-Month Randomized Controlled Clinical Trial. J. Periodont. 2017, 88, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Nickles, K.; Dannewitz, B.; Gallenbach, K.; Ramich, T.; Scharf, S.; Röllke, L.; Schacher, B.; Eickholz, P. Long-Term Stability After Regenerative Treatment of Infrabony Defects: A Retrospective Case Series. J. Periodontol. 2017, 88, 536–542. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Q.; Sculean, A.; Buser, D.; Pippenger, B.E.; Dard, M.; Shirakata, Y.; Chandad, F.; Zhang, Y. Osteoinductive potential of 4 commonly employed bone grafts. Clin. Oral Investig. 2016, 20, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Szatmári, P.; Gera, I. Treatment of localized intrabony periodontal defects with enamel matrix derivative (Emdogain). Case series. Fogorv. Sz. 2014, 107, 15–28. [Google Scholar]
- Fileto Mazzonetto, A.L.; Casarin, R.C.V.; Santamaria, M.P.; Andere, N.M.R.B.; Araújo, C.F.; Videira Clima da Silva, R.; Purisaca, J.E.V.; Sallum, E.A.; Sallum, A.W. Clinical, radiographic, and patient-centered outcomes after use of enamel matrix proteins for the treatment of intrabony defects in patients with aggressive periodontitis: A 12-month multicenter clinical trial. J. Periodont. 2021, 92, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Machot, E.; Hoffmann, T.; Lorenz, K.; Khalili, I.; Noack, B. Clinical Outcomes after Treatment of Periodontal Intrabony Defects with Nanocrystalline Hydroxyapatite (Ostin) or Enamel Matrix Derivatives (Emdogain): A Randomized Controlled Clinical Trial. BioMed Res. Int. 2014, 2014, 786353. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.A.; Marini, L.; Pilloni, A.; Sahrmann, P. Early wound healing outcomes after regenerative periodontal surgery with enamel matrix derivatives or guided tissue regeneration: A systematic review. BMC Oral Health 2019, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, A.; Bertl, K.; Spineli, L.M.; Sculean, A.; Cortellini, P.; Tonetti, M. Medium- and long-term clinical benefits of periodontal regenerative/reconstructive procedures in intrabony defects: Systematic review and network meta-analysis of randomized controlled clinical studies. J. Clin. Periodont. 2021, 48, 410–430. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.J.; Jhingran, R.; Gupta, V.; Bains, V.K.; Madan, R.; Rizvi, I. Efficacy of platelet-rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: A clinical and cone beam computed tomography study. J. Int. Acad. Periodontol. 2014, 16, 86–96. [Google Scholar]
- Matarasso, M.; Iorio-Siciliano, V.; Blasi, A.; Ramaglia, L.; Salvi, G.E.; Sculean, A. Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects. A systematic review and meta-analysis. Clin. Oral Investig. 2015, 19, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Alberti, A.; Calciolari, E.; Taschieri, S.; Francetti, L. Enamel matrix derivative for the treatment of partially contained intrabony defects: 12-month results. Aust. Dent. J. 2019, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Buduneli, N.; Cortellini, P. Clinical outcomes of the entire papilla preservation technique with and without biomaterials in the treatment of isolated intrabony defects: A randomized controlled clinical trial. J. Clin. Periodont. 2020, 47, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Pietruska, M.; Pietruski, J.; Nagy, K.; Brecx, M.; Arweiler, N.B.; Sculean, A. Four-year results following treatment of intrabony periodontal defects with an enamel matrix derivative alone or combined with a biphasic calcium phosphate. Clin. Oral Investig. 2012, 16, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal Regeneration with Enamel Matrix Derivative in Reconstructive Periodontal Therapy: A Systematic Review. J. Periodont. 2012, 83, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, L.; Hu, J. The use of enamel matrix derivative alone versus in combination with bone grafts to treat patients with periodontal intrabony defects: A meta-analysis. J. Am. Dent. Assoc. 2012, 143, e46–e56. [Google Scholar] [CrossRef]
