Optimizing Endodontic Surgery: A Systematic Review of Guided Tissue Regeneration, Grafting, and Platelet Concentrates vs. No Intervention
Abstract
1. Introduction
2. Method
- Population: Individuals with permanent teeth undergoing endodontic surgery.
- Interventions: Endodontic surgery with placement of bone grafts, membranes, or platelet concentrates.
- Comparisons: Endodontic surgery without the placement of bone grafts, membranes, or platelet concentrates.
- Outcomes: Improved clinical and radiographic outcomes one-year post-surgery.
- Study Design: RCTs and prospective clinical trials.
2.1. Search Methods for the Identification of Studies
2.2. Screening and Data Extraction
2.3. Risk of Bias Assessment
2.4. Quality of Evidence
2.5. Quantitative Analyses
3. Results
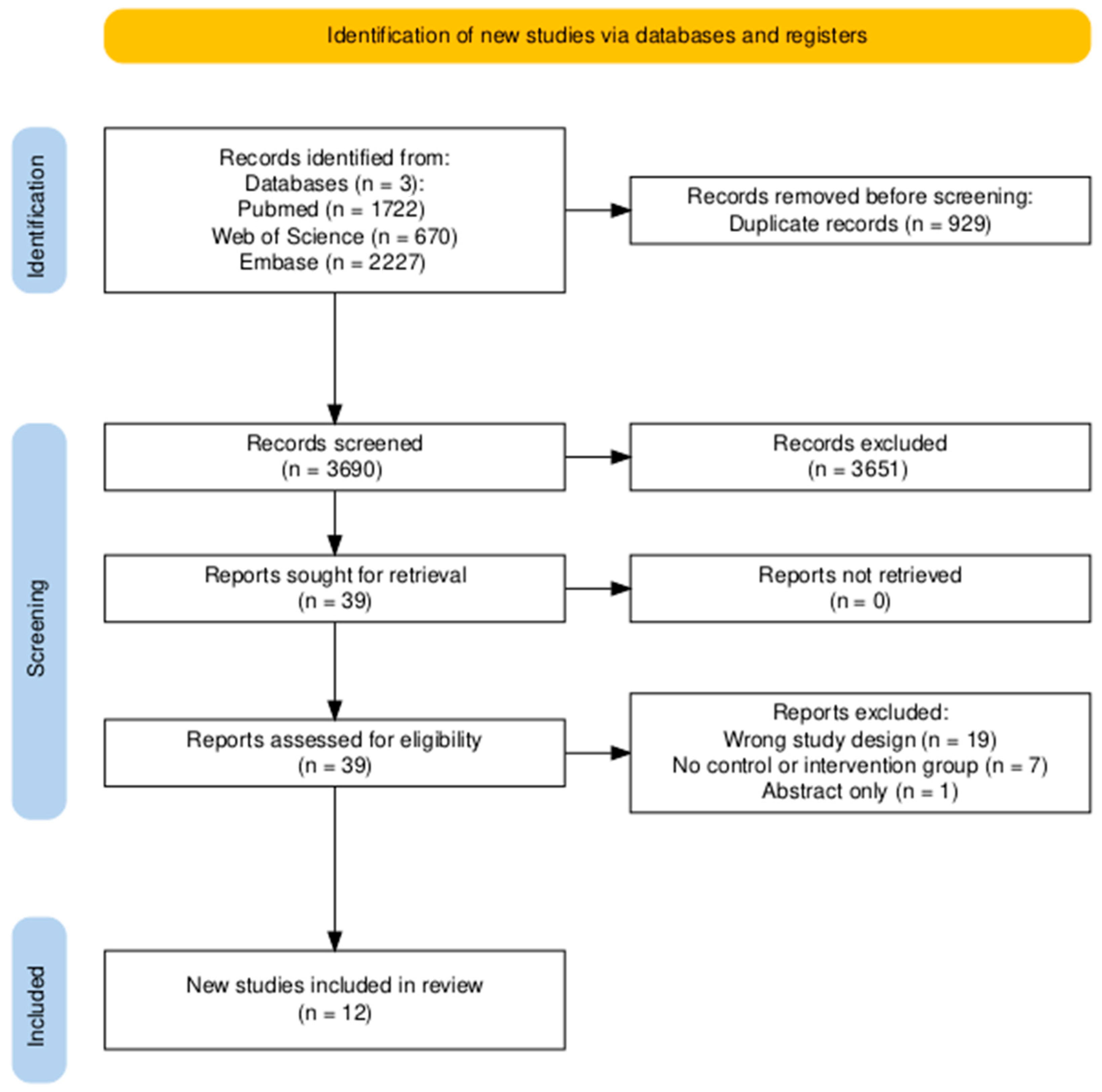
3.1. Search Results
3.2. Study Characteristics
3.2.1. Population
3.2.2. Intervention
3.2.3. Outcome Assessment
3.3. Risk of Bias
4. Data Synthesis
4.1. Primary Analysis
4.2. Subgroup Analysis
- Bone graft only: Intervention group n = 45 and control group n = 40 in three studies [24,28,31]. The success rate of endodontic surgery with graft was estimated to be 4.48 (OR: 4.48, 95% CI: 0.84–23.97) compared to the control group (Figure 6). The statistical heterogeneity for this group was moderately significant (I-squared: 35.6%, Q: 3.10, p-value: 0.212). However, this difference was not statistically significant.
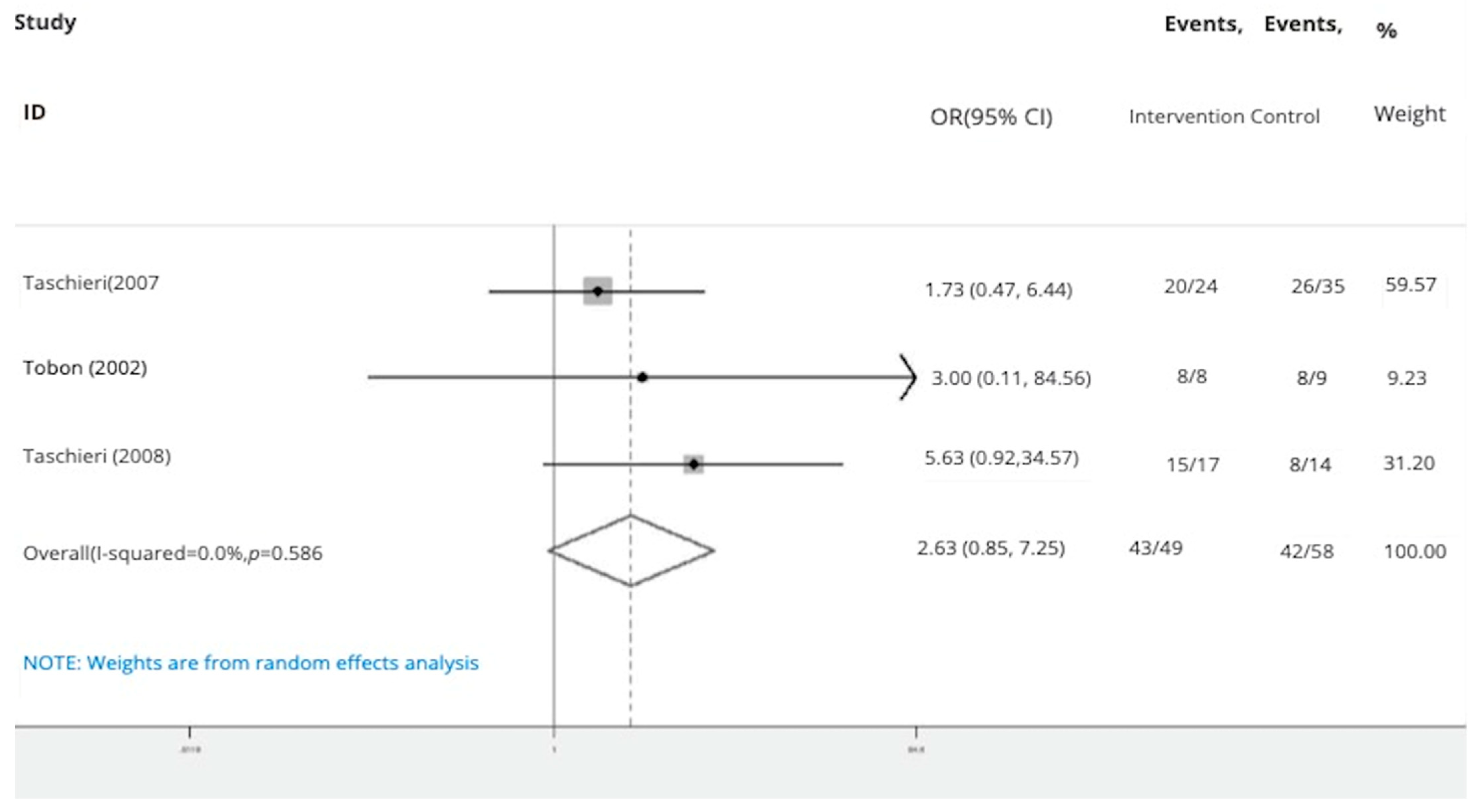
- Bone graft with membrane: In three studies (n = 49 intervention, n = 58 control) [2,29,30], the success rate of endodontic surgery with graft and membrane was estimated to be 2.63 times higher (OR: 2.63, 95% CI: 0.95–7.25) compared to the control group (Figure 7). Heterogeneity indices indicate that the heterogeneity between the results of the primary studies is very low (I-squared: 0%, Q: 1.07, p-value: 0.586). This difference was not statistically significant.
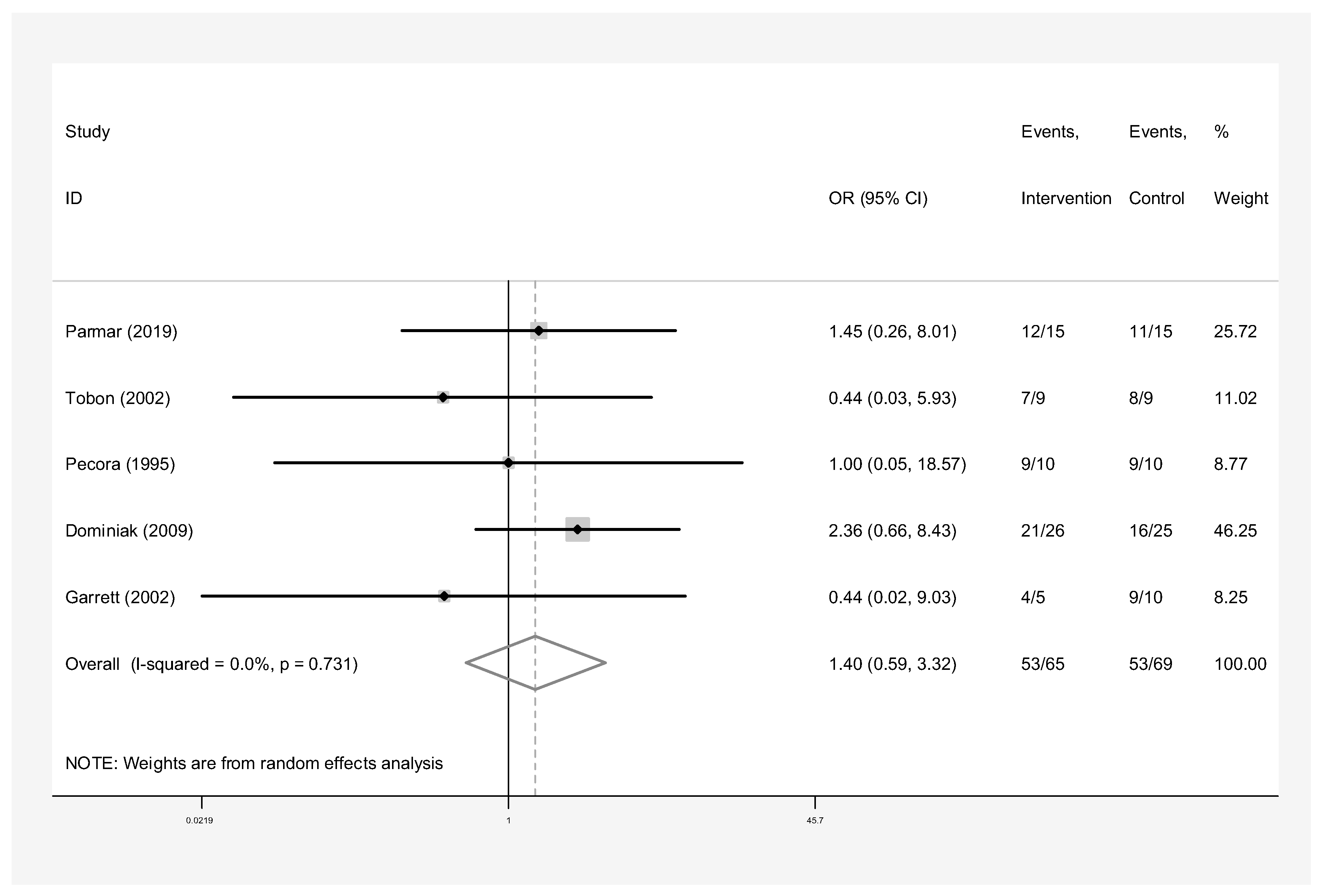
- Membrane only: Intervention group n = 65 and control group n = 69 in five studies [24,25,26,27,30]. The success rate of endodontic surgery with membrane only was estimated to be 1.40 (OR: 1.40, 95% CI: 0.59–3.32) compared to the control group (Figure 8). Heterogeneity indices indicate that the heterogeneity between the results of the primary studies was very low (I-squared: 0%, Q: 2.03, p-value: 0.731). This difference was not statistically significant.
- Concentrated Growth Factor Only: Intervention group n = 47 and control group n = 48 in four studies [11,12,31,32]. The success rate of endodontic surgery with CGF only was estimated to be 1.98 (OR: 1.98, 95% CI: 0.28–14.15) compared to the control group (Figure 9). The statistical heterogeneity was moderate (I2: 44.9%, Q: 5.45, p-value: 0.142). However, this difference was not statistically significant.
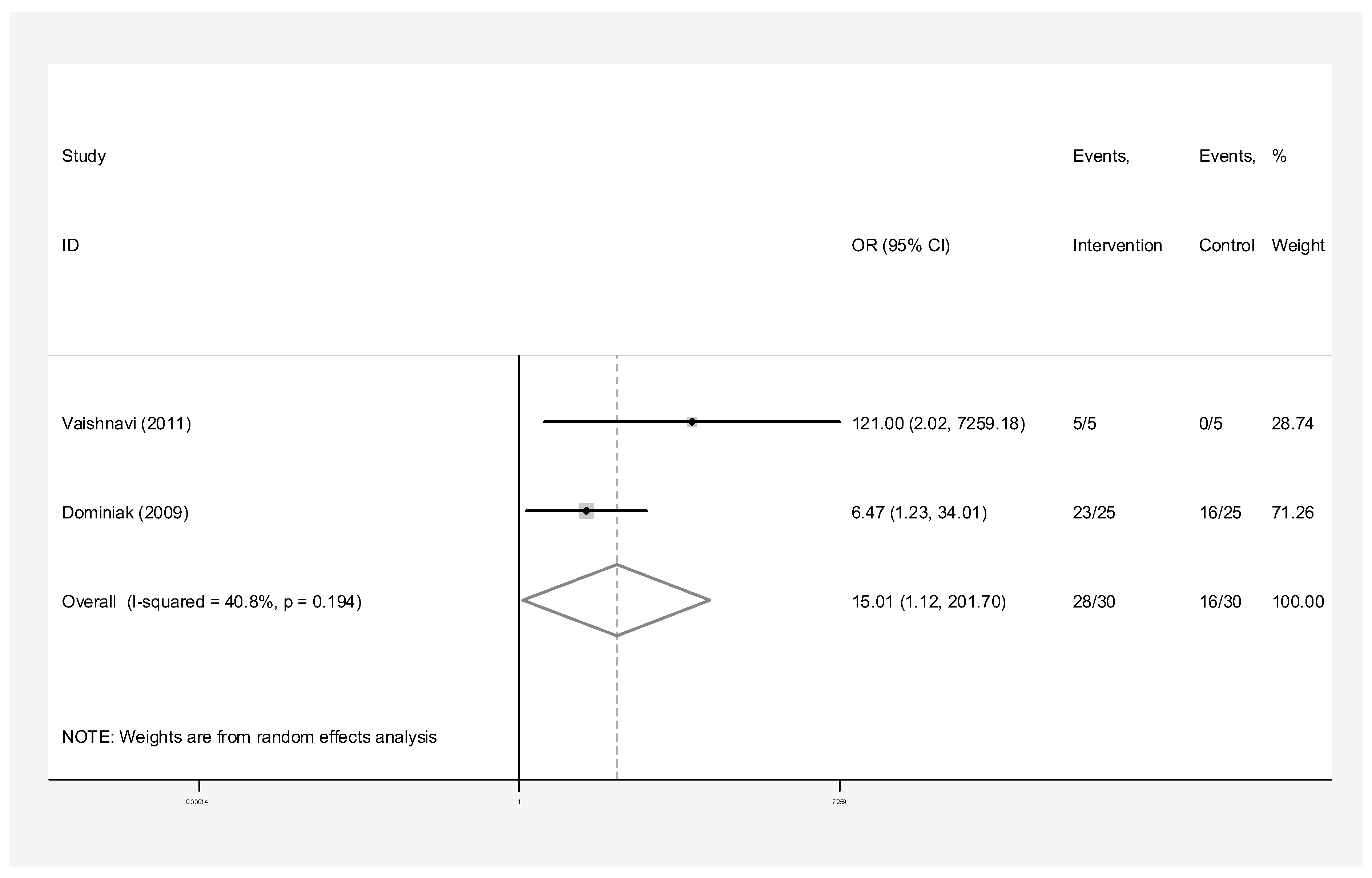
- Concentrated Growth Factor with Bone Graft: Intervention group n = 30 and control group n = 30 in two studies [24,31]. The success rate of endodontic surgery with CGF and bone graft was estimated to be 15.01 (OR: 15.01, 95% CI: 1.12–271.70) compared to the control group (Figure 10). The statistical heterogeneity was moderate (I-squared: 40.8%, Q: 1.69, p-value: 0.194). However, this difference was not statistically significant.
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutmann, J.L. Perspectives on root-end resection. J. Histodontol. 1999, 47, 135. [Google Scholar]
- Taschieri, S.; Del Fabbro, M.; Testori, T.; Saita, M.; Weinstein, R. Efficacy of guided tissue regeneration in the management of through-and-through lesions following surgical endodontics: A preliminary study. Int. J. Periodontics Restor. Dent. 2008, 28, 265–271. [Google Scholar]
- Bashutski, J.D.; Wang, H.L. Periodontal and endodontic regeneration. J. Endod. 2009, 35, 321–328. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, X.; Yang, J.; Jiang, H.; Yan, P. The effect of regeneration techniques on periapical surgery with different protocols for different lesion types: A meta-analysis. J. Oral Maxillofac. Surg. 2016, 74, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, A.; Sánchez-Garcés, M.; Gay-Escoda, C. Materials and prognostic factors of bone regeneration in peri-apical surgery: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2014, 19, e419–e425. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Stassen, L.F.; Hislop, W.S.; Still, D.M.; Moos, K.F. Use of anorganic bone in periapical defects following apical surgery—A prospective trial. Br. J. Oral Maxillofac. Surg. 1994, 32, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef]
- Kanno, T.; Takahashi, T.; Tsujisawa, T.; Ariyoshi, W.; Nishihara, T. Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J. Oral Maxillofac. Surg. 2005, 63, 362–369. [Google Scholar] [CrossRef]
- Goyal, B.; Tewari, S.; Duhan, J.; Sehgal, P.K. Comparative evaluation of platelet-rich plasma and guided tissue regeneration membrane in the healing of apicomarginal defects: A clinical study. J. Endod. 2011, 37, 773. [Google Scholar] [CrossRef]
- Dhiman, M.; Kumar, S.; Duhan, J.; Sangwan, P.; Tewari, S. Effect of platelet-rich fibrin on healing of apicomarginal defects: A randomized controlled trial. J. Endod. 2015, 41, 985–991. [Google Scholar] [CrossRef]
- Dhamija, R.; Tewari, S.; Sangwan, P.; Duhan, J.; Mittal, S. Impact of platelet-rich plasma in the healing of through-and-through periapical lesions using 2-dimensional and 3-dimensional evaluation: A randomized controlled trial. J. Endod. 2020, 46, 1167–1184. [Google Scholar] [CrossRef]
- Sharma, A.; Pradeep, A.R. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J. Periodontol. 2011, 82, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.A.; Albanyan, H.; Azim, K.A.; Piasecki, L. The Buffalo study: Outcome and associated predictors in endodontic microsurgery—A cohort study. Int. Endod. J. 2021, 54, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.; Foschi, F.; Maloney, B.; Creavin, G.; Duncan, H.F. The impact of bone grafting with/without barrier membrane placement on the outcome of apical surgery: A systematic review and meta-analysis. Int. Endod. J. 2024, 57, 1006–1020. [Google Scholar] [CrossRef]
- Liu, T.J.; Zhou, J.N.; Guo, L.H. Impact of different regenerative techniques and materials on the healing outcome of endodontic surgery: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 536–555. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, M.; Ihsan, M.S.; Kharat, P.; Azarpazhooh, A. The effect of hard tissue defects on the clinical outcome of endodontic microsurgery: A systematic review and meta-analysis. Clin. Oral Investig. 2023, 27, 7079–7089. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Elkabbany, A.; Del Fabbro, M.; von Arx, T. Guided Tissue Regeneration Using a Barrier Membrane in Endodontic Surgery. Swiss Dent. J. 2016, 126, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Von Arx, T.; Alsaeed, M. The use of regenerative techniques in apical surgery: A literature review. Saudi Dent. J. 2011, 23, 113–127. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods. 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H. A Grade Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef]
