Abstract
Background/Objective: Many novel solutions for a range of dental problems are emerging as a result of the quick development of nanotechnology and nanocomplex synthetic techniques. The effectiveness, quality, and negative consequences of these advancements are occasionally debatable, though. This systematic review sought to better summarize the existing additions of nanoparticles to dental adhesive systems in order to improve their performance and properties, evaluate their quality, and examine the results that have been published. Materials and methods: The present systematic review was carried out according to PRISMA guidelines. The search was carried out on PubMed central, Cochrane collaboration, Science direct and Scopus scientific engines. Selected MeSH keywords (nanoparticles, adhesive resin, enamel demineralization) were used for data extraction. A total of 13 full-text original articles were included in the final analysis, and these articles were based on adding nanoparticles to the adhesive resin to evaluate their effects on enamel demineralization. Results: The literature search resulted in a total of 13 original studies/articles up until November 2024. The text articles comprised in vitro studies with robust inclusion and exclusion criteria. The review included various types of adhesives and nanoparticles, with amorphous calcium phosphate (ACP) being the most common. Other nanoparticles included polydopamine–Ag, bioactive glass, and silver. Most studies assessed the effects of nanoparticles on adhesive shear bond strength (SBS), microbial growth, and microhardness. Only three studies investigated the effects of nanoparticles on microhardness using Vickers tests. Conclusions: The review found that adding nanoparticles to orthodontic dental adhesives enhances their antibacterial and anticariogenic properties without affecting the shear bond strength. This could prevent enamel demineralization during orthodontic therapy. Future research could benefit from these positive properties, necessitating an interdisciplinary approach.
1. Introduction
Tiny particles with sizes ranging from 1 to 100 nanometres (nm), where 1 nm is one-billionth of a meter, are called nanoparticles. Nanoparticles have distinct physical and chemical characteristics that set them apart from their bulk counterparts because of their small size [1]. Because of their high surface area to volume ratio, nanoparticles are more reactive and interact better with other materials. Quantum effects become important at the nanoscale, affecting the optical, electrical, and magnetic characteristics of nanoparticles. Metals, ceramics, polymers, and composites are just a few of the materials that can be used to create them [2].
Because of their special qualities at the nanoscale, nanoparticles provide numerous advantages in a variety of industries. Drug delivery methods can be enhanced by nanoparticles, increasing the efficacy and precision of treatments. For instance, they can minimize harm to healthy cells by delivering chemotherapy medications straight to cancer cells. Materials can become stronger, lighter, and more resilient by incorporating nanoparticles [3,4]. The sports equipment, automobile, and aerospace industries can all benefit from this. Electronic gadgets may now be produced more quickly, efficiently, and in smaller sizes thanks to nanotechnology. This covers anything from sophisticated computer systems to cell phones. Water filtration systems can employ nanoparticles to more efficiently remove impurities. Additionally, they contribute to energy conversion and storage, as shown in more effective solar panels and batteries [5]. The addition of nanoparticles improves commonplace products like antimicrobial surfaces, scratch-resistant coatings, and stain-resistant textiles. Nanoparticles can function as catalysts in chemical reactions to quicken them, increasing industrial operations’ efficiency and lowering the requirement for harsh chemicals [6].
