Pathophysiological Responses of Oral Keratinocytes After Exposure to Flavored E-Cigarette Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Supplies
2.2. Preparation of E-Liquids
2.3. Preparation of Human Saliva
2.4. Preparation of Cell Culture Media
2.5. Cell Cultures
2.6. OKF6/TERT-2 Cell Morphology and Supernatant pH
2.7. Wound Healing Assay
2.8. GSH Extraction
2.9. HPLC Determinations of Total GSH
2.10. Mucins and Tight Junction Gene Expression
2.11. Released Glycoprotein Concentration and SDS-Page
2.12. ELISA Determination of TNFα, IL-6, and IL-8
2.13. Statistical Analysis
3. Results
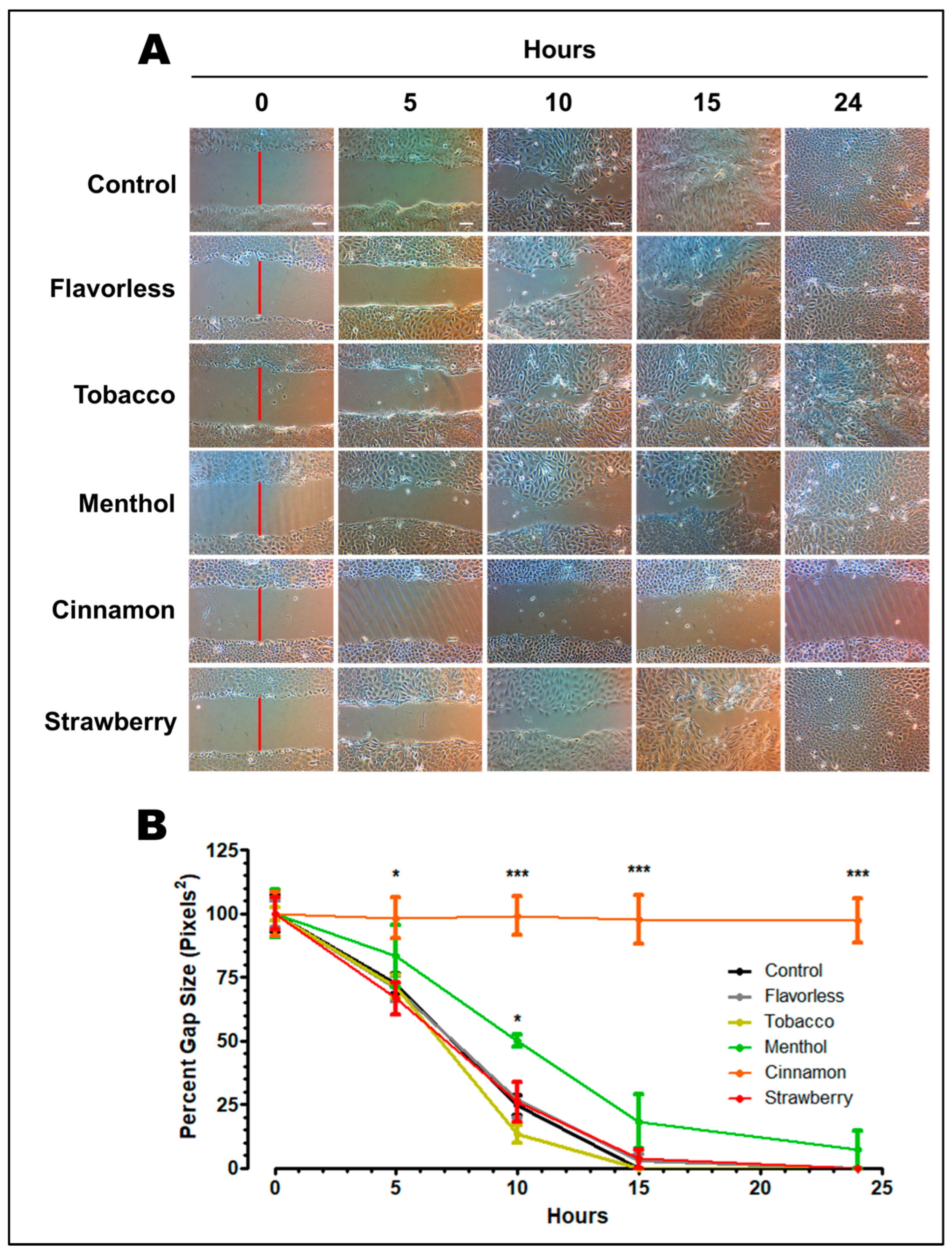
3.1. OKF6/TERT-2 Cell Morphology
3.2. Wound Healing Assay
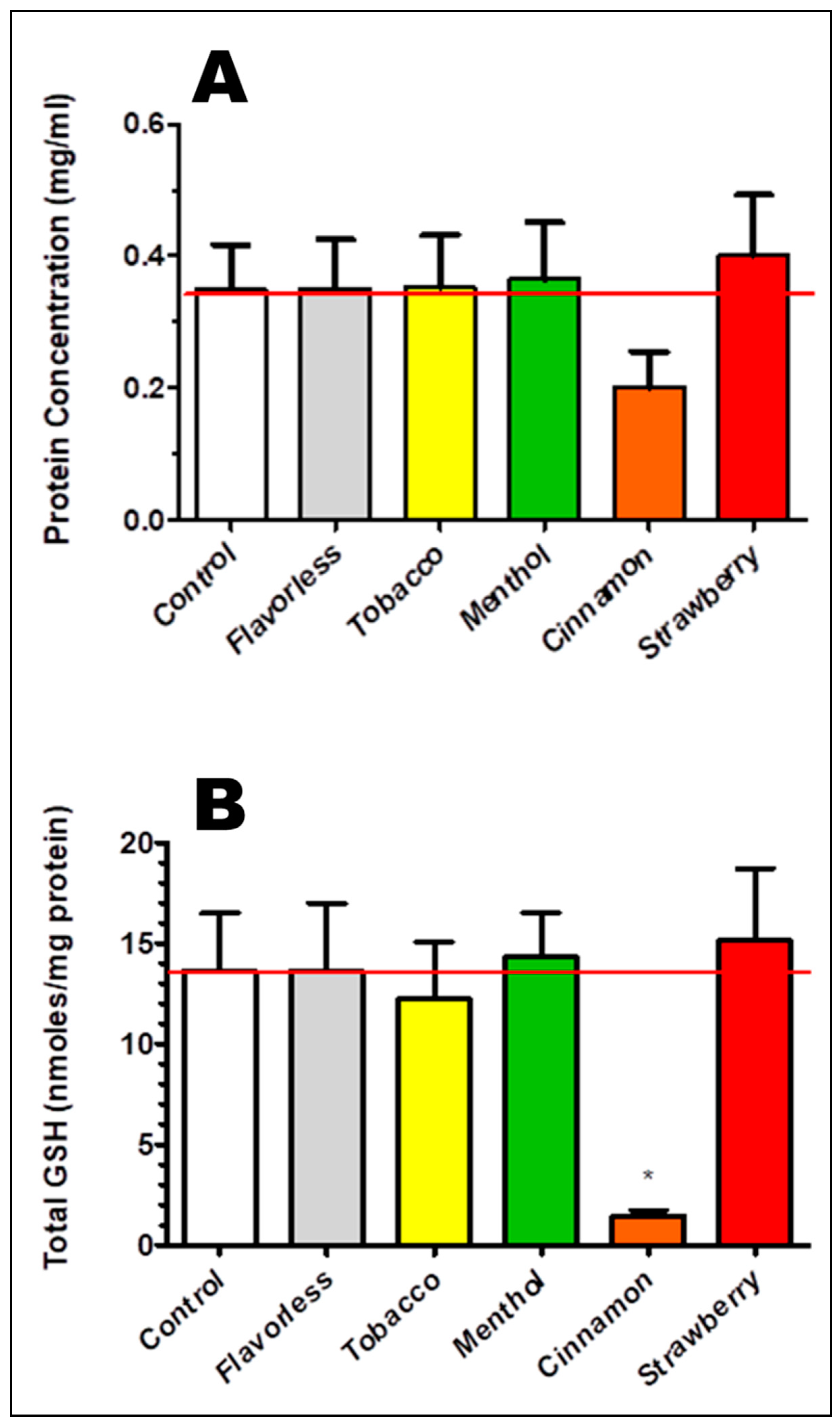
3.3. Oxidative Stress as Indexed by Total GSH
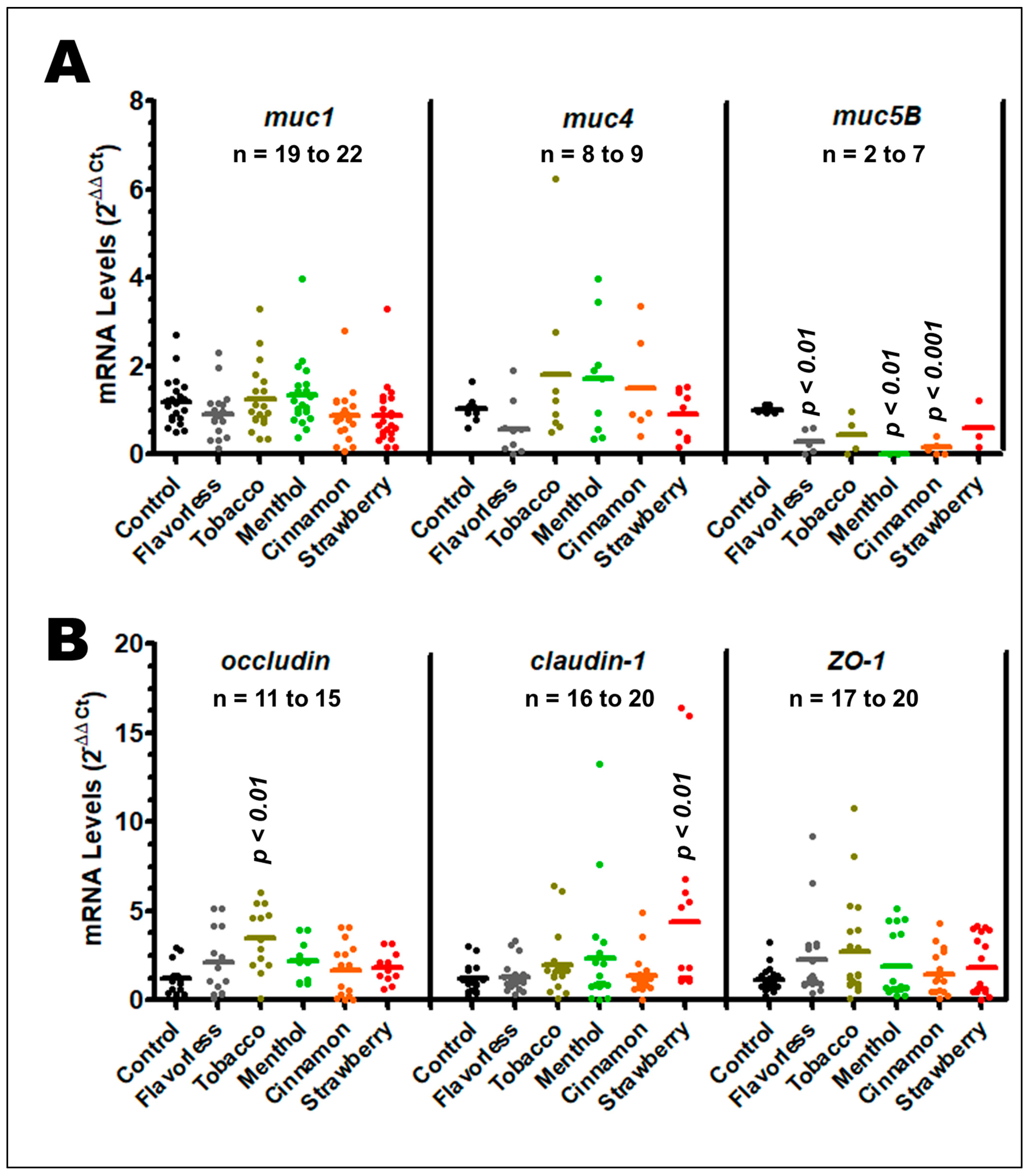
3.4. Gene Expression of Mucins and Tight Junction Genes
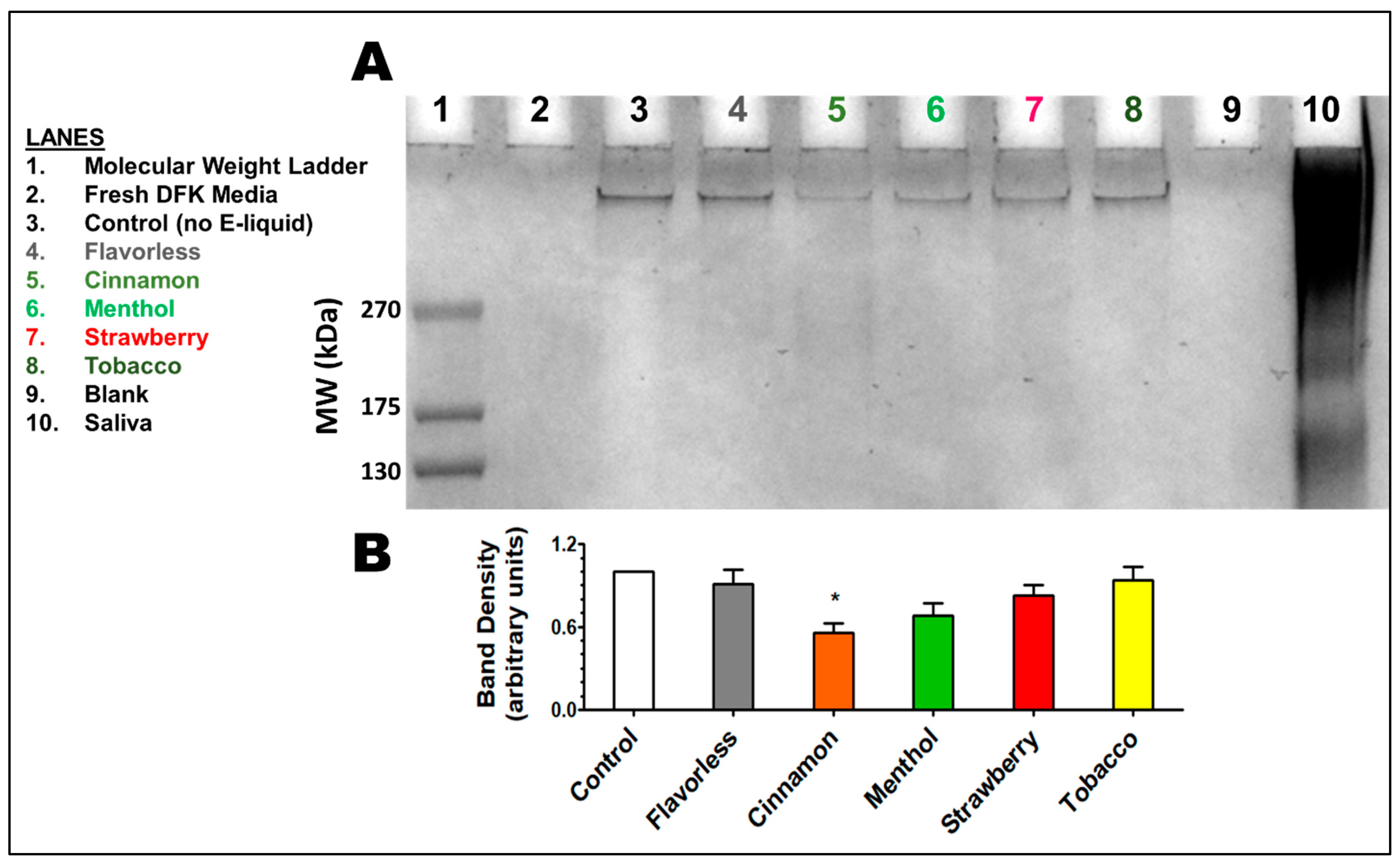
3.5. Released Glycoproteins
3.6. Imflammatory Response as Indexed by IL-6 and IL-8
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichtenberg, K. E-Cigarettes: Current Evidence and Policy. Mo. Med. 2017, 114, 335–338. [Google Scholar] [PubMed]
- Palazzolo, D.L. Electronic Cigarettes and Vaping: A New Challenge in Clinical Medicine and Public Health. A Literature Review. Front. Public Health 2013, 1, 56. [Google Scholar] [CrossRef]
- Dutra, L.M.; Grana, R.; Glantz, S.A. Philip Morris Research on Precursors to the Modern E-Cigarette Since 1990. Tob. Control 2017, 26, e97–e105. [Google Scholar] [CrossRef]
- Eltorai, A.E.; Choi, A.R.; Eltorai, A.S. Impact of Electronic Cigarettes on Various Organ Systems. Respir. Care 2019, 64, 328–336. [Google Scholar] [CrossRef]
- Isik Andrikopoulos, G.; Farsalinos, K.; Poulas, K. Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health. Toxics 2019, 7, 61. [Google Scholar] [CrossRef]
- Agaku, I.T.; King, B.A.; Husten, C.G.; Bunnell, R.; Ambrose, B.K.; Hu, S.S.; Holder-Hayes, E.; Day, H.R. Tobacco Product Use Among Adults—United States, 2012–2013. Morb. Mortal. Wkly. Rep. 2014, 63, 542–547. [Google Scholar]
- Bunnell, R.E.; Agaku, I.T.; Arrazola, R.A.; Apelberg, B.J.; Caraballo, R.S.; Corey, C.G.; Coleman, B.N.; Dube, S.R.; King, B.A. Intentions to Smoke Cigarettes Among Never-Smoking US Middle and High School Electronic Cigarette Users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob. Res. 2015, 17, 228–235. [Google Scholar] [CrossRef] [PubMed]
- King, B.A.; Alam, S.; Promoff, G.; Arrazola, R.; Dube, S.R. Awareness and Ever-Use of Electronic Cigarettes among U.S. Adults, 2010–2011. Nicotine Tob. Res. 2013, 15, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Newcombe, R.; Walton, D. The Prevalence, Correlates and Reasons for Using Electronic Cigarettes Among New Zealand Adults. Addict. Behav. 2015, 45, 245–251. [Google Scholar] [CrossRef]
- McMillen, R.C.; Gottlieb, M.A.; Shaefer, R.M.W.; Winickoff, J.P.; Klein, J.D. Trends in Electronic Cigarette Use Among U.S. Adults: Use Is Increasing in Both Smokers and Nonsmokers. Nicotine Tob. Res. 2015, 17, 1195–1202. [Google Scholar] [CrossRef]
- Regan, A.K.; Promoff, G.; Dube, S.R.; Arrazola, R. Electronic Nicotine Delivery Systems: Adult Use and Awareness of the “e-Cigarette” in the USA. Tob. Control 2013, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Siu, A.L. Behavioral and Pharmacotherapy Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Women: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2015, 163, 622. [Google Scholar] [CrossRef] [PubMed]
- Holliday, R.; Kist, R.; Bauld, L. E-Cigarette Vapour Is Not Inert and Exposure Can Lead to Cell Damage. Evid. Based Dent. 2016, 17, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.P.; Luo, W.; Pankow, J.F.; Strongin, R.M.; Peyton, D.H. Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med. 2015, 372, 392–394. [Google Scholar] [CrossRef]
- Kim, S.A.; Smith, S.; Beauchamp, C.; Song, Y.; Chiang, M.; Giuseppetti, A.; Frukhtbeyn, S.; Shaffer, I.; Wilhide, J.; Routkevitch, D.; et al. Cariogenic Potential of Sweet Flavors in Electronic-Cigarette Liquids. PLoS ONE 2018, 13, e0203717. [Google Scholar] [CrossRef]
- Yu, V.; Rahimy, M.; Korrapati, A.; Xuan, Y.; Zou, A.E.; Krishnan, A.R.; Tsui, T.; Aguilera, J.A.; Advani, S.; Crotty Alexander, L.E.; et al. Electronic Cigarettes Induce DNA Strand Breaks and Cell Death Independently of Nicotine in Cell Lines. Oral Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Bullen, C. Rise in E-Cigarette Use Linked to Increase in Smoking Cessation Rates. BMJ 2017, 358, j3506. [Google Scholar] [CrossRef]
- Gorukanti, A.; Delucchi, K.; Ling, P.; Fisher-Travis, R.; Halpern-Felsher, B. Adolescents’ Attitudes towards e-Cigarette Ingredients, Safety, Addictive Properties, Social Norms, and Regulation. Prev. Med. 2017, 94, 65–71. [Google Scholar] [CrossRef]
- Schneider, S.; Diehl, K. Vaping as a Catalyst for Smoking? An Initial Model on the Initiation of Electronic Cigarette Use and the Transition to Tobacco Smoking Among Adolescents. Nicotine Tob. Res. 2016, 18, 647–653. [Google Scholar] [CrossRef]
- Soneji, S.; Barrington-Trimis, J.L.; Wills, T.A.; Leventhal, A.M.; Unger, J.B.; Gibson, L.A.; Yang, J.; Primack, B.A.; Andrews, J.A.; Miech, R.A.; et al. Association Between Initial Use of E-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2017, 171, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Soneji, S.S.; Sung, H.-Y.; Primack, B.A.; Pierce, J.P.; Sargent, J.D. Quantifying Population-Level Health Benefits and Harms of e-Cigarette Use in the United States. PLoS ONE 2018, 13, e0193328. [Google Scholar] [CrossRef]
- Zhu, S.-H.; Zhuang, Y.-L.; Wong, S.; Cummins, S.E.; Tedeschi, G.J. E-Cigarette Use and Associated Changes in Population Smoking Cessation: Evidence from US Current Population Surveys. BMJ 2017, 358, j3262. [Google Scholar] [CrossRef]
- Chun, L.F.; Moazed, F.; Calfee, C.S.; Matthay, M.A.; Gotts, J.E. Pulmonary Toxicity of E-Cigarettes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L193–L206. [Google Scholar] [CrossRef] [PubMed]
- Bahl, V.; Lin, S.; Xu, N.; Davis, B.; Wang, Y.; Talbot, P. Comparison of Electronic Cigarette Refill Fluid Cytotoxicity Using Embryonic and Adult Models. Reprod. Toxicol. 2012, 34, 529–537. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Romagna, G.; Allifranchini, E.; Ripamonti, E.; Bocchietto, E.; Todeschi, S.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells. Int. J. Environ. Res. Public Health 2013, 10, 5146–5162. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Leverette, R.D.; Cooper, B.T.; Bennett, M.B.; Brown, S.E. Comparative In Vitro Toxicity Profile of Electronic and Tobacco Cigarettes, Smokeless Tobacco and Nicotine Replacement Therapy Products: E-Liquids, Extracts and Collected Aerosols. Int. J. Environ. Res. Public Health 2014, 11, 11325–11347. [Google Scholar] [CrossRef] [PubMed]
- Sancilio, S.; Gallorini, M.; Cataldi, A.; di Giacomo, V. Cytotoxicity and Apoptosis Induction by E-Cigarette Fluids in Human Gingival Fibroblasts. Clin. Oral Investig. 2016, 20, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, S.; Dieken, H.; Krischenowski, O.; Förster, C.; Branscheid, D.; Aufderheide, M. Evaluation of E-Cigarette Liquid Vapor and Mainstream Cigarette Smoke after Direct Exposure of Primary Human Bronchial Epithelial Cells. Int. J. Environ. Res. Public Health 2015, 12, 3915–3925. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lyes, M.; Sladewski, K.; Enany, S.; McEachern, E.; Mathew, D.P.; Das, S.; Moshensky, A.; Bapat, S.; Pride, D.T.; et al. Electronic Cigarette Inhalation Alters Innate Immunity and Airway Cytokines While Increasing the Virulence of Colonizing Bacteria. J. Mol. Med. 2016, 94, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Cuadra, G.A.; Shamim, A.; Shah, R.; Morgan, J.; Palazzolo, D.L. Comparison of Culture Media for In Vitro Expansion of Oral Epithelial Keratinocytes. Appl. Biosci. 2023, 2, 308–327. [Google Scholar] [CrossRef]
- Kreslake, J.M.; Wayne, G.F.; Alpert, H.R.; Koh, H.K.; Connolly, G.N. Tobacco Industry Control of Menthol in Cigarettes and Targeting of Adolescents and Young Adults. Am. J. Public Health 2008, 98, 1685–1692. [Google Scholar] [CrossRef]
- Czoli, C.D.; Goniewicz, M.; Islam, T.; Kotnowski, K.; Hammond, D. Consumer Preferences for Electronic Cigarettes: Results from a Discrete Choice Experiment. Tob. Control 2016, 25, e30–e36. [Google Scholar] [CrossRef] [PubMed]
- Tackett, A.P.; Lechner, W.V.; Meier, E.; Grant, D.M.; Driskill, L.M.; Tahirkheli, N.N.; Wagener, T.L. Biochemically Verified Smoking Cessation and Vaping Beliefs among Vape Store Customers. Addiction 2015, 110, 868–874. [Google Scholar] [CrossRef]
- Morean, M.E.; Butler, E.R.; Bold, K.W.; Kong, G.; Camenga, D.R.; Cavallo, D.A.; Simon, P.; O’Malley, S.S.; Krishnan-Sarin, S. Preferring More E-Cigarette Flavors Is Associated with e-Cigarette Use Frequency among Adolescents but Not Adults. PLoS ONE 2018, 13, e0189015. [Google Scholar] [CrossRef]
- Yerger, V.B. Menthol’s Potential Effects on Nicotine Dependence: A Tobacco Industry Perspective. Tob. Control 2011, 20, ii29–ii36. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, W.J.; Caskey, N.H.; Jarvik, M.E.; Gross, T.M.; Rosenblatt, M.R.; Carpenter, C. Menthol vs. Nonmenthol Cigarettes: Effects on Smoking Behavior. Am. J. Public Health 1995, 85, 67–72. [Google Scholar] [CrossRef]
- Lewis, M.J.; Wackowski, O. Dealing with an Innovative Industry: A Look at Flavored Cigarettes Promoted by Mainstream Brands. Am. J. Public Health 2006, 96, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, A.M.; Goldenson, N.I.; Cho, J.; Kirkpatrick, M.G.; McConnell, R.S.; Stone, M.D.; Pang, R.D.; Audrain-McGovern, J.; Barrington-Trimis, J.L. Flavored E-Cigarette Use and Progression of Vaping in Adolescents. Pediatrics 2019, 144, e20190789. [Google Scholar] [CrossRef]
- Muthumalage, T.; Prinz, M.; Ansah, K.O.; Gerloff, J.; Sundar, I.K.; Rahman, I. Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used E-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Front. Physiol. 2018, 8, 1130. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors Produced by Electronic Cigarettes and E-Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef]
- Behar, R.Z.; Davis, B.; Wang, Y.; Bahl, V.; Lin, S.; Talbot, P. Identification of Toxicants in Cinnamon-Flavored Electronic Cigarette Refill Fluids. Toxicol. Vitr. 2014, 28, 198–208. [Google Scholar] [CrossRef]
- Ka, H.; Park, H.-J.; Jung, H.-J.; Choi, J.-W.; Cho, K.-S.; Ha, J.; Lee, K.-T. Cinnamaldehyde Induces Apoptosis by ROS-Mediated Mitochondrial Permeability Transition in Human Promyelocytic Leukemia HL-60 Cells. Cancer Lett. 2003, 196, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, J.; Sundar, I.K.; Freter, R.; Sekera, E.R.; Friedman, A.E.; Robinson, R.; Pagano, T.; Rahman, I. Inflammatory Response and Barrier Dysfunction by Different E-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl. Vitr. Toxicol. 2017, 3, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.M.; Escobedo Martínez, M.F.; Barbeito Castro, E.; Junquera Olay, S.; Olay García, S.; Junquera Gutiérrez, L.M. Effects of Vape Use on Oral Health: A Review of the Literature. Medicina 2024, 60, 365. [Google Scholar] [CrossRef] [PubMed]
- Effah, F.; Taiwo, B.; Baines, D.; Bailey, A.; Marczylo, T. Pulmonary Effects of E-Liquid Flavors: A Systematic Review. J. Toxicol. Environ. Health Part B 2022, 25, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Zielinska-Blizniewska, H.; Malinowska, K.; Zajdel, K.; Zakonnik, L.; Zajdel, R. A Summary of In Vitro and In Vivo Studies Evaluating the Impact of E-Cigarette Exposure on Living Organisms and the Environment. Int. J. Mol. Sci. 2020, 21, 652. [Google Scholar] [CrossRef]
- Chen, H.; Li, G.; Chan, Y.L.; Chapman, D.G.; Sukjamnong, S.; Nguyen, T.; Annissa, T.; McGrath, K.C.; Sharma, P.; Oliver, B.G. Maternal E-Cigarette Exposure in Mice Alters DNA Methylation and Lung Cytokine Expression in Offspring. Am. J. Respir. Cell Mol. Biol. 2018, 58, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Coakley, R.D.; Ghio, A.J.; Muhlebach, M.S.; Esther, C.R.; Alexis, N.E.; Tarran, R. Chronic E-Cigarette Use Increases Neutrophil Elastase and Matrix Metalloprotease Levels in the Lung. Am. J. Respir. Crit. Care Med. 2019, 200, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Coakley, R.C.; Mascenik, T.; Rowell, T.R.; Davis, E.S.; Rogers, K.; Webster, M.J.; Dang, H.; Herring, L.E.; Sassano, M.F.; et al. Chronic E-Cigarette Exposure Alters the Human Bronchial Epithelial Proteome. Am. J. Respir. Crit. Care Med. 2018, 198, 67–76. [Google Scholar] [CrossRef]
- Glynos, C.; Bibli, S.-I.; Katsaounou, P.; Pavlidou, A.; Magkou, C.; Karavana, V.; Topouzis, S.; Kalomenidis, I.; Zakynthinos, S.; Papapetropoulos, A. Comparison of the Effects of E-Cigarette Vapor with Cigarette Smoke on Lung Function and Inflammation in Mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L662–L672. [Google Scholar] [CrossRef]
- Reidel, B.; Radicioni, G.; Clapp, P.W.; Ford, A.A.; Abdelwahab, S.; Rebuli, M.E.; Haridass, P.; Alexis, N.E.; Jaspers, I.; Kesimer, M. E-Cigarette Use Causes a Unique Innate Immune Response in the Lung, Involving Increased Neutrophilic Activation and Altered Mucin Secretion. Am. J. Respir. Crit. Care Med. 2018, 197, 492–501. [Google Scholar] [CrossRef]
- Higham, A.; Bostock, D.; Booth, G.; Dungwa, J.V.; Singh, D. The Effect of Electronic Cigarette and Tobacco Smoke Exposure on COPD Bronchial Epithelial Cell Inflammatory Responses. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Cichońska, D.; Kusiak, A.; Kochańska, B.; Ochocińska, J.; Świetlik, D. Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva. Int. J. Environ. Res. Public Health 2019, 16, 4433. [Google Scholar] [CrossRef]
- Ji, E.H.; Elzakra, N.; Chen, W.; Cui, L.; Lee, E.S.; Sun, B.; Messadi, D.; Xia, T.; Zhu, Y.; Hu, S. E-Cigarette Aerosols Induce Unfolded Protein Response in Normal Human Oral Keratinocytes. J. Cancer 2019, 10, 6915–6924. [Google Scholar] [CrossRef]
- Tommasi, S.; Caliri, A.W.; Caceres, A.; Moreno, D.E.; Li, M.; Chen, Y.; Siegmund, K.D.; Besaratinia, A. Deregulation of Biologically Significant Genes and Associated Molecular Pathways in the Oral Epithelium of Electronic Cigarette Users. Int. J. Mol. Sci. 2019, 20, 738. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Javed, F.; Romanos, G.E.; Rahman, I. E-Cigarettes and Flavorings Induce Inflammatory and pro-Senescence Responses in Oral Epithelial Cells and Periodontal Fibroblasts. Oncotarget 2016, 7, 77196–77204. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Kellesarian, S.V.; Sundar, I.K.; Romanos, G.E.; Rahman, I. Recent Updates on Electronic Cigarette Aerosol and Inhaled Nicotine Effects on Periodontal and Pulmonary Tissues. Oral Dis. 2017, 23, 1052–1057. [Google Scholar] [CrossRef]
- Leigh, N.J.; Tran, P.L.; O’Connor, R.J.; Goniewicz, M.L. Cytotoxic Effects of Heated Tobacco Products (HTP) on Human Bronchial Epithelial Cells. Tob. Control 2018, 27, s26–s29. [Google Scholar] [CrossRef] [PubMed]
- Leigh, N.J.; Lawton, R.I.; Hershberger, P.A.; Goniewicz, M.L. Flavourings Significantly Affect Inhalation Toxicity of Aerosol Generated from Electronic Nicotine Delivery Systems (ENDS). Tob. Control 2016, 25, ii81–ii87. [Google Scholar] [CrossRef]
- Majid, O.W. Preliminary Evidence of Impaired Oral Wound Healing in E-Cigarette Users: A Call for Perioperative Vaping Cessation. Evid. Based Dent. 2024, 25, 63–64. [Google Scholar] [CrossRef]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the Role of Glutathione on Oxidative Stress and Infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.B.; Butterfield, D.A. Measurement of Oxidized/Reduced Glutathione Ratio. Methods Mol. Biol. 2010, 648, 269–277. [Google Scholar] [CrossRef]
- Seo, Y.J.; Lee, J.W.; Lee, E.H.; Lee, H.K.; Kim, H.W.; Kim, Y.-H. Role of Glutathione in the Adaptive Tolerance to H2O2. Free Radic. Biol. Med. 2004, 37, 1272–1281. [Google Scholar] [CrossRef]
- Rahman, I.; Bel, A.; Mulier, B.; Donaldson, K.; MacNee, W. Differential Regulation of Glutathione by Oxidants and Dexamethasone in Alveolar Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 1998, 275, L80–L86. [Google Scholar] [CrossRef]
- Ji, E.H.; Sun, B.; Zhao, T.; Shu, S.; Chang, C.H.; Messadi, D.; Xia, T.; Zhu, Y.; Hu, S. Characterization of Electronic Cigarette Aerosol and Its Induction of Oxidative Stress Response in Oral Keratinocytes. PLoS ONE 2016, 11, e0154447. [Google Scholar] [CrossRef]
- Franco, R.; DeHaven, W.I.; Sifre, M.I.; Bortner, C.D.; Cidlowski, J.A. Glutathione Depletion and Disruption of Intracellular Ionic Homeostasis Regulate Lymphoid Cell Apoptosis. J. Biol. Chem. 2008, 283, 36071–36087. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- King, M.W. Glycoproteins: Roles in Cellular Homeostasis and Disease. In Reviews in Cell Biology and Molecular Medicine; John Wiley & Sons, Ltd.: West Sussex, UK, 2006; ISBN 978-3-527-60090-8. [Google Scholar]
- Wang, S.-S.; Tang, Y.-L.; Pang, X.; Zheng, M.; Tang, Y.-J.; Liang, X.-H. The Maintenance of an Oral Epithelial Barrier. Life Sci. 2019, 227, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Meyle, J. Oral Mucosal Epithelial Cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic Diseases Caused by Oral Infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Fischman, J.S.; Sista, S.; Lee, D.; Cuadra, G.A.; Palazzolo, D.L. Flavorless vs. Flavored Electronic Cigarette-Generated Aerosol and E-Liquid on the Growth of Common Oral Commensal Streptococci. Front. Physiol. 2020, 11, 585416. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.P.; Palazzolo, D.L.; Cuadra, G.A. Mechanistic Effects of E-Liquids on Biofilm Formation and Growth of Oral Commensal Streptococcal Communities: Effect of Flavoring Agents. Dent. J. 2022, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- What Flavour Percentage Should I Use? Available online: https://vapable.com/what-flavour-percentage-should-i-use/ (accessed on 23 January 2025).
- Christian, N.; Burden, D.; Emam, A.; Brenk, A.; Sperber, S.; Kalu, M.; Cuadra, G.; Palazzolo, D. Effects of E-Liquids and Their Aerosols on Biofilm Formation and Growth of Oral Commensal Streptococcal Communities: Effect of Cinnamon and Menthol Flavors. Dent. J. 2024, 12, 232. [Google Scholar] [CrossRef]
- Dickson, M.A.; Hahn, W.C.; Ino, Y.; Ronfard, V.; Wu, J.Y.; Weinberg, R.A.; Louis, D.N.; Li, F.P.; Rheinwald, J.G. Human Keratinocytes That Express hTERT and Also Bypass a P16(INK4a)-Enforced Mechanism That Limits Life Span Become Immortal yet Retain Normal Growth and Differentiation Characteristics. Mol. Cell. Biol. 2000, 20, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ Ecosystem: An Open Platform for Biomedical Image Analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Francioso, A.; Fanelli, S.; Cavallaro, R.A.; Fontana, M.; Mattioli, R.; D’Erme, M.; Mosca, L. Fluorometric Optimized Determination of Total Glutathione in Erythrocytes. Separations 2021, 8, 83. [Google Scholar] [CrossRef]
- Hohnholt, M.C.; Dringen, R. Short Time Exposure to Hydrogen Peroxide Induces Sustained Glutathione Export from Cultured Neurons. Free Radic. Biol. Med. 2014, 70, 33–44. [Google Scholar] [CrossRef]
- Otręba, M.; Kośmider, L.; Knysak, J.; Warncke, J.D.; Sobczak, A. E-Cigarettes: Voltage- and Concentration-Dependent Loss in Human Lung Adenocarcinoma Viability. J. Appl. Toxicol. 2018, 38, 1135–1143. [Google Scholar] [CrossRef]
- Leslie, L.J.; Bathrinarayanan, P.V.; Jackson, P.; Muanda, J.A.M.M.; Pallett, R.; Stillman, C.J.P.; Marshall, L.J. A Comparative Study of Electronic Cigarette Vapor Extracts on Airway-Related Cell Lines in Vitro. Inhal. Toxicol. 2017, 29, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Ferri, E.; Labra, M.; Mantecca, P. Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used. Int. J. Environ. Res. Public Health 2017, 14, 1254. [Google Scholar] [CrossRef]
- Behar, R.Z.; Luo, W.; Lin, S.C.; Wang, Y.; Valle, J.; Pankow, J.F.; Talbot, P. Distribution, Quantification and Toxicity of Cinnamaldehyde in Electronic Cigarette Refill Fluids and Aerosols. Tob. Control 2016, 25, ii94–ii102. [Google Scholar] [CrossRef]
- Cinnamaldehyde Safety Data Sheet; Sigma Aldrich: St. Louis, MO, USA, 2025; p. 63103.
- Guo, J.; Yan, S.; Jiang, X.; Su, Z.; Zhang, F.; Xie, J.; Hao, E.; Yao, C. Advances in Pharmacological Effects and Mechanism of Action of Cinnamaldehyde. Front. Pharmacol. 2024, 15, 1365949. [Google Scholar] [CrossRef]
- Gordan, V.V.; Garvan, C.W.; Ottenga, M.E.; Schulte, R.; Harris, P.A.; McEdward, D.; Magnusson, I. Could Alkali Production Be Considered an Approach for Caries Control? Caries Res. 2011, 44, 547–554. [Google Scholar] [CrossRef]
- Shaikh, Z.N.; Alqahtani, A.; Almela, T.; Franklin, K.; Tayebi, L.; Moharamzadeh, K. Effects of Electronic Cigarette Liquid on Monolayer and 3D Tissue-Engineered Models of Human Gingival Mucosa. J. Adv. Periodontol. Implant Dent. 2019, 11, 54–62. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Heller, Z.A.; Ms, E.C.A.; Dmd, J.E.P. Implications of Electronic Cigarettes on the Safe Administration of Sedation and General Anesthesia in the Outpatient Dental Setting. Anesth. Prog. 2022, 69, 41–52. [Google Scholar] [CrossRef]
- Amerongen, A.V.N.; Veerman, E.C.I. Saliva—The Defender of the Oral Cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef]
- Rereddy, S.K.; Cao, A.C.; Blackwell, B.; Poling-Skutvik, R.; Arratia, P.E.; Mirza, N. Rheology of Saliva in Health and Disease. Biorheology 2023, 59, 19–27. [Google Scholar] [CrossRef]
- Faruque, M.; Wanschers, M.; Ligtenberg, A.J.; Laine, M.L.; Bikker, F.J. A Review on the Role of Salivary MUC5B in Oral Health. J. Oral Biosci. 2022, 64, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hou, L.; Peng, X.; Tang, F. The Prevalence of Xerostomia among E-Cigarette or Combustible Tobacco Users: A Systematic Review and Meta-Analysis. Tob. Induc. Dis. 2023, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Merikallio, H.; Kaarteenaho, R.; Lindén, S.; Padra, M.; Karimi, R.; Li, C.-X.; Lappi-Blanco, E.; Wheelock, Å.M.; Sköld, M.C. Smoking-Associated Increase in Mucins 1 and 4 in Human Airways. Respir. Res. 2020, 21, 239. [Google Scholar] [CrossRef]
- Go, Y.Y.; Mun, J.Y.; Chae, S.-W.; Chang, J.; Song, J.-J. Comparison between in Vitro Toxicities of Tobacco- and Menthol-Flavored Electronic Cigarette Liquids on Human Middle Ear Epithelial Cells. Sci. Rep. 2020, 10, 2544. [Google Scholar] [CrossRef]
- Narashiman, S.; Narasimhan, M.; Venkatraman, G. Expression of Mucin 4 in Leukoplakia and Oral Squamous Cell Carcinoma: An Immunohistochemical Study. J. Oral Maxillofac. Pathol. 2014, 18, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Shivam, A.K.; Ahuja, P.; Dutta, J. Mucin-4: A Novel Marker for Oral Cancer. J. Oral Maxillofac. Pathol. 2019, 23, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Tang, V.W.; Goodenough, D.A. Paracellular Ion Channel at the Tight Junction. Biophys. J. 2003, 84, 1660–1673. [Google Scholar] [CrossRef]
- Gruber, S.; Cini, N.; Kowald, L.-M.; Mayer, J.; Rohorzka, A.; Kuess, P.; Dörr, W. Upregulated Epithelial Junction Expression Represents a Novel Parameter of the Epithelial Radiation Response to Fractionated Irradiation in Oral Mucosa. Strahlenther. Onkol. 2018, 194, 771–779. [Google Scholar] [CrossRef]
- Sappayatosok, K.; Phattarataratip, E. Overexpression of Claudin-1 Is Associated with Advanced Clinical Stage and Invasive Pathologic Characteristics of Oral Squamous Cell Carcinoma. Head Neck Pathol. 2014, 9, 173–180. [Google Scholar] [CrossRef]
- Zejc, T.; Piontek, J.; Schulzke, J.-D.; Fromm, M.; Ervens, J.; Rosenthal, R. Clinical Significance of Claudin Expression in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 11234. [Google Scholar] [CrossRef]