- Döri, F.; Arweiler, N.B.; Szántó, E.; Ágics, A.; Gera, I.; Sculean, A. Ten-Year Results Following Treatment of Intrabony Defects With an Enamel Matrix Protein Derivative Combined With Either a Natural Bone Mineral or a β-Tricalcium Phosphate. J. Periodont. 2013, 84, 749–757. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Andreuccetti, G.; Blasi, A.; Matarasso, M.; Sculean, A.; Salvi, G.E. Clinical outcomes following regenerative therapy of non-contained intrabony defects using a deproteinized bovine bone mineral combined with either enamel matrix derivative or collagen membrane. J. Periodontol. 2014, 85, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Parashis, A.O.; Polychronopoulou, A.; Tsiklakis, K.; Tatakis, D.N. Enamel Matrix Derivative in Intrabony Defects: Prognostic Parameters of Clinical and Radiographic Treatment Outcomes. J. Periodont. 2012, 83, 1346–1352. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Romano, F.; Quirico, A.; Di Bella, M.; Pallotti, S.; Aimetti, M. Effectiveness of Enamel Matrix Derivative in Conjunction with Particulate Autologous Bone in the Treatment of Noncontained Intrabony Defects: A 2-Year Prospective Case Series. Int. J. Periodontics. Restor. Dent. 2018, 38, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Wei, L.; Bosshardt, D.D.; Buser, D.; Sculean, A.; Zhang, Y. Effects of enamel matrix proteins in combination with a bovine-derived natural bone mineral for the repair of bone defects. Clin. Oral Investig. 2014, 18, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, C.; Labriola, A.; Generali, L. Dental root surface treatment with ethylenediaminetetraacetic acid does not improve enamel matrix derivative peptide treatment within intrabony defects: A retrospective study. J. Biol. Regul. Homeost. Agents 2019, 33, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Al-Machot, E.; Meyle, J.; Jervøe-Storm, P.-M.; Jepsen, S. Three-year results following regenerative periodontal surgery of advanced intrabony defects with enamel matrix derivative alone or combined with a synthetic bone graft. Clin. Oral Investig. 2016, 20, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Vecchiatini, R.; Maietti, E.; Simonelli, A. A simplified composite outcome measure to assess the effect of periodontal regenerative treatment in intraosseous defects. J. Periodont 2020, 91, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Duan, D.; Xin, Y.; Bai, L.; Li, T.; Li, C.; Xu, Y. A review of the literature: Antibiotic usage and its relevance to the infection in periodontal flaps. Acta Odontol. Scand. 2017, 75, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Döri, F.; Arweiler, N.; Húszár, T.; Gera, I.; Miron, R.J.; Sculean, A. Five-Year Results Evaluating the Effects of Platelet-Rich Plasma on the Healing of Intrabony Defects Treated With Enamel Matrix Derivative and Natural Bone Mineral. J. Periodont. 2013, 84, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Agrali, O.; Kuru, B.E.; Yarat, A.; Kuru, L. Evaluation of gingival crevicular fluid transforming growth factor-β1 level after treatment of intrabony periodontal defects with enamel matrix derivatives and autogenous bone graft: A randomized controlled clinical trial. Nig. J. Clin. Pract. 2016, 19, 535. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Ferrarotti, F.; Mariani, G.M.; Romano, F. A novel flapless approach versus minimally invasive surgery in periodontal regeneration with enamel matrix derivative proteins: A 24-month randomized controlled clinical trial. Clin. Oral Investig. 2017, 21, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Mitani, A.; Takasu, H.; Horibe, T.; Furuta, H.; Nagasaka, T.; Aino, M.; Fukuda, M.; Fujimura, T.; Mogi, M.; Noguchi, T. Five-year clinical results for treatment of intrabony defects with EMD, guided tissue regeneration and open-flap debridement: A case series. J. Periodont. Res. 2014, 50, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Aydemir Turkal, H.; Demirer, S.; Dolgun, A.; Keceli, H.G. Evaluation of the adjunctive effect of platelet-rich fibrin to enamel matrix derivative in the treatment of intrabony defects. Six-month results of a randomized, split-mouth, controlled clinical study. J. Clin. Periodont. 2016, 43, 955–964. [Google Scholar] [CrossRef]
- Anoixiadou, S.; Parashis, A.; Vouros, I. Enamel matrix derivative as an adjunct to minimally invasive non-surgical treatment of intrabony defects: A randomized clinical trial. J. Clin. Periodont. 2022, 49, 134–143. [Google Scholar] [CrossRef]
- Ogihara, S.; Tarnow, D.P. Efficacy of Enamel Matrix Derivative With Freeze-Dried Bone Allograft or Demineralized Freeze-Dried Bone Allograft in Intrabony Defects: A Randomized Trial. J. Periodontol. 2014, 85, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Buduneli, N.; Cortellini, P. Entire papilla preservation technique in the regenerative treatment of deep intrabony defects: 1-Year results. J. Clin. Periodont. 2017, 44, 926–932. [Google Scholar] [CrossRef]
- Bhutda, G.; Deo, V. Five years clinical results following treatment of human intra-bony defects with an enamel matrix derivative: A randomized controlled trial. Acta Odontol. Scand. 2013, 71, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Scaffold Requirements for Periodontal Regeneration with Enamel Matrix Derivative Proteins. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28531879/ (accessed on 13 June 2024).
- Aimetti, M.; Ferrarotti, F.; Mariani, G.; Fratini, A.; Giraudi, M.; Romano, F. Enamel Matrix Derivative Proteins in Combination with a Flapless Approach for Periodontal Regeneration of Intrabony Defects: A 2-Year Prospective Case Series. Int. J. Periodont. Restor. Dent. 2016, 36, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Jing, D.; Shuang, Y.; Miron, R.J. Enamel matrix derivative improves gingival fibroblast cell behavior cultured on titanium surfaces. Clin. Oral Investig. 2016, 20, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Clinical and CBCT Evaluation of Combined Periodontal Regenerative Therapies Using Enamel Matrix Derivative and Deproteinized Bovine Bone Mineral With or Without Collagen Membrane. Available online: http://quintpub.com/journals/prd/abstract.php?iss2_id=1525&article_id=18331#.YpI_RXXP1PY (accessed on 28 May 2024).
- De Leonardis, D.; Paolantonio, M. Enamel Matrix Derivative, Alone or Associated With a Synthetic Bone Substitute, in the Treatment of 1- to 2-Wall Periodontal Defects. J. Periodontol. 2013, 84, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- de Sanctis, M.; Goracci, C.; Zucchelli, G. Long-term effect on tooth vitality of regenerative therapy in deep periodontal bony defects: A retrospective study. Int. J. Periodont. Restor. Dent. 2013, 33, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mikami, R.; Mizutani, K.; Shioyama, H.; Matsuura, T.; Aoyama, N.; Suda, T.; Kusunoki, Y.; Takeda, K.; Izumi, Y.; Aida, J.; et al. Influence of aging on periodontal regenerative therapy using enamel matrix derivative: A 3-year prospective cohort study. J. Clin. Periodontol. 2021, 49, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Artzi, Z.; Tal, H.; Platner, O.; Wasersprung, N.; Weinberg, E.; Slutzkey, S.; Gozali, N.; Carmeli, G.; Herzberg, R.; Kozlovsky, A. Deproteinized bovine bone in association with guided tissue regeneration or enamel matrix derivatives procedures in aggressive periodontitis patients: A 1-year retrospective study. J. Clin. Periodontol. 2015, 42, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Sculean, A.; Kantarci, A. Regenerative Periodontal Therapy in Intrabony Defects and Long-Term Tooth Prognosis. Dent. Clin. N. Am. 2022, 66, 103–109. [Google Scholar] [CrossRef]
- University of Bern. Effect of Emdogain on Wound Healing After Gingival Recession Coverage Using Connective Tissue Graft: A Pilot Study 2017. Available online: https://pubmed.ncbi.nlm.nih.gov/31290017/ (accessed on 20 June 2024).
- NCT02810054; Using MIST With or Without EMD in Treatment of Intrabony Periodontal Defects. The University of Manchester: Manchester, UK, 2018. Available online: https://clinicaltrials.gov/study/NCT02810054 (accessed on 15 May 2024).
- NCT05029089; Modified Entire Papilla Preservation Technique for Treatment of Intrabony Defects. Medical University of Warsaw: Warszawa, Poland, 2021. Available online: https://clinicaltrials.gov/study/NCT05029089 (accessed on 21 May 2024).
- Lee, J.H.; Kim, D.H.; Jeong, S.N. Adjunctive Use of Enamel Matrix Derivatives to Porcine Derived Xenograft for the Treatment of One-Wall Intrabony Defects: Two-Year Longitudinal Results of a Randomized Controlled Clinical Trial. J. Periodontol. 2019, 91, 880–889. [Google Scholar] [CrossRef]
- JPRN-UMIN000008231; A Non-Inferiority Study Evaluating Fibroblast Growth Factor-2 (KCB-1D) to Enamel Matrix Derivative (Emdogain(R)Gel) for Periodontal Tissue Regeneration. Ogawa Red Cross Hospital: Saitama, Japan, 2019. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000008231 (accessed on 20 June 2024).
- IRCT2017022716141N3; Comparative Evaluation of Two Different Regenerative Materials in Reconstruction of Periodontal Defects. Hamedan University of Medical Sciences: Hamedan, Iran, 2018. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT2017022716141N3 (accessed on 10 June 2024).
- ISRCTN18159776; Treatment of Peri-Implant Diseases with Enamel Matrix Proteins. ThinkingPerio Research: Bilbao, Spain, 2021. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN18159776 (accessed on 10 June 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).