Because of their potent antibacterial qualities, nanoparticles—like silver nanoparticles—are utilized in medical device coatings and wound dressings to help stop infections. To encourage quicker and more efficient wound and burn healing, nanoparticles can be added to creams and dressings [7]. Zinc oxide nanoparticles and natural extracts, for instance, have demonstrated encouraging outcomes in the treatment of skin burns [8]. Innovative immunological treatments for illnesses like cancer are being developed using nanoparticles. In order to better identify and combat cancer cells, they can aid in immune system modulation [3,4]. To treat genetic problems, nanoparticles can introduce genetic material into cells. This approach is being investigated for the treatment of muscular dystrophy and cystic fibrosis [9]. These uses demonstrate how nanoparticles can revolutionize medical interventions and enhance patient care.
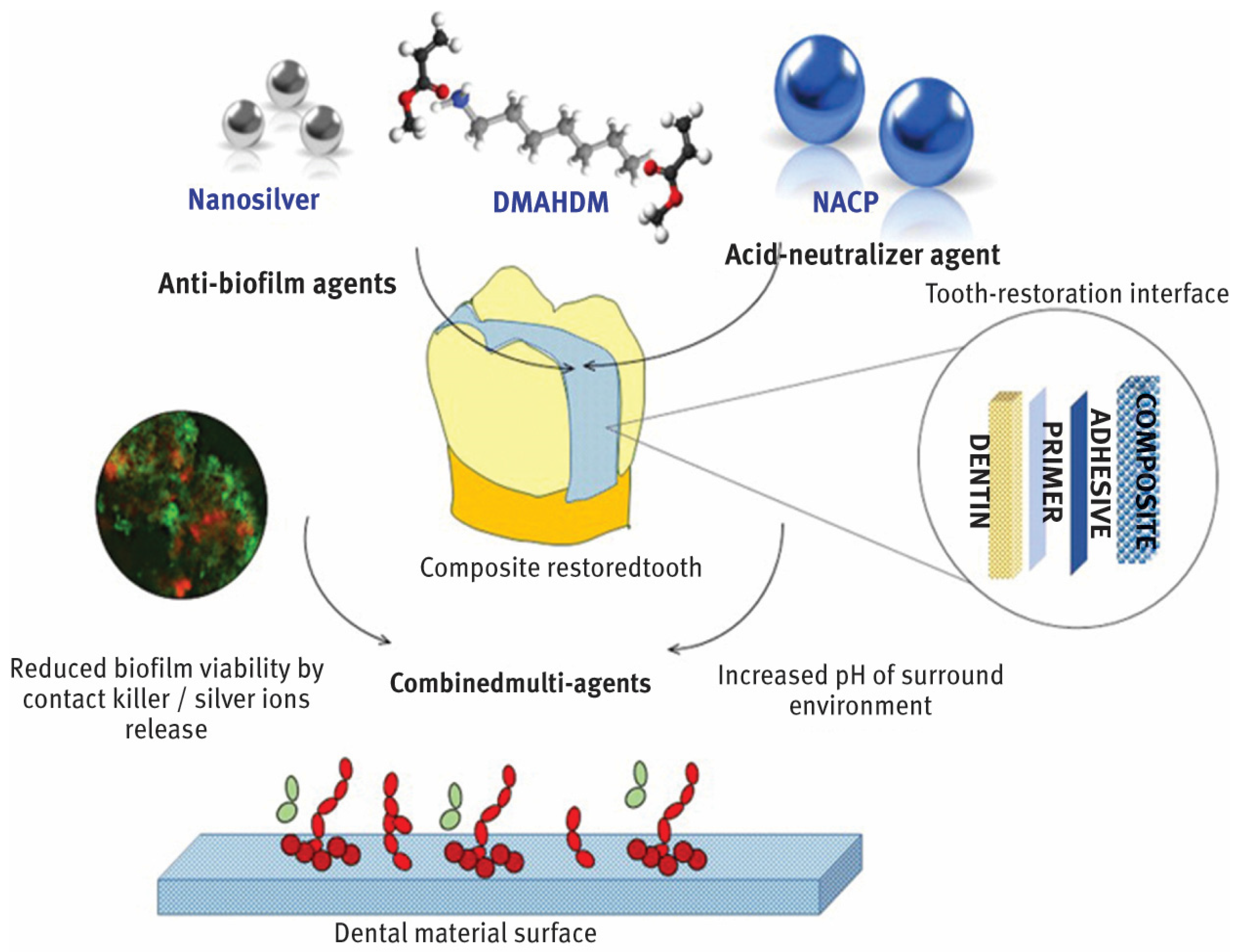
In dentistry, nanoparticles are advancing significantly and providing creative fixes for a range of tooth problems. By blocking the growth of dangerous bacteria, nanoparticles with potent antibacterial qualities, including zinc oxide and silver, help to prevent dental caries or tooth decay as shown in Figure 1 [10,11,12]. In order to improve the treatment of gum disorders, periodontal therapy can employ nanoparticles to transport medications straight to the afflicted areas [13]. Implants and prostheses can be made more resilient to wear and strain by adding nanoparticles to dental materials to improve their mechanical qualities. In order to increase the strength, durability, and aesthetic appeal of fillings and restorations, nanocomposites are utilized [14]. Better adhesion and fracture resistance are offered by these materials [15]. The early diagnosis of dental disorders is made possible by nanoparticles, which increase the sensitivity and precision of diagnostic instruments like imaging techniques [16]. To lower the risk of infections and enhance general hygiene in dental offices, nanoparticles can be applied to dental tools and surfaces. Dental nanotechnology, sometimes known as “nanodentistry”, is constantly developing and holds the promise of more effective and efficient dental treatment options down the road [10,11,12,13,14,15,16].
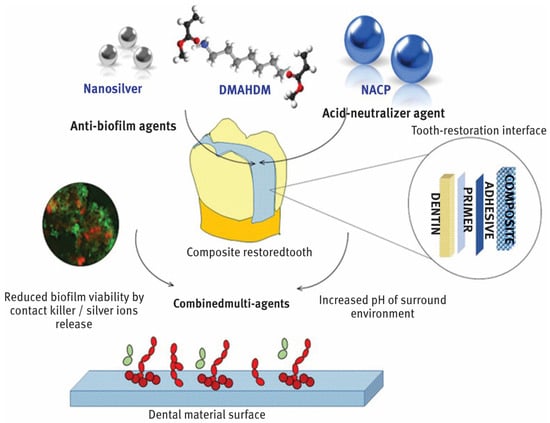
Figure 1.
Incorporation of AgNPs into dental structure. From “Designing Multiagent Dental Materials for Enhanced Resistance to Biofilm Damage at the Bonded Interface”, by Melo, MA et al. [10], ACS Applied Materials & Interfaces 2016 8 (18), 11779–11787 copywritted by ACS publications [10].
Recent research has focused on adding various nanoparticles to dental adhesives to improve their physical characteristics and antibacterial capabilities [11,12]. Elastomeric ligatured supported NPs like Benzocaine and silver have shown anticariogenic and antimicrobial effects, preventing dental decay and promoting oral health. Combining silver NPs with zinc oxide and chlorhexidine enhances tensile strength and anti-inflammatory properties, improving orthodontic material durability [16]. Gold nanoparticles have shown improved antibacterial properties, preventing enamel demineralization and maintaining adhesive integrity [13,14]. Titanium dioxide NPs enhance antibacterial efficacy without compromising adhesive shear bond strength [15]. Calcium phosphate NPs are stable and cost-effective, inhibiting bacterial growth and making them an attractive option for future development in dental adhesives [16,17].
The rationale for a review study on the application of nanoparticles in dental adhesives stems from the growing body of evidence that demonstrates the wide-ranging benefits of incorporating NPs into dental materials in addition to the limited evidence on how this affects enamel demineralization. By synthesizing and evaluating these studies, the present review aims to provide a comprehensive understanding of the mechanisms behind the enhanced properties of nanoparticle-enhanced adhesives, while also highlighting the potential for further innovation in the field. The growing interest in antimicrobial, mechanical, and anti-inflammatory effects makes this an important area of research with the potential to significantly improve dental care outcomes, particularly in orthodontics and preventive dentistry.
2. Material and Methods
This research project has been recognized and approved by the College of Dentistry Research Center (CDRC) at King Saud University, with registration number FR 0723.
2.1. Research Question
In this systematic review, we followed preferred reporting items from the systematic review and meta-analysis (PRISMA) guidelines. The main question was “Is there any impact of incorporating nanoparticles to adhesive resin on the demineralization of enamel?”
2.2. Search Strategy
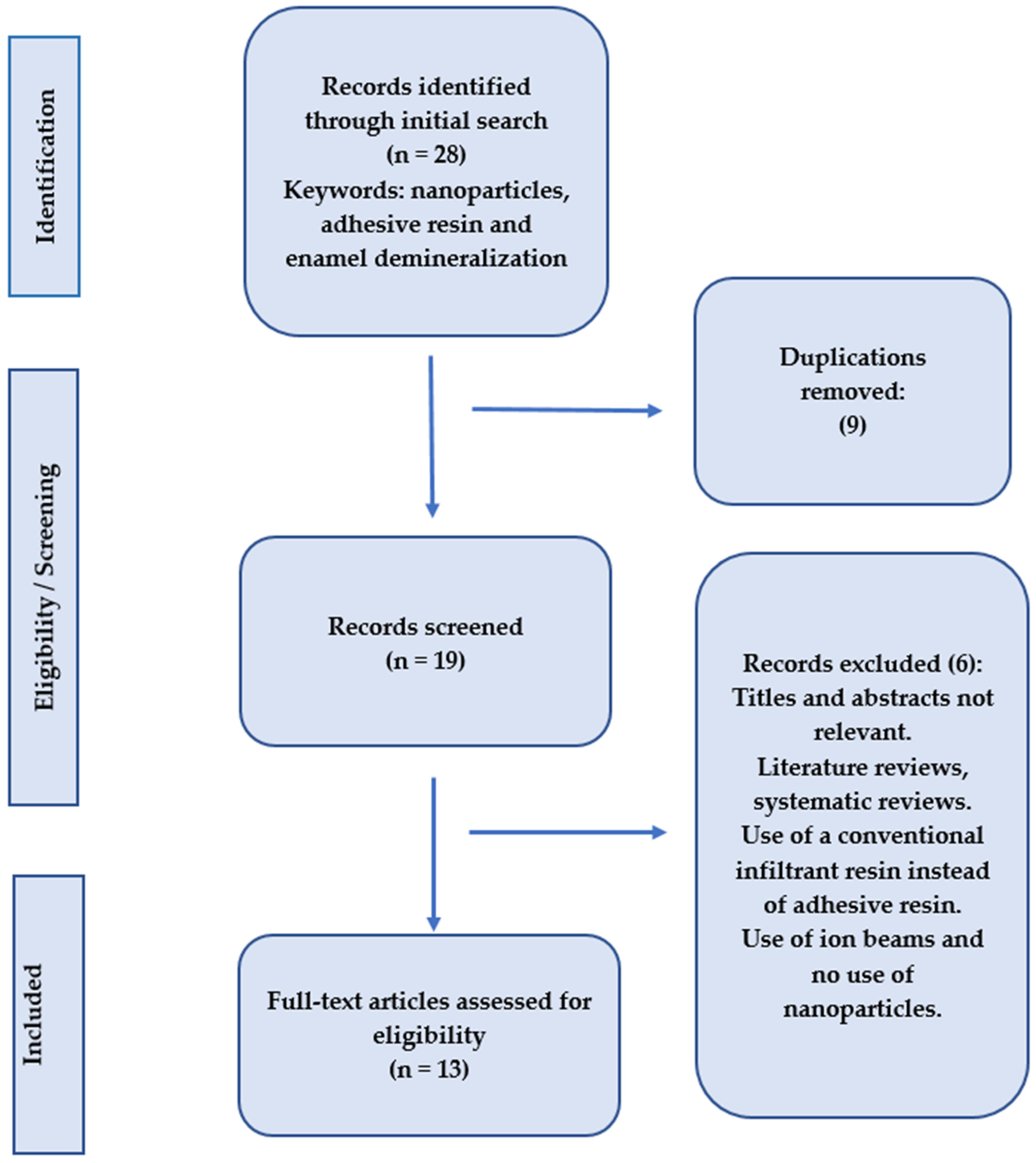
The PubMed Central, Cochrane Collaboration, Science Direct, and Scopus databases were searched for abstracts and full texts of papers, related to the subject, that were accessible online until November 2024, without regard for language limitations, as part of this systematic review. Keywords associated with “adhesive resin”, “enamel demineralization”, and “nanoparticles” were the main focus of the search. By carefully examining the references from the included publications, the search was further broadened. After carefully gathering all pertinent information, the principal investigator examined the findings. Only original research articles with full texts were included; after reviewing the abstracts and titles, any repeated studies were eliminated (Figure 2).
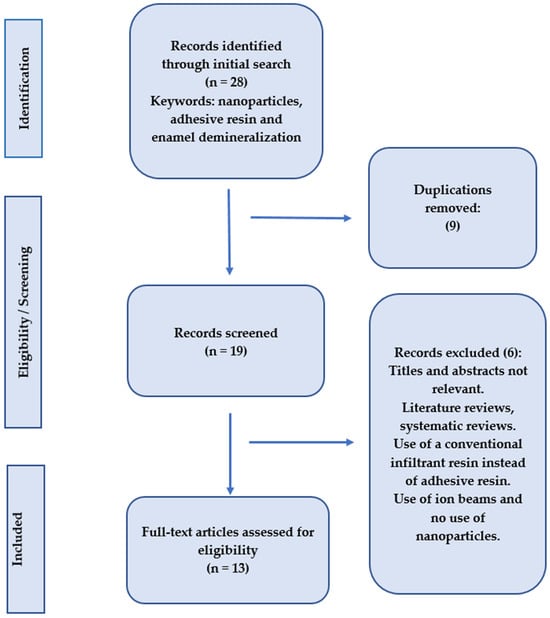
Figure 2.
Search methodolgy employed in the present systematic review.
2.3. Eligibility Criteria for Literature Search
The following criteria were utilized to evaluate each included article and extract the data: study design, adhesive systems used, authors’ names, journal and year of publication, study outcomes, and nanoparticles added to the adhesives (Table 1 and Table 2).

Table 1.
General characteristics of selected studies included in the systematic review.

Table 2.
Types of the nanoparticles incorporated, study outcomes and conclusions of the studies included in the systematic review.
2.4. Exclusion Criteria
After reviewing the titles and abstracts, studies that were surveys, clinical reports, literature reviews, or systematic reviews were eliminated. Studies with insufficient information or those that did not concentrate on adding nanoparticles to adhesives were not included in our review (Table 3).

Table 3.
General characteristics of selected studies excluded in the systematic review.
3. Results
3.1. General Characteristics of Included Studies
All studies included were in vitro research studies.
Different types of adhesives and nanoparticles were used in the studies included in the review. The most common adhesive used was Transbond™ XT (Transbond™ XT Light Cure Adhesive, 3M, Unitek, Dubai, UAE), which is typically used to bond metal and ceramic brackets to enamel surfaces. Six studies included in the review used this adhesive. Other adhesives used in the studies were Scotchbond™ (3M™ Scotchbond™ Universal Plus Adhesive, 3M, Unitek, Dubai, UAE), Clearfil S3 Bond™ (CLEARFIL™ S³ BOND PLUS, Kuraray, Noritake, Europe GmbH, Hattersheim, Germany), Adper Single Bond 2™ (3M™ Adper™ Single Bond 2, 3M, North Ryde, NSW, Australia), and Ortho Connect™ (GC Ortho Connect™, GC Orthodontics, Alsip, IL, USA).
The most prevalent nanoparticle added to adhesives in the trials included in the review was amorphous calcium phosphate (ACP), which was used in four investigations. Polydopamine-Ag, bioactive glass containing 2.5% fluoride, quaternary ammonium resin monomer, arginine-loaded mesoporous silica, silver and silver diamine fluoride, nano-hydroxyapatite, silica nanofillers, titanium oxide (TiO2), nano-bioactive glass–silver and Emodin were some of the other nanoparticles used in different studies.
Most of the studies looked at how adding nanoparticles to adhesives affected the shear bond strength (SBS) of the adhesive to enamel, with 11 studies testing SBS. Nine studies tested the effects of nanoparticles on microbial growth using colony counting and cell viability tests to check for cytotoxicity. Only three studies examined the impacts of nanoparticles on microhardness, using the Vickers test.
3.2. General Outcomes of Included Studies
Most studies found that adding nanoparticles to adhesives did not significantly affect the shear bond strength (SBS) between the adhesive and enamel, but it did improve the anti-cariogenic properties. Amorphous calcium phosphate (ACP) was added as a nanoparticle in many of the studies, and the SBS was similar to that in the control group [16,17,18,19]. In one study by Jia A et al. (2023) [17], the SBS of an adhesive with 0.2% amorphous calcium phosphate–polydopamine–silver nanoparticles was 11.89 ± 1.27 MPa, which achieved the minimum clinical bond strength of 7.8 MPa. The anti-demineralization test showed that adding 2.5% fluoride bioactive glass as a nanomaterial to the adhesives helped prevent enamel demineralization. When exposed to fluorescent mineralizing adhesives, both demineralized enamel and dentin, along with reconstituted type I collagen, could undergo remineralization at the extra- and intra-fibrillar levels [20].
Adhesives with arginine-loaded mesoporous silica nanoparticles showed significantly stronger antibacterial activity and better acid inhibition without compromising bonding strength or biocompatibility, compared to conventional adhesives [21]. When silver nanoparticles and silver diamine fluoride nanoparticles were added, there was no noticeable difference in performance compared to adhesives without these anti-caries compounds. However, adding silver nanoparticles improved the microhardness of the teeth. These antimicrobial compounds could potentially be used as a pretreatment to prevent caries before resin restoration [22,23]. Additionally, adding hydroxyapatite nanoparticles to orthodontic composites increased the mineral content and microhardness of surrounding enamel, but the shear bond strength (SBS) decreased as the nanoparticle amount increased [24,25].
Experimental composite adhesives with silica nanofillers and silver nanoparticles showed no significant difference in shear bond strength (SBS) or bond failure compared to regular adhesives [26]. Twenty-four hours after curing, there was no noticeable difference in SBS between regular adhesives and those with titanium oxide (TiO2) nanoparticles (p = 0.58). However, the TiO2 nanoparticles showed better antibacterial activity than the regular adhesives, with a significant difference between the two groups (p = 0.03) [27]. The 2% concentration of Emodin nanoparticles also performed much better than the control group in fighting S. mutans (p < 0.05) [28].
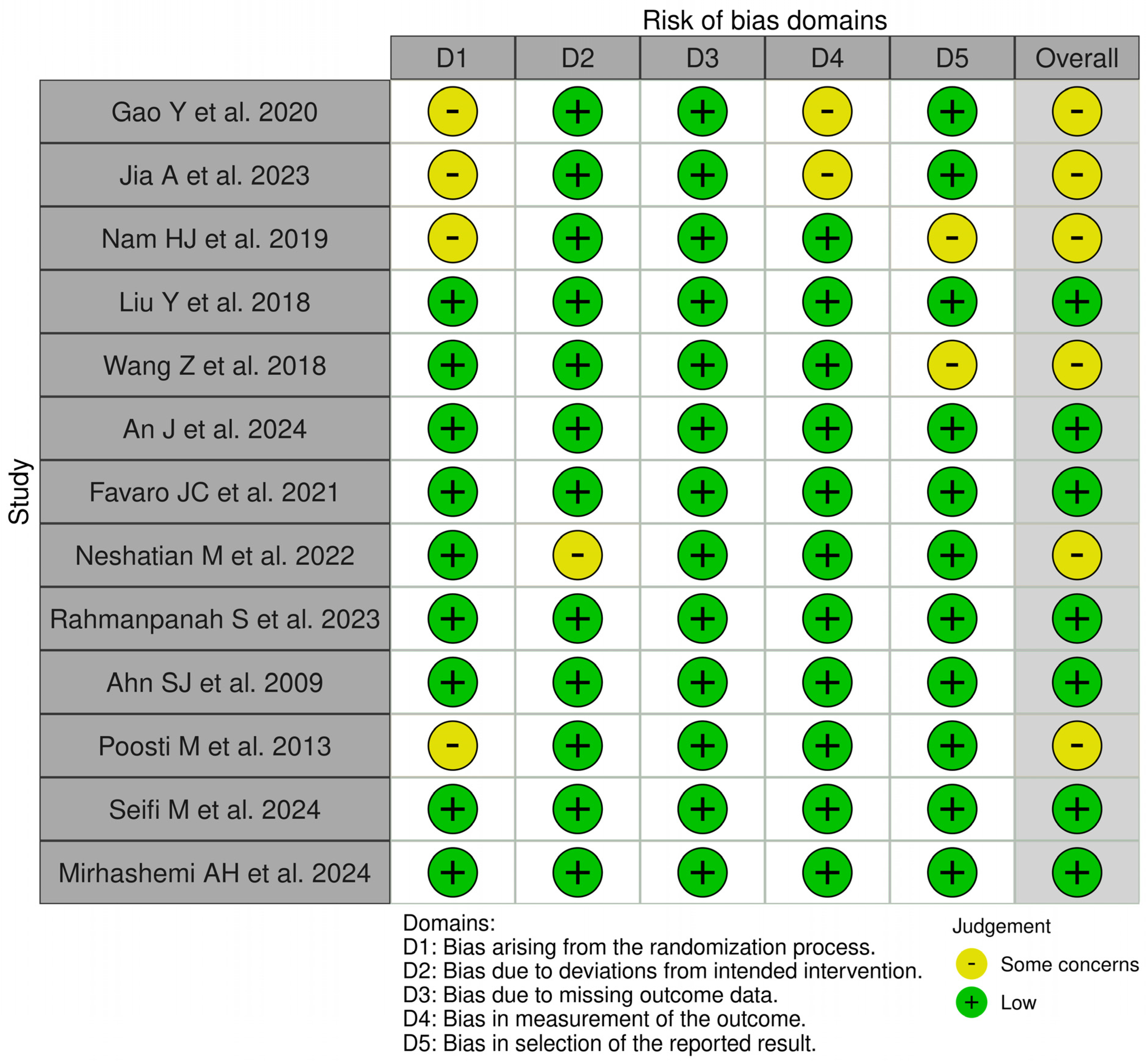
3.3. Risk of Bias Assessment
Figure 3 displays the risk of bias assessments for the studies included in this systematic review. The findings reveal that while some of the investigations raised concerns and had the potential for bias, the majority of the studies included had a low risk of bias. In general, all included studies had a low risk of bias.
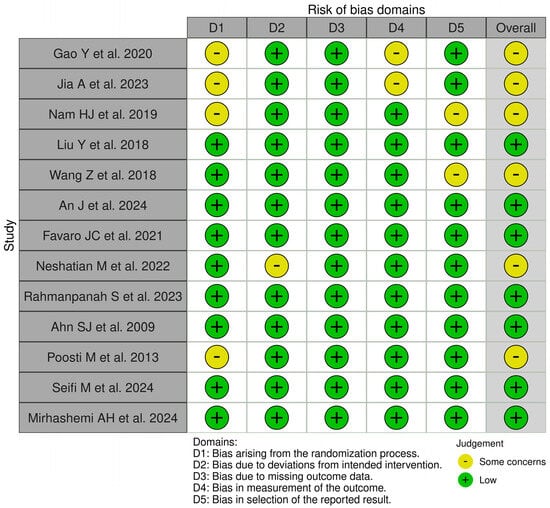
Figure 3.
Qualitative analysis of the included studies by Robvis© (Cochrane Methods Bias, The Cochrane Collaboration, London, United Kingdom) [16,17,18,19,20,21,22,23,24,25,26,27,28].
4. Discussion
In recent years, nanotechnology has evolved, and nanoparticles have been added to several materials to enhance their mechanical as well as biological properties [10,11,12,13,14,15]. Dental adhesives are being enriched with nanoparticles to improve their performance and properties [16,17]. Nano-hydroxyapatite and silica penetrate dentinal tubules, creating stronger bonds and reducing bond failure risk [24]. They minimize shrinkage during polymerization, preventing gaps and secondary caries. Nanoparticles also enhance mechanical properties like elastic modulus and tensile strength, making them more durable. Some nanoparticles, like silver and zinc oxide, have antibacterial properties, preventing bacterial growth and infections [22]. Nano-hydroxyapatite releases fluoride ions, remineralizing tooth structure and preventing decay. Different types of nanoparticles include nano-hydroxyapatite, silica, silver, and zinc oxide [14]. These advancements make dental treatments more effective and long-lasting. Therefore, this systematic review was conducted to analyze and compare the existing research articles on the additions of nanoparticles to dental adhesive systems in order to improve their performance and properties, evaluate their quality, and examine the results that have been published.
In orthodontics, braces come in a variety of forms. Compared to bands, bonded brackets offer a number of benefits, including improved appearance, simplicity in placement, and accessibility and removal for dental care [34]. Although it is challenging to clean by regular brushing, the bracket adhesive–enamel junction is the most common location for bacterial adherence and biofilm formation [35]. White spots, tooth cavities, and enamel decalcification are frequent outcomes of the plaque buildup surrounding orthodontic brackets [36]. Additionally, eliminating microbial growth surrounding orthodontic appliances is challenging. The material of the bracket, the design of the orthodontic brackets, and the ligating technique all play significant roles in its adherence to the permanent appliance. Surface roughness and surface-free energy are two of the many variables that affect plaque quantity and quality [34,35,36]. Microorganisms’ adherence to surfaces is also influenced by van der Waal forces and electrostatic attractions [37].
Dental adhesives are enhanced by nanoparticles, which improve their properties through various mechanisms. These nanoparticles, like silica, titanium dioxide, or hydroxyapatite, act as fillers in the adhesive matrix, enhancing its stiffness, tensile strength, and durability. They create a more uniform bond between the adhesive and the enamel/dentin surface, promoting better adhesion [38,39]. Nanoparticles like silver, copper, and titanium oxide have natural antimicrobial effects, reducing bacterial growth and preventing decay and inflammation. Some nanoparticles, like amorphous calcium phosphate (ACP), release calcium and phosphate ions, protecting enamel from demineralization. They also promote the remineralization of the enamel by releasing minerals that restore mineral content in demineralized areas, strengthening the tooth structure [40]. Nanoparticles like hydroxyapatite or bioactive glass increase the microhardness of both the adhesive and the surrounding enamel, enhancing wear resistance and bond strength. Some nanoparticles are biocompatible and less toxic, reducing the risk of adverse reactions in the oral cavity and improving the overall safety of the adhesive [41]. Nanoparticles, typically 1–100 nm, have a higher surface area, enhancing bonding strength and antibacterial activity. Their material, like silver, titanium dioxide, or hydroxyapatite, determines their effect. The concentration of nanoparticles must be controlled to ensure desired properties. Even dispersion in the adhesive matrix is crucial for consistent performance. Modifying the surface of nanoparticles can improve compatibility with the matrix. The pretreatment of the tooth surface, specific properties of enamel and dentin, and curing processes also impact nanoparticle integration. The oral environment’s pH and moisture can also affect nanoparticle stability and effectiveness [1,2].
The demineralization of enamel can be considerably impacted by the addition of nanoparticles to adhesive resins. Research has indicated that the characteristics of resin infiltrants can be improved by the addition of nanoparticles, such as nano-silica (NS) [26]. For example, it has been discovered that adding NS to resin infiltrants increases the mineral density of demineralized enamel while simultaneously decreasing water sorption and solubility. As a result, the resin is better able to penetrate and stabilize the enamel, possibly stopping additional demineralization [26]. Furthermore, it has been demonstrated that adding amorphous calcium phosphate, silver nanoparticles, hydroxyapatite nanoparticles and titatium dioxide (TiO2) to adhesive resins improves their cytocompatibility and maintains clinically acceptable shear bond strength levels [16,17,18,19,20,21,22,23,24,25,26,27,28]. This implies that these changes can enhance dental adhesives’ overall functionality and robustness, increasing their ability to shield enamel from demineralization.
Glucans and a variety of bacteria, the most prevalent and most cariogenic of which is Streptococcus mutans (S. mutans), make up adult dental plaque [42,43]. Researchers have investigated the connection between S. mutans CFUs on the surfaces of several orthodontic material types. The adherence of S. mutans to ceramic, plastic, and stainless steel brackets was not significantly different, according to research by Juvvadi et al. [44], Fournier et al. [45], Papaioannou et al. [46] and Brusca et al. [47]. The findings of Ahn et al. were very different [48]. Compared to ceramic and plastic brackets, stainless steel brackets had more CFUs. Compared to stainless steel brackets, titanium and gold brackets displayed fewer CFUs. A lower number of CFUs S. mutans was found in gold brackets, which may be due to the inert qualities of gold [44]. Because they have the highest critical surface tension (more surface energy), metallic brackets have a higher level of bacterial adherence than ceramic brackets. Microorganisms were more likely to adhere to stainless steel. When used properly, a substance with a high surface free energy will draw more germs than one with a low one [44,47,48].
The ability of orthodontic adhesives to hold onto cariogenic streptococci was greater than that of bracket materials [48]. When orthodontic brackets were fixed with nano-filled adhesives, prior short-term (24 h) in vitro investigations showed equivalent or lower (but nevertheless adequate) shear strength [49]. The use of nanofillers decreased the adhesive’s surface roughness in comparison to conventional orthodontic adhesives; however, this was not the case when silver nanoparticles were added to the combination. However, it has not yet been determined how well adhesives with nanofillers work over the long run to stop enamel demineralization during orthodontic treatment, especially under orthodontic bands and around brackets [50].
The contact between the polymeric resin and the tooth can deteriorate due to bacterial acids, microleakage, and cyclic stressors. It is a challenging and thought-provoking task to conduct research on the integration of state-of-the-art antimicrobial agents for the creation of novel, durable, bioactive resin-based dental materials [37]. When added to dental materials like dental adhesives, the antibacterial agents released, such as silver nanoparticles, amorphous calcium phosphate nanoparticles, titanium oxide nanoparticles, and several others, have demonstrated promise [16,17,18,19,20,21,22,23,24,25,26,27,28]. Melo and colleagues [51] conducted a thorough examination of the synthesis of silver nanoparticles and their potential incorporation into dental primers, adhesives, and composites, all of which are necessary for performing composite restoration [51].
Most of the studies in the present review were in vitro studies, and the results were not correlated with in vivo investigations. None of the publications included data from human clinical trials that would have indicated the effectiveness of the adhesives with nanoparticles used in orthodontics. The usage of dental adhesives in clinical settings has expanded due to the development of nanotechnologies, which promote contact between nanoparticles and oral microbiota. We suggest that more researchers look into and address clinical trials, in vivo studies and clinical correlation to predict the actual outcomes of the functional uses of dental adhesives loaded with nanoparticles in dental clinics, especially orthodontic clinics, in order to examine these limitations and strengthen the scientific evidence.
5. Conclusions
According to the review’s findings, adding nanoparticles to orthodontic dental adhesives improved their antibacterial and anticariogenic qualities while having no discernible impact on the shear bond strength between the tested dental adhesives and enamel. In order to stop enamel demineralization during orthodontic therapy, nanoparticles can be effectively added to orthodontic adhesives. Future orthodontics research could be greatly aided by the recent development of positive antibacterial properties. The effectiveness of orthodontic therapy has increased due to the physicochemical characteristics that nanomaterials have acquired. It is expected that the use of nanomaterials in dentistry, particularly in orthodontics, will increase further, necessitating an interdisciplinary approach that emphasizes knowledge of both dentistry and nanomaterial research.
Funding
This research received no external funding.
Acknowledgments
The author would like to thank and acknowledge Syed Rashid Habib and Aljazi Aldweesh from the College of Dentistry at King Saud University for their critical assessment during the writing of this review study.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Payal; Pandey, P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Pat. Nanotechnol. 2022, 16, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull 2022, 79, 5747–5771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rybka, M.; Mazurek, Ł.; Konop, M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing-From Experimental Studies to Clinical Practice. Life 2022, 13, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herranz, F.; Almarza, E.; Rodríguez, I.; Salinas, B.; Rosell, Y.; Desco, M.; Bulte, J.W.; Ruiz-Cabello, J. The application of nanoparticles in gene therapy and magnetic resonance imaging. Microsc. Res. Tech. 2011, 74, 577–591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melo, M.A.; Orrego, S.; Weir, M.D.; Xu, H.H.; Arola, D.D. Designing Multiagent Dental Materials for Enhanced Resistance to Biofilm Damage at the Bonded Interface. ACS Appl. Mater. Interfaces 2016, 8, 11779–11787. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, S.; Waheed, Y. Emerging Applications of Nanotechnology in Dentistry. Dent. J. 2023, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, K.; Masoumi, S.M.; Amini, S.; Goudarzi, M.; Tafreshi, S.M.; Bagheri, A.; Yasamineh, S.; Alwan, M.; Arellano, M.T.C.; Gholizadeh, O. Recent advances in metal nanoparticles to treat periodontitis. J. Nanobiotechnol. 2023, 21, 283. [Google Scholar] [CrossRef]
- Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.-A.; Popa, M.; Desbrieres, J. The Benefits of Smart Nanoparticles in Dental Applications. Int. J. Mol. Sci. 2021, 22, 2585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Budi, H.S.; Jameel, M.F.; Widjaja, G.; Alasady, M.S.; Mahmudiono, T.; Mustafa, Y.F.; Fardeeva, I.; Kuznetsova, M. Study on the role of nano antibacterial materials in orthodontics (a review). Braz. J. Biol. Rev. Brasleira Biol. 2022, 84, e257070. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liang, K.; Weir, M.D.; Gao, J.; Imazato, S.; Tay, F.R.; Lynch, C.D.; Oates, T.W.; Li, J.; Xu, H.H. Enamel remineralization via poly(amido amine) and adhesive resin containing calcium phosphate nanoparticles. J. Dent. 2020, 92, 103262. [Google Scholar] [CrossRef]
- Jia, A.; Wang, P.; Tong, F.; Chen, Z.; Deng, Y.; Yao, H.; Wang, L.; Liu, Y.; Ge, H. Developing a Novel Enamel Adhesive with Amorphous Calcium Phosphate and Silver Nanoparticles to Prevent Demineralization during Orthodontic Treatment. J. Funct. Biomater. 2023, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials 2019, 12, 1813. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Niu, L.-N.; Yu, T.; Xu, H.H.; Weir, M.D.; Oates, T.W.; Tay, F.R.; Chen, J.-H. Antibacterial and remineralizing orthodontic adhesive containing quaternary ammonium resin monomer and amorphous calcium phosphate nanoparticles. J. Dent. 2018, 72, 53–63. [Google Scholar] [CrossRef]
- Wang, Z.; Ouyang, Y.; Wu, Z.; Zhang, L.; Shao, C.; Fan, J.; Zhang, L.; Shi, Y.; Zhou, Z.; Pan, H.; et al. A novel fluorescent adhesive-assisted biomimetic mineralization. Nanoscale 2018, 10, 18980–18987. [Google Scholar] [CrossRef]
- An, J.; Shen, X.; Peng, T.; Qiao, M.; Xu, B. Formulation of arginine-loaded mesoporous silica nanoparticles (Arg@MSNs) modified orthodontic adhesive. J. Dent. 2024, 145, 104992. [Google Scholar] [CrossRef] [PubMed]
- Favaro, J.C.; Peixoto, Y.C.T.d.M.; Geha, O.; Dias, F.A.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Can silver diamine fluoride or silver nanoparticle-based anticaries agents to affect enamel bond strength? Restor. Dent. Endod. 2021, 46, e7. [Google Scholar] [CrossRef] [PubMed]
- Neshatian, M.; Holcroft, J.; Kishen, A.; De Souza, G.; Ganss, B. Promoting mineralization at biological interfaces Ex Vivo with novel amelotin-based bio-nano complexes. Mater. Today Bio 2022, 14, 100255. [Google Scholar] [CrossRef] [PubMed]
- Rahmanpanah, S.; Seifi, M.; Gharavi, Z.; Sadighnia, N.; Amdjadi, P. Evaluation of shear bond strength and enamel remineralizing effect of experimental orthodontic composite containing nano-hydroxyapatite: An in vitro study. Int. Orthod. 2023, 21, 100725. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Lee, S.J.; Kook, J.K.; Lim, B.S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent. Mater. 2009, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Poosti, M.; Ramazanzadeh, B.; Zebarjad, M.; Javadzadeh, P.; Naderinasab, M.; Shakeri, M.T. Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur. J. Orthod. 2013, 35, 676–679. [Google Scholar] [CrossRef]
- Seifi, M.; Eskandarloo, F.; Amdjadi, P.; Farmany, A. Investigation of mechanical properties, remineralization, antibacterial effect, and cellular toxicity of composite orthodontic adhesive combined with silver-containing nanostructured bioactive glass. BMC Oral Health 2024, 24, 650. [Google Scholar] [CrossRef] [PubMed]
- Mirhashemi, A.H.; Pourhajibagher, M.; Zebardast, B.; Bahrami, R.; Kharazi Fard, M.J. In Vitro effects of antimicrobial properties and shear bond strength of different concentrations of Emodin nanoparticles incorporated orthodontic composites. Int. Orthod. 2024, 22, 100836. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhao, I.S.; Mei, M.L.; Li, Q.; Yu, O.Y.; Chu, C.H. Use of Silver Nanomaterials for Caries Prevention: A Concise Review. Int. J. Nanomed. 2020, 15, 3181–3191. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A.; Borzabadi, E.; Lynch, E. Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol. Scand. 2014, 72, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van Landuyt, K.L.; Van Meerbeek, B.; De Munck, J. TEM interfacial characterization of an experimental self-adhesive filling material bonded to enamel/dentin. Dent. Mater. 2011, 27, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Leimer, M.R.; Hartmann, J.; Harm, S.; Pasztorek, M.; Ulrich, I.B. Ex Vivo investigation on internal tunnel approach/internal resin infiltration and external nanosilver-modified resin infiltration of proximal caries exceeding into dentin. PLoS ONE 2020, 15, e0228249. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.; Jarmar, T.; Svahn, F.; Neves, A.; Verlinden, B.; Van Meerbeek, B.; Engqvist, H. Ultrastructural characterization of tooth-biomaterial interfaces prepared with broad and focused ion beams. Dent. Mater. 2009, 25, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Kalha, A. Bond or band? Evid. Based Dent. 2007, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Hennig, C.-L.; Blochberger, B.; Symmank, J.; Nitzsche, Á.; Nietzsche, S.; Steiniger, F.; Dederichs, M.; Güllmar, A.; Reise, M.; Schulze-Späte, U.; et al. Effects of reducing excess dental adhesive on bacterial adhesion in the bracket periphery. Clin. Oral Investig. 2023, 27, 1993–2001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kachuie, M.; Khoroushi, M. Prevention and Treatment of White Spot Lesions in Orthodontic Patients. Contemp. Clin. Dent. 2017, 8, 11–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreve, S.; Reis, A.C.D. Bacterial adhesion to biomaterials: What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shadlou, S.; Ahmadi-Moghadam, B.; Taheri, F. Nano-Enhanced Adhesives. Rev. Adhes. Adhes. 2014, 2, 371–412. [Google Scholar] [CrossRef]
- Bourgi, R.; Doumandji, Z.; Cuevas-Suárez, C.E.; Ben Ammar, T.; Laporte, C.; Kharouf, N.; Haikel, Y. Exploring the Role of Nanoparticles in Dental Materials: A Comprehensive Review. Coatings 2025, 15, 33. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mistry, S.; Kundu, D.; Datta, S.; Basu, D. Effects of bioactive glass, hydroxyapatite and bioactive glass-Hydroxyapatite composite graft particles in the treatment of infrabony defects. J. Indian Soc. Periodontol. 2012, 16, 241–246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albani, R.; Habib, S.R.; AlHelal, A.A.; Alrabiah, M. Streptococcus-mutans and Porphyromonas-gingivalis adhesion to glazed/polished surfaces of CAD/CAM restorations. Heliyon 2024, 10, e40276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habib, S.; Nakshabandi, A.; Al Shawi, A.; Allohaidan, F.; Al Kurdi, R.; AlSarhan, M. Degree of Streptococcus mutans Colonization on Common Restorative Materials Subjected to Wear Cycle. Int. J. Prosthodont. 2021, 34, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, S.R.; Rammohan, S.N.; Gandikota, C.S.; Challa, P.; Manne, R.; Mathur, A. Adherence of Streptococcus mutans and Candida albicans to different bracket materials. J. Pharm. Bioallied. Sci. 2012, 4 (Suppl. S2), S212–S216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fournier, A.; Payant, L.; Bouclin, R. Adherence of Streptococcus mutans to orthodontic brackets. Am. J. Orthod Dentofac. Orthop 1998, 114, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, W.; Gizani, S.; Nassika, M.; Kontou, E.; Nakou, M. Adhesion of Streptococcus mutans to different types of brackets. Angle Orthod. 2007, 77, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Brusca, M.I.; Chara, O.; Sterin-Borda, L.; Rosa, A.C. Influence of different orthodontic brackets on adherence of microorganisms in vitro. Angle Orthod. 2007, 77, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Lim, B.S.; Yang, H.C.; Chang, Y.I. Quantitative analysis of the adhesion of cariogenic streptococci to orthodontic metal brackets. Angle Orthod. 2005, 75, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials Application in Orthodontics. Nanomaterials 2021, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Madian, A.; Elfouly, D.; El-Harouni, N. The effect of silver nanoparticles on the shear bond strength of orthodontic bonding system: A systematic review. Clin. Investig. Orthod. 2022, 81, 187–194. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Weir, M.D.; Hsia, R.; Rodrigues, L.K.A.; Xu, H.H. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J. Biomed Mater Res. B Appl. Biomater. 2013, 101, 620–629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).