- Gallagher, K.P.D.; Vargas, P.A.; Santos-Silva, A.R. The Use of E-Cigarettes as a Risk Factor for Oral Potentially Malignant Disorders and Oral Cancer: A Rapid Review of Clinical Evidence. Med. Oral Patol. Oral Cir. Bucal 2024, 29, e18–e26. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, K.; Fang, X.; Zhu, Z.; Chen, Q.; Li, C.; Hua, H. Oral Mucosal Changes in Tight Junction Proteins in Patients with Oral Lichen Planus. Oral Dis. 2024, 30, 4367–4375. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin Regulates Macromolecule Flux across the Intestinal Epithelial Tight Junction Barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef] [PubMed]
- Raduka, A.; Gao, N.; Chatburn, R.L.; Rezaee, F. Electronic Cigarette Exposure Disrupts Airway Epithelial Barrier Function and Exacerbates Viral Infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L580–L593. [Google Scholar] [CrossRef]
- Takehara, S.; Wright, F.; Kawaguchi, Y.; Morio, I.; Ishida, Y.; Tagami, J. The Impact of Outbound Exchange Programs on Japanese Dental Students. J. Med. Dent. Sci. 2018, 65, 99–105. [Google Scholar] [CrossRef]
- Zalewska, A.; Zwierz, K.; Zółkowski, K.; Gindzieński, A. Structure and Biosynthesis of Human Salivary Mucins. Acta Biochim. Pol. 2000, 47, 1067–1079. [Google Scholar] [CrossRef]
- Itzek, A.; Chen, Z.; Merritt, J.; Kreth, J. Effect of Salivary Agglutination on Oral Streptococcal Clearance by Human Polymorphonuclear Neutrophil Granulocytes. Mol. Oral Microbiol. 2017, 32, 197–210. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What Interactions Drive the Salivary Mucosal Pellicle Formation? Colloids Surf. B Biointerfaces 2014, 120, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Cheng, Y.; Guo, W.; Sun, S.; Chen, X.; Zhang, T.; Cheng, H.; Hao, J.; Lu, Y.; Wang, X.; et al. High Expression Level of α2-3-Linked Sialic Acids on Salivary Glycoproteins of Breastfeeding Women May Help to Protect Them from Avian Influenza Virus Infection. Molecules 2022, 27, 4285. [Google Scholar] [CrossRef] [PubMed]
- Takehara, S.; Yanagishita, M.; Podyma-Inoue, K.A.; Kawaguchi, Y. Degradation of MUC7 and MUC5B in Human Saliva. PLoS ONE 2013, 8, e69059. [Google Scholar] [CrossRef] [PubMed]
- Culp, D.J.; Robinson, B.; Cash, M.N.; Bhattacharyya, I.; Stewart, C.; Cuadra-Saenz, G. Salivary Mucin 19 Glycoproteins: Innate Immune Functions in Streptococcus Mutans-Induced Caries in Mice and Evidence for Expression in Human Saliva. J. Biol. Chem. 2015, 290, 2993–3008. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, J.; Bojanowski, C.M.; Shin, J.; Moshensky, A.; Fuentes, A.L.; Bonde, S.S.; Chuki, D.; Pride, D.T.; Crotty Alexander, L.E. Compositional Differences in the Oral Microbiome of E-Cigarette Users. Front. Microbiol. 2021, 12, 599664. [Google Scholar] [CrossRef]
- Charde, P.; Ali, K.; Hamdan, N. Effects of E-Cigarette Smoking on Periodontal Health: A Scoping Review. PLoS Glob. Public Health 2024, 4, e0002311. [Google Scholar] [CrossRef]
- Lee, W.H.; Ong, S.-G.; Zhou, Y.; Tian, L.; Bae, H.R.; Baker, N.; Whitlatch, A.; Mohammadi, L.; Guo, H.; Nadeau, K.C.; et al. Modeling Cardiovascular Risks of E-Cigarettes with Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J. Am. Coll. Cardiol. 2019, 73, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Clapp, P.W.; Lavrich, K.S.; van Heusden, C.A.; Lazarowski, E.R.; Carson, J.L.; Jaspers, I. Cinnamaldehyde in Flavored E-Cigarette Liquids Temporarily Suppresses Bronchial Epithelial Cell Ciliary Motility by Dysregulation of Mitochondrial Function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L470–L486. [Google Scholar] [CrossRef]
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored E-Cigarette Liquids and Cinnamaldehyde Impair Respiratory Innate Immune Cell Function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef] [PubMed]
- Hickman, E.; Herrera, C.A.; Jaspers, I. Common E-Cigarette Flavoring Chemicals Impair Neutrophil Phagocytosis and Oxidative Burst. Chem. Res. Toxicol. 2019, 32, 982–985. [Google Scholar] [CrossRef]
- Begic, A.; Djuric, A.; Gobeljic, B.; Stevanovic, I.; Lukic, V.; Stanojevic, I.; Ninkovic, M.; Saso, L.; Vojvodic, D.; Djukic, M. The Simple Isocratic HPLC—UV Method for the Simultaneous Determination of Reduced and Oxidized Glutathione in Animal Tissue. Acta Chromatogr. 2017, 29, 67–84. [Google Scholar] [CrossRef]
- Colombo, G.; Dalle-Donne, I.; Orioli, M.; Giustarini, D.; Rossi, R.; Clerici, M.; Regazzoni, L.; Aldini, G.; Milzani, A.; Butterfield, D.A.; et al. Oxidative Damage in Human Gingival Fibroblasts Exposed to Cigarette Smoke. Free Radic. Biol. Med. 2012, 52, 1584–1596. [Google Scholar] [CrossRef]
- Cereser, C.; Guichard, J.; Drai, J.; Bannier, E.; Garcia, I.; Boget, S.; Parvaz, P.; Revol, A. Quantitation of Reduced and Total Glutathione at the Femtomole Level by High-Performance Liquid Chromatography with Fluorescence Detection: Application to Red Blood Cells and Cultured Fibroblasts. J. Chromatogr. B Biomed. Sci. Appl. 2001, 752, 123–132. [Google Scholar] [CrossRef]
- Haddad, J.J.; Olver, R.E.; Land, S.C. Antioxidant/pro-Oxidant Equilibrium Regulates HIF-1alpha and NF-Kappa B Redox Sensitivity. Evidence for Inhibition by Glutathione Oxidation in Alveolar Epithelial Cells. J. Biol. Chem. 2000, 275, 21130–21139. [Google Scholar] [CrossRef]
- Kaushik, G.; Kaushik, T.; Khanduja, S.; Pathak, C.M.; Khanduja, K.L. Cigarette Smoke Condensate Promotes Cell Proliferation through Disturbance in Cellular Redox Homeostasis of Transformed Lung Epithelial Type-II Cells. Cancer Lett. 2008, 270, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, M.; Oberley, T.D.; Sempf, J.M.; Nel, A.E. Comparison of the Pro-Oxidative and Proinflammatory Effects of Organic Diesel Exhaust Particle Chemicals in Bronchial Epithelial Cells and Macrophages1. J. Immunol. 2002, 169, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Cipollina, C.; Gerbino, S.; Cigna, D.; Caputo, V.; Balsamo, R.; Lanata, L.; Gjomarkaj, M. Comparative Cytoprotective Effects of Carbocysteine and Fluticasone Propionate in Cigarette Smoke Extract-Stimulated Bronchial Epithelial Cells. Cell Stress Chaperones 2013, 18, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Moon, H.W.; Oh, Y.; Kim, K.; Kim, D.-D.; Lim, C.-J. Defensive Properties of Ginsenoside Re against UV-B-Induced Oxidative Stress through Up-Regulating Glutathione and Superoxide Dismutase in HaCaT Keratinocytes. Iran. J. Pharm. Res. 2018, 17, 249–260. [Google Scholar]
