Reference Values for Permanent-Tooth Emergence in Hungarian Children: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Study Setting
2.2. Ethics
2.3. Sample Population
2.4. Data Collection
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorski, J.P.; Marks, S.C., Jr.; Cahill, D.R.; Wise, G.E. Developmental changes in the extracellular matrix of the dental follicle during tooth eruption. Connect. Tissue Res. 1988, 18, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E.; Frazier-Bowers, S.; D’Souza, R.N. Cellular, molecular, and genetic determinants of tooth eruption. Crit. Rev. Oral Biol. Med. 2002, 13, 323–334. [Google Scholar] [CrossRef]
- Geller, F.; Feenstra, B.; Zhang, H.; Shaffer, J.R.; Hansen, T.; Esserlind, A.L.; Boyd, H.A.; Nohr, E.A.; Timpson, N.J.; Fatemifar, G.; et al. Genome-wide association study identifies four loci associated with eruption of permanent teeth. PLoS Genet. 2011, 7, e1002275. [Google Scholar] [CrossRef]
- Brin, I.; Camasuvi, S.; Dali, N.; Aizenbud, D. Comparison of second molar eruption patterns in patients with skeletal Class II and skeletal Class I malocclusions. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 746–751. [Google Scholar] [CrossRef]
- Lee, E.; Jeong, T.; Shin, J. Positional Differences of the Mandibular Canal in Relation to Permanent Mandibular First Molars with Eruption Disturbances in Children. Children 2020, 7, 206. [Google Scholar] [CrossRef]
- Diéguez-Pérez, M.; Paz-Cortés, M.M.; Muñoz-Cano, L. Evaluation of the Relationship between the Weight and Height Percentiles and the Sequence and Chronology of Eruption in Permanent Dentition. Healthcare 2022, 10, 1363. [Google Scholar] [CrossRef]
- Mohamedhussein, N.; Busuttil-Naudi, A.; Mohammed, H.; UlHaq, A. Association of obesity with the eruption of first and second permanent molars in children: A systematic review. Eur. Arch. Paediatr. Dent. 2020, 21, 13–23. [Google Scholar] [CrossRef]
- Enwonwu, C.O. Influence of socio-economic conditions on dental development in Nigerian children. Arch. Oral Biol. 1973, 18, 95–107. [Google Scholar] [CrossRef]
- Clements, E.M.; Davies-Thomas, E.; Pickett, K.G. Time of eruption of permanent teeth in British children at independent, rural, and urban schools. Br. Med. J. 1957, 1, 1511–1513. [Google Scholar] [CrossRef]
- Adler, P. Studies on the eruption of the permanent teeth. IV. The effect upon the eruption of the permanent teeth of caries in the deciduous dentition, and of urbanization. Acta Genet. Stat. Med. 1958, 8, 78–94. [Google Scholar]
- Leroy, R.; Bogaerts, K.; Lesaffre, E.; Declerck, D. The effect of fluorides and caries in primary teeth on permanent tooth emergence. Community Dent. Oral Epidemiol. 2003, 31, 463–470. [Google Scholar] [CrossRef]
- Eskeli, R.; Lösönen, M.; Ikävalko, T.; Myllykangas, R.; Lakka, T.; Laine-Alava, M.T. Secular trends affect timing of emergence of permanent teeth. Angle Orthod. 2016, 86, 53–58. [Google Scholar] [CrossRef]
- Almonaitiene, R.; Balciuniene, I.; Tutkuviene, J. Factors influencing permanent teeth eruption. Part one–general factors. Stomatologija 2010, 12, 67–72. [Google Scholar]
- Choukroune, C. Tooth eruption disorders associated with systemic and genetic diseases: Clinical guide. J. Dentofac. Anom. Orthod. 2017, 20, 402. [Google Scholar] [CrossRef]
- Shaweesh, A.I.; Al-Omiri, M.K.; Alsoleihat, F.D. Variation in time of emergence of permanent teeth among urban and rural Jordanian school children. Saudi Med. J. 2011, 32, 1066–1072. [Google Scholar] [PubMed]
- Dhamo, B.; Kragt, L.; Grgic, O.; Ongkosuwito, E.M.; Wolvius, E.B.; Raat, H.; Jaddoe, V.W.V.; Hofman, A.; Tiemeier, H.; van Wijk, A.J. Ancestry and dental development: A geographic and genetic perspective. Am. J. Phys. Anthropol. 2018, 165, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Vandana, S.; Muthu, M.S.; Akila, G.; Anusha, M.; Kandaswamy, D.; Aswath Narayanan, M.B. Global variations in eruption chronology of permanent teeth: A systematic review and meta-analysis. Am. J. Hum. Biol. 2024, 36, e24060. [Google Scholar] [CrossRef]
- Hassanali, J.; Odhiambo, J.W. Ages of eruption of the permanent teeth in Kenyan African and Asian children. Ann. Hum. Biol. 1981, 8, 425–434. [Google Scholar] [CrossRef]
- Garn, S.M.; Sandusky, S.T.; Nagy, J.M.; Trowbridge, F.L. Negro-Caucasoid differences in permanent tooth emergence at a constant income level. Arch. Oral Biol. 1973, 18, 609–615. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Dental growth and development. In Reference Manual of Pediatric Dentistry; AAPD: Chicago, IL, USA, 2022; p. 580. [Google Scholar]
- Proffit, W.R.; Fields, H.W.; Larson, B.E.; Sarver, D.M. Contemporary Orthodontics, 6th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 74–83. [Google Scholar]
- Shaweesh, A.I. Timing and sequence of emergence of permanent teeth in the Jordanian population. Arch. Oral Biol. 2012, 57, 122–130. [Google Scholar] [CrossRef]
- Rajić, Z.; Rajić-Mestrović, S.; Verzak, Z. Chronology, dynamics and period of permanent tooth eruption in Zagreb children (Part II). Coll. Antropol. 2000, 24, 137–143. [Google Scholar] [PubMed]
- Almonaitiene, R.; Balciuniene, I.; Tutkuviene, J. Standards for permanent teeth emergence time and sequence in Lithuanian children, residents of Vilnius city. Stomatologija 2012, 14, 93–100. [Google Scholar]
- Šindelářová, R.; Žáková, L.; Broukal, Z. Standards for permanent tooth emergence in Czech children. BMC Oral Health 2017, 17, 140. [Google Scholar] [CrossRef]
- Moslemi, M. An epidemiological survey of the time and sequence of eruption of permanent teeth in 4–15-year-olds in Tehran, Iran. Int. J. Paediatr. Dent. 2004, 14, 432–438. [Google Scholar] [CrossRef]
- Carter, K.; Worthington, S. Morphologic and Demographic Predictors of Third Molar Agenesis: A Systematic Review and Meta-analysis. J. Dent. Res. 2015, 94, 886–894. [Google Scholar] [CrossRef]
- Heidmann, J. Comparison of different methods for estimating human tