Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe® aMMP-8 POCT, Lumoral® 2× PDT- and Lingora® Fermented Lingonberry Oral Rinse-Treatments
Abstract
1. Introduction
2. Hidden Periodontitis Uncovered?
3. A Tool Against Systemic Diseases
4. Bacteria Spread Through the Gums
5. How Can One Prevent Dysbiotic Bacterial Buildup?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol. 2000 2020, 84, 14–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010: A Systematic Review and Meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef]
- Liccardo, D.; Marzano, F.; Carraturo, F.; Guida, M.; Femminella, G.D.; Bencivenga, L.; Agrimi, J.; Addonizio, A.; Melino, I.; Valletta, A.; et al. Potential Bidirectional Relationship Between Periodontitis and Alzheimer’s Disease. Front. Physiol. 2020, 11, 683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef]
- Yu, J.; Ploner, A.; Chen, M.S.; Zhang, J.; Sandborgh-Englund, G.; Ye, W. Poor dental health and risk of pancreatic cancer: A nationwide registry-based cohort study in Sweden, 2009–2016. Br. J. Cancer 2022, 127, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N.; Bıyıkoğlu, B.; Kinane, D.F. Utility of gingival crevicular fluid components for periodontal diagnosis. Periodontol. 2000 2024, 95, 156–175. [Google Scholar] [CrossRef]
- Sorsa, T.; Alassiri, S.; Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Nwhator, S.O.; Gieselmann, D.R.; Sakellari, D. Active MMP-8 (aMMP-8) as a Grading and Staging Biomarker in the Periodontitis Classification. Diagnostics 2020, 10, 61. [Google Scholar] [CrossRef]
- Lee, W.; Aitken, S.; Sodek, J.; McCulloch, C.A. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: Role of active enzyme in human periodontitis. J. Periodontal Res. 1995, 30, 23–33. [Google Scholar] [CrossRef]
- Romanelli, R.; Mancini, S.; Laschinger, C.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Activation of neutrophil collagenase in perio- dontitis. Infect. Immun. 1999, 67, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Romanelli, R.; Laschinger, C.A.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J. Periodontol. 1999, 70, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Aji, N.R.A.S.; Räisänen, I.T.; Rathnayake, N.; Lundy, F.T.; Mc Crudden, M.T.C.; Goyal, L.; Sorsa, T.; Gupta, S. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease. J. Clin. Periodontol. 