- Park, W.H. The Effects of Exogenous H2O2 on Cell Death, Reactive Oxygen Species and Glutathione Levels in Calf Pulmonary Artery and Human Umbilical Vein Endothelial Cells. Int. J. Mol. Med. 2013, 31, 471–476. [Google Scholar] [CrossRef]
- Wavreil, F.D.M.; Heggland, S.J. Cinnamon-Flavored Electronic Cigarette Liquids and Aerosols Induce Oxidative Stress in Human Osteoblast-like MG-63 Cells. Toxicol. Rep. 2019, 7, 23–29. [Google Scholar] [CrossRef]
- Kerasioti, E.; Veskoukis, A.S.; Skaperda, Z.; Zacharias, A.; Poulas, K.; Lazopoulos, G.; Kouretas, D. The Flavoring and Not the Nicotine Content Is a Decisive Factor for the Effects of Refill Liquids of Electronic Cigarette on the Redox Status of Endothelial Cells. Toxicol. Rep. 2020, 7, 1095–1102. [Google Scholar] [CrossRef]
- Herbert, J.; Kelty, J.S.; Laskin, J.D.; Laskin, D.L.; Gow, A.J. Menthol Flavoring in E-Cigarette Condensate Causes Pulmonary Dysfunction and Cytotoxicity in Precision Cut Lung Slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L345–L357. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Ghosh, A. Carbonyl Profiles of Electronic Nicotine Delivery System (ENDS) Aerosols Reflect Both the Chemical Composition and the Numbers of E-Liquid Ingredients–Focus on the In Vitro Toxicity of Strawberry and Vanilla Flavors. Int. J. Environ. Res. Public Health 2022, 19, 16774. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Schneider, A.; Fox, S.C.; Meyer, M.; Meboldt, M.; Attin, T.; Schmidlin, P.R. Cytotoxic and Inflammatory Effects of Electronic and Traditional Cigarettes on Oral Gingival Cells Using a Novel Automated Smoking Instrument: An In Vitro Study. Toxics 2022, 10, 179. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, W.; Zhang, D.; Wang, M.; Aprecio, R.; Ji, N.; Mohamed, O.; Li, Y.; Ding, Y.; Wang, Q. 25-Hydroxyvitamin D3-Enhanced PTPN2 Positively Regulates Periodontal Inflammation through the JAK/STAT Pathway in Human Oral Keratinocytes and a Mouse Model of Type 2 Diabetes Mellitus. J. Periodontal Res. 2018, 53, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Diala, I.; Sato, S.; Usui, M.; Nakashima, K.; Nishihara, T.; Takenaka, S. Development of a Membrane-Based Microwave-Mediated Electrochemical ELISA Method for TNF-α Detection in Patients with Periodontitis. Anal. Sci. 2013, 29, 927–930. [Google Scholar] [CrossRef]
- Rai, A.K.; Panda, M.; Das, A.K.; Rahman, T.; Das, R.; Das, K.; Sarma, A.; Kataki, A.C.; Chattopadhyay, I. Dysbiosis of Salivary Microbiome and Cytokines Influence Oral Squamous Cell Carcinoma through Inflammation. Arch. Microbiol. 2021, 203, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Brierly, G.; Celentano, A.; Breik, O.; Moslemivayeghan, E.; Patini, R.; McCullough, M.; Yap, T. Tumour Necrosis Factor Alpha (TNF-α) and Oral Squamous Cell Carcinoma. Cancers 2023, 15, 1841. [Google Scholar] [CrossRef]
- Vyhnalova, T.; Danek, Z.; Gachova, D.; Linhartova, P.B. The Role of the Oral Microbiota in the Etiopathogenesis of Oral Squamous Cell Carcinoma. Microorganisms 2021, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- Gualtero, D.F.; Lafaurie, G.I.; Buitrago, D.M.; Castillo, Y.; Vargas-Sanchez, P.K.; Castillo, D.M. Oral Microbiome Mediated Inflammation, a Potential Inductor of Vascular Diseases: A Comprehensive Review. Front. Cardiovasc. Med. 2023, 10, 1250263. [Google Scholar] [CrossRef]




| Standard Curve Statistics | ||||||
|---|---|---|---|---|---|---|
| Compound | Injected Concentration (µM) | Retention Time (min) * | Measured Concentration (µM) * | % Deviation from Injected Concentration | When Area Under Peak Is … | ±95% Confidence Interval |
| Total Glutathione | 3.125 6.250 12.500 25.000 50.000 | 5.775 ± 0.051 5.814 ± 0.049 5.821 ± 0.051 5.841 ± 0.052 5.873 ± 0.061 | 2.957 ± 0.056 4.667 ± 0.044 12.438 ± 0.024 22.496 ± 0.170 51.476 ± 0.150 | −5.367 −25.328 −0.499 −10.015 2.952 | 9816 18,650 39,263 70,017 157,052 ± 665 | ±782 ±1487 ±3130 ±6299 ±12,519 |
| Glutathione Straight Line Equation is Y = 3141X (line forced through zero); LOD (µM) = 0.00027; LOQ (µM) = 0.00083; R2 = 0.995%; % RSD = 11.162 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamim, A.; Herzog, H.; Shah, R.; Pecorelli, S.; Nisbet, V.; George, A.; Cuadra, G.A.; Palazzolo, D.L. Pathophysiological Responses of Oral Keratinocytes After Exposure to Flavored E-Cigarette Liquids. Dent. J. 2025, 13, 60. https://doi.org/10.3390/dj13020060
Shamim A, Herzog H, Shah R, Pecorelli S, Nisbet V, George A, Cuadra GA, Palazzolo DL. Pathophysiological Responses of Oral Keratinocytes After Exposure to Flavored E-Cigarette Liquids. Dentistry Journal. 2025; 13(2):60. https://doi.org/10.3390/dj13020060
Chicago/Turabian StyleShamim, Abrar, Hannah Herzog, Raivat Shah, Sara Pecorelli, Virginia Nisbet, Ann George, Giancarlo A. Cuadra, and Dominic L. Palazzolo. 2025. "Pathophysiological Responses of Oral Keratinocytes After Exposure to Flavored E-Cigarette Liquids" Dentistry Journal 13, no. 2: 60. https://doi.org/10.3390/dj13020060
APA StyleShamim, A., Herzog, H., Shah, R., Pecorelli, S., Nisbet, V., George, A., Cuadra, G. A., & Palazzolo, D. L. (2025). Pathophysiological Responses of Oral Keratinocytes After Exposure to Flavored E-Cigarette Liquids. Dentistry Journal, 13(2), 60. https://doi.org/10.3390/dj13020060