- StataCorp. Stata: Statistical Software, Release 14; StataCorp LLC: College Station, TX, USA, 2015. [Google Scholar]
- Dominiak, M.; Lysiak-Drwal, K.; Gedrange, T.; Zietek, M. Efficacy of healing process of bone defects after apicectomy: Results after 6 and 12 months. J. Physiol. Pharmacol. 2009, 60 (Suppl. S8), 51–55. [Google Scholar] [PubMed]
- Garrett, K.; Kerr, M.; Hartwell, G.; O’Sullivan, S.; Mayer, P. The effect of a bioresorbable matrix barrier in endodontic surgery on the rate of periapical healing: An in vivo study. J. Endod. 2002, 28, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.D.; Dhamija, R.; Tewari, S.; Sangwan, P.; Gupta, A.; Duhan, J.; Mittal, S. 2D and 3D radiographic outcome assessment of the effect of guided tissue regeneration using resorbable collagen membrane in the healing of through-and-through periapical lesions—A randomized controlled trial. Int. Endod. J. 2019, 52, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Pecora, G.; Kim, S.; Celletti, R.; Davarpanah, M. The guided tissue regeneration principle in endodontic surgery: One-year postoperative results of large periapical lesions. Int. Endod. J. 1995, 28, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Pecora, G.; De Leonardis, D.; Ibrahim, N.; Bovi, M.; Cornelini, R. The use of calcium sulphate in the surgical treatment of a ‘through and through’ periradicular lesion. Int. Endod. J. 2001, 34, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Taschieri, S.; Del Fabbro, M.; Testori, T.; Weinstein, R. Efficacy of xenogeneic bone grafting with guided tissue regeneration in the management of bone defects after surgical endodontics. J. Oral Maxillofac. Surg. 2007, 65, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Tobón, S.I.; Arismendi, J.A.; Marín, M.L.; Mesa, A.L.; Valencia, J.A. Comparison between a conventional technique and two bone regeneration techniques in periradicular surgery. Int. Endod. J. 2002, 35, 635–641. [Google Scholar] [CrossRef]
- Vaishnavi, C.; Mohan, B.; Narayanan, L.L. Treatment of endodontically induced periapical lesions using hydroxyapatite, platelet-rich plasma, and a combination of both: An in vivo study. J. Conserv. Dent. 2011, 14, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Yahata, Y.; Handa, K.; Ohkura, N.; Okamoto, M.; Ohshima, J.; Itoh, S.; Kawashima, N.; Tanaka, T.; Sato, N.; Noiri, Y.; et al. Autologous concentrated growth factor mediated accelerated bone healing in root-end microsurgery: A multicenter randomized clinical trial. Regener. Ther. 2023, 24, 377–384. [Google Scholar] [CrossRef]
- Tsesis, I.; Rosen, E.; Tamse, A.; Taschieri, S.; Del Fabbro, M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment: A systematic review and meta-analysis. J. Endod. 2011, 37, 1039. [Google Scholar] [CrossRef]
- Meschi, N.; Castro, A.B.; Vandamme, K.; Quirynen, M. The impact of autologous platelet concentrates on endodontic healing: A systematic review. Platelets 2016, 27, 613–633. [Google Scholar] [CrossRef] [PubMed]





| Intervention Group | Control Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Date | Age Range | Lesion Size/Type | Tooth Type | Regenerative Material | Cases | Success | Failure | Cases | Success | Failure |
| Dominiak 2009 [24] | 9 to 60 | Von Arx 1a | Maxillary/Mandibular Anterior/Posterior | Resorbable collagen membranes (BioGide®, NJ, USA) | 26 | 21 | 5 | 25 | 16 | 9 |