2021, 48, 1051–1065. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Authors’ Response: “Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease”. J. Clin. Periodontol. 2021, 48, 1499–1500. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Wei, S.; Xu, M.; Shi, J.; Lai, H.; Tonetti, M.S. Diagnostic accuracy of active matrix metalloproteinase-8 point-of-care test for the discrimination of periodontal health status: Comparison of saliva and oral rinse samples. J. Periodontal Res. 2022, 57, 768–779. [Google Scholar] [CrossRef]
- Wei, S.; Lin, T.; Sáenz-Ravello, G.; Gao, H.; Zhang, Y.; Tonetti, M.S.; Deng, K. Diagnostic accuracy of salivary active matrix metalloproteinase (aMMP)-8 point-of-care test for detecting periodontitis in adults: A systematic review and meta-analysis. J. Clin. Periodontol. 2024, 51, 1093–1108. [Google Scholar] [CrossRef]
- Guarnieri, R.; Reda, R.; Zanza, A.; Xhajanka, E.; Patil, S.; Di Nardo, D.; Testarelli, L. Relationship between gingival and peri-implant sulcular fluid active matrix metalloproteinase-8 concentration and clinical indices in healthy and diseased conditions. Explor. Med. 2024, 5, 243–256. [Google Scholar] [CrossRef]
- Tschesche, H.; Wenzel, H. Neutrophil Collagenase. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 725–734. [Google Scholar] [CrossRef]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar]
- Bornes, R.; Montero, J.; Correia, A.; Marques, T.; Rosa, N. Peri-implant diseases diagnosis, prognosis and dental implant monitoring: A narrative review of novel strategies and clinical impact. BMC Oral Health 2023, 23, 183. [Google Scholar] [CrossRef]
- Guarnieri, R.; Reda, R.; Zanza, A.; Miccoli, G.; Nardo, D.D.; Testarelli, L. Can Peri-Implant Marginal Bone Loss Progression and a-MMP-8 Be Considered Indicators of the Subsequent Onset of Peri-Implantitis? A 5-Year Study. Diagnostics 2022, 12, 2599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gellibolian, R.; Miller, C.S.; Markaryan, A.N.; Weltman, R.L.; Van Dyke, T.E.; Ebersole, J.L. Precision periodontics: Quantitative measures of disease progression. J. Am. Dent. Assoc. 2022, 153, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.S.; Abdulkareem, A.A.; Sha, A.M.; Rawlinson, A. Diagnostic Accuracy of Oral Fluids Biomarker Profile to Determine the Current and Future Status of Periodontal and Peri-Implant Diseases. Diagnostics 2020, 10, 838. [Google Scholar] [CrossRef]
- Mc Crudden, M.T.C.; Irwin, C.R.; El Karim, I.; Linden, G.J.; Lundy, F.T. Matrix metalloproteinase-8 activity in gingival crevicular fluid: Development of a novel assay. J. Periodontal Res. 2017, 52, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, I.T.; Aji, N.R.A.S.; Sakellari, D.; Grigoriadis, A.; Rantala, I.; Pätilä, T.; Heikkilä, P.; Gupta, S.; Sorsa, T. Active Matrix Metalloproteinase-8 (aMMP-8) Versus Total MMP-8 in Periodontal and Peri-Implant Disease Point-of-Care Diagnostics. Biomedicines 2023, 11, 2885. [Google Scholar] [CrossRef]
- Lassenius, M.I.; Pietiläinen, K.H.; Kaartinen, K.; Pussinen, P.J.; Syrjänen, J.; Forsblom, C.; Pörsti, I.; Rissanen, A.; Kaprio, J.; Mustonen, J.; et al. Bacterial Endotoxin Activity in Human Serum Is Associated With Dyslipidemia, Insulin Resistance, Obesity, and Chronic Inflammation. Diabetes Care 2011, 34, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Izadi Borujeni, S.; Mayer, M.; Eickholz, P. Activated matrix metalloproteinase-8 in saliva as diagnostic test for periodontal disease? A case-control study. Med. Microbiol. Immunol. 2015, 204, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Ebersole, J.L.; Kryscio, R.J.; Dawson, D., 3rd; Al-Sabbagh, M.; Miller, C.S. Rapid assessment of salivary MMP-8 and periodontal disease using lateral flow immunoassay. Oral Dis. 2016, 22, 681–687. [Google Scholar] [CrossRef]
- Lorenz, K.; Keller, T.; Noack, B.; Freitag, A.; Netuschil, L.; Hoffmann, T. Evaluation of a novel point-of-care test for active matrix metalloproteinase-8: Agreement between qualitative and quantitative measurements and relation to periodontal inflammation. J. Periodontal Res. 2017, 52, 277–284. [Google Scholar] [CrossRef]
- Schmalz, G.; Hübscher, A.E.; Angermann, H.; Schmidt, J.; Schmickler, J.; Legler, T.J.; Ziebolz, D. Associations of chairside salivary aMMP-8 findings with periodontal parameters, potentially periodontal pathogenic bacteria and selected blood parameters in systemically healthy adults. Diagn. Microbiol. Infect. Dis. 2019, 95, 179–184. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Kumar, M.S.; Vamsi, G.; Sripriya, R.; Sehgal, P.K. Expression of matrix metalloproteinases (MMP-8 and -9) in chronic periodontitis patients with and without diabetes mellitus. J. Periodontol. 2006, 77, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of Family Doctors (WONCA Europe). J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef] [PubMed]
- Gangbar, S.; Overall, C.M.; McCulloch, C.A.; Sodek, J. Identification of polymorphonuclear leukocyte collagenase and gelatinase activities in mouthrinse samples: Correlation with periodontal disease activity in adult and juvenile periodontitis. J. Periodontal Res. 1990, 25, 257–267. [Google Scholar] [CrossRef]
- Uitto, V.J.; Nieminen, A.; Coil, J.; Hurttia, H.; Larjava, H. Oral fluid elastase as an indicator of periodontal health. J. Clin. Periodontol. 1996, 23, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Manoil, D.; Parga, A.; Bostanci, N.; Belibasakis, G.N. Microbial diagnostics in periodontal diseases. Periodontol. 2000 2024, 95, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2015, 21, 172–183. [Google Scholar] [CrossRef]
- Pakarinen, S.; Saarela, R.K.; Välimaa, H.; Heikkinen, A.M.; Kankuri, E.; Noponen, M.; Alapulli, H.; Tervahartiala, T.; Räisänen, I.T.; Sorsa, T.; et al. Home-applied dual-light photodynamic therapy in the treatment of stable chronic periodontitis (HOPE-CP)—Three-month interim results. Dent. J. 2022, 10, 206. [Google Scholar] [CrossRef]
- Deumer, J.; Frentzen, M.; Meinke, M.C. Investigation of active matrix- metaloproteinase-8 (aMMP-8) as a reference parameter for path control in antimicrobial photothermal therapy (aPTT) using a split-mouth design. Heliyon 2019, 5, e01661. [Google Scholar] [CrossRef]
- Pärnänen, P.; Nikula-Ijäs, P.; Sorsa, T. Antimicrobial and anti-inflammatory lingonberry mouthwash—A clinical pilot study in the oral cavity. Microorganisms 2019, 7, 331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pärnänen, P.; Sorsa, T.; Tervahartiala, T.; Nikula-Ijäs, P. Isolation, characterization and regulation of moonlighting proteases from Candida glabrata cell wall. Microb. Pathog. 2020, 149, 104547. [Google Scholar] [CrossRef] [PubMed]
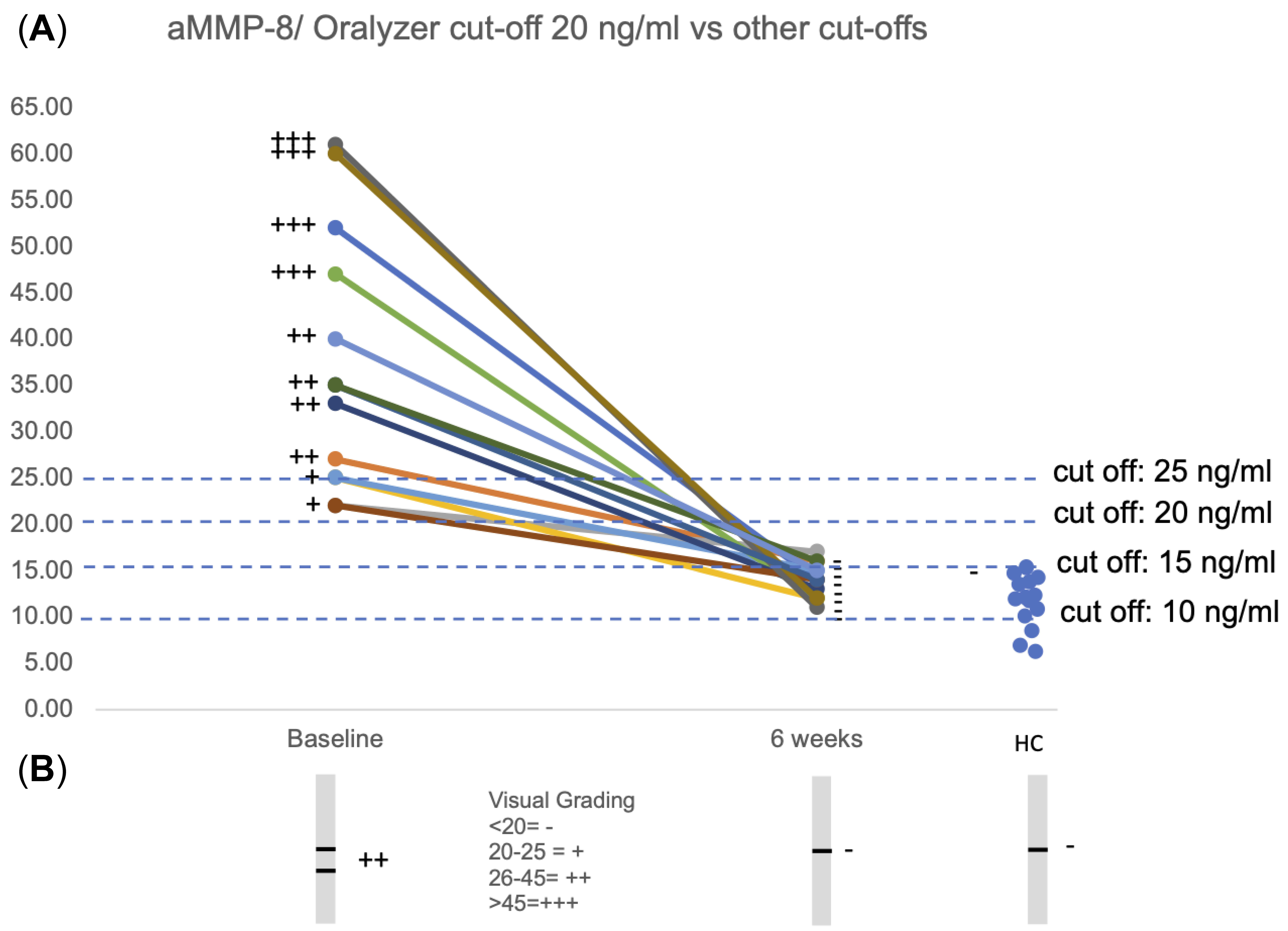
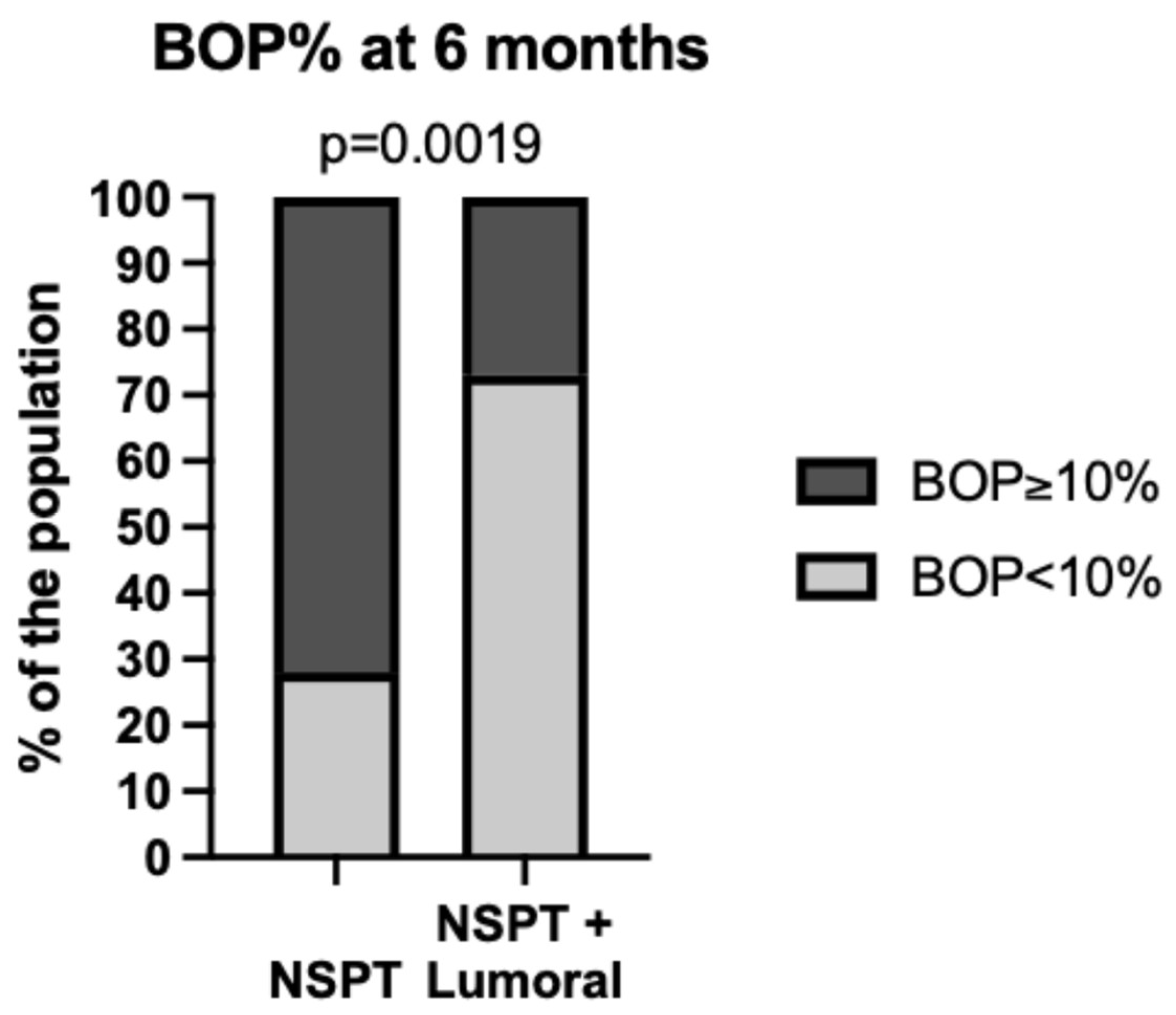

| Grade A (N = 14) (Median ± IQR, 95% CI, [min, max]) | Grade B (N = 91) (Median ± IQR, 95% CI, [min, max]) | Grade C (N = 14) (Median ± IQR, 95% CI, [min, max]) | p-Value | Significant Pairwise Differences | |
|---|---|---|---|---|---|
| aMMP-8 (ng/mL) | 15.0 ± 10.0, 5.0–15.0, [5.0, 20.1] | 15.0 ± 18.9, 15.0–23.4, [5.0, 86.7] | 28.3 ± 29.1, 15.0–53.5, [10.2, 73.1] | <0.001 | Between grade A and B, grade A and C |
| aMMP-8 (ng/mL)/NTP | 0.59 ± 0.51, 0.21–0.75, [0.18, 0.91] | 0.77 ± 0.70, 0.63–0.90, [0.18, 4.3] | 1.30 ± 1.30, 0.58–2.3, [0.46, 4.9] | 0.002 | Between grade A and B, grade A and C |
| Total MMP-8 (ng/mL) | 38.8 ± 37.5, 22.9–62.4, [7.3, 136.4] | 39.4 ± 74.8, 26.2–61.1, [0.22, 685.9] | 60.2 ± 140.5, 24.3–174.7, [3.8, 289.3] | 0.499 | - |
| HbA1c (%) | 5.35 ± 0.65, 4.8–5.6, [4.5, 6.2] | 5.30 ± 0.60, 5.1–5.4, [4.1, 8.1] | 5.95 ± 1.35, 5.4–6.9, [5.0, 8.9] | 0.001 | Between grade A and C, grade B and C |
| LPS-activity, LAL (EU/mL) | 2760.5 ± 4095.0, 257.9–5531.8, [164.5, 6710.6] | 1609.3 ± 3336.1, 1032.9–2582.1, [154.2, 7966.0] | 991.2 ± 4147.7, 230.3–4781.8, [193.7, 7308.9] | 0.683 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aji, N.R.A.S.; Sahni, V.; Penttala, M.T.; Sakellari, D.; Grigoriadis, A.; Pätilä, T.; Pärnänen, P.; Neefs, D.; Pfützner, A.; Gupta, S.; et al. Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe® aMMP-8 POCT, Lumoral® 2× PDT- and Lingora® Fermented Lingonberry Oral Rinse-Treatments. Dent. J. 2025, 13, 127. https://doi.org/10.3390/dj13030127
Aji NRAS, Sahni V, Penttala MT, Sakellari D, Grigoriadis A, Pätilä T, Pärnänen P, Neefs D, Pfützner A, Gupta S, et al. Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe® aMMP-8 POCT, Lumoral® 2× PDT- and Lingora® Fermented Lingonberry Oral Rinse-Treatments. Dentistry Journal. 2025; 13(3):127. https://doi.org/10.3390/dj13030127
Chicago/Turabian StyleAji, Nur Rahman Ahmad Seno, Vaibhav Sahni, Miika T. Penttala, Dimitra Sakellari, Andreas Grigoriadis, Tommi Pätilä, Pirjo Pärnänen, Dirk Neefs, Andreas Pfützner, Shipra Gupta, and et al. 2025. "Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe® aMMP-8 POCT, Lumoral® 2× PDT- and Lingora® Fermented Lingonberry Oral Rinse-Treatments" Dentistry Journal 13, no. 3: 127. https://doi.org/10.3390/dj13030127
APA StyleAji, N. R. A. S., Sahni, V., Penttala, M. T., Sakellari, D., Grigoriadis, A., Pätilä, T., Pärnänen, P., Neefs, D., Pfützner, A., Gupta, S., Sorsa, T., & Räisänen, I. T. (2025). Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe® aMMP-8 POCT, Lumoral® 2× PDT- and Lingora® Fermented Lingonberry Oral Rinse-Treatments. Dentistry Journal, 13(3), 127. https://doi.org/10.3390/dj13030127