| Xenogenic Bio-Oss collagen graft®, NJ USA | 30 | 25 | 5 | |||||||
| Xenogenic Bio-Oss collagen® material in combination with platelet-rich plasma (PRP) | 25 | 23 | 2 | |||||||
| Dhamija 2020 [12] | 16 years and older | Through and through | Maxillary/Mandibular Tooth type not specified | Platelet-rich plasma (PRP) | 16 | 15 | 1 | 16 | 15 | 1 |
| Dhiman 2015 [11] | 17 to 47 | Not defined | Maxillary/Mandibular Tooth type not specified | Platelet-rich fibrin (PRF) | 15 | 13 | 2 | 15 | 12 | 3 |
| Garrett 2002 [25] | 24 to 67 | Von Arx 1a | Tooth type not specified | Bioresorbable polylactic acid membrane | 10 | 9 | 1 | 5 | 4 | 1 |
| Parmar 2019 [26] | 16 years and older | Through and through | Maxillary/Mandibular Anterior/Posterior | Resorbable collagen membrane | 15 | 12 | 3 | 15 | 11 | 4 |
| Pecora 1995 [27] | 27 to 50 | Through and through | Tooth type not specified | e-PTFE membrane (Gortex) | 10 | 9 | 1 | 10 | 9 | 1 |
| Pecora 2001 [28] | 47 to 50 | Von Arx 1a Through and through | Tooth type not specified | Calcium sulphate graft | 10 | 9 | 1 | 10 | 8 | 2 |
| Taschieri 2008 [2] | Mean age Women–32 Men 47 | Through and through | Maxillary/Mandibular Anterior/Posterior | Anorganic bovine hydroxyapatite graft with resorbable collagen membrane | 17 | 15 | 2 | 14 | 8 | 6 |
| Taschieri 2007 [29] | Mean age Women–d36 Men 43 | Through and through and 4 wall defects | Maxillary/Mandibular Anterior/Posterior | Anorganic bovine hydroxyapatite graft with resorbable collagen membrane | 24 | 20 | 4 | 35 | 26 | 4 |
| Tobón 2002 [30] | 14 to 74 | Not defined | Maxillary/Mandibular Anterior/Posterior | Synthetic bioactive resorbable graft of hydroxylapatite (Osteogen) with nonbioabsorbable GoreTex1 augmentation membrane | 8 | 8 | 0 | 9 | 8 | 1 |
| Not defined | Nonbioabsorbable GoreTex1 augmentation membrane | 9 | 7 | 2 | ||||||
| Vaishnavi 2011 [31] | 20 to 40 | Bony defect had to be confined to the apical area with the bone covering the entire root surface coronally and had an intact lingual cortical plate | Tooth type not specified | Platelet-rich plasma (PRP) and hydroxyapatite | 5 | 5 | 0 | 5 | 0 | 5 |
| Platelet-rich plasma (PRP) | 5 | 5 | 0 | |||||||
| Replacement with hydroxyapatite | 5 | 5 | 0 | |||||||
| Yahata 2023 [32] | 20 to 70 | Lesion size ≥5 mm in diameter on periapical radiography | Maxillary/Mandibular Anterior/Posterior | Concentrated growth factor (CGF) | 11 | 10 | 1 | 12 | 12 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabeti, M.; Black, N.; Ramazani, M.; Zarenejaddivkolahei, N.; Moosazadeh, M. Optimizing Endodontic Surgery: A Systematic Review of Guided Tissue Regeneration, Grafting, and Platelet Concentrates vs. No Intervention. Dent. J. 2025, 13, 91. https://doi.org/10.3390/dj13030091
Sabeti M, Black N, Ramazani M, Zarenejaddivkolahei N, Moosazadeh M. Optimizing Endodontic Surgery: A Systematic Review of Guided Tissue Regeneration, Grafting, and Platelet Concentrates vs. No Intervention. Dentistry Journal. 2025; 13(3):91. https://doi.org/10.3390/dj13030091
Chicago/Turabian StyleSabeti, Mohammad, Natalie Black, Mohsen Ramazani, Nafiseh Zarenejaddivkolahei, and Mahmood Moosazadeh. 2025. "Optimizing Endodontic Surgery: A Systematic Review of Guided Tissue Regeneration, Grafting, and Platelet Concentrates vs. No Intervention" Dentistry Journal 13, no. 3: 91. https://doi.org/10.3390/dj13030091
APA StyleSabeti, M., Black, N., Ramazani, M., Zarenejaddivkolahei, N., & Moosazadeh, M. (2025). Optimizing Endodontic Surgery: A Systematic Review of Guided Tissue Regeneration, Grafting, and Platelet Concentrates vs. No Intervention. Dentistry Journal, 13(3), 91. https://doi.org/10.3390/dj13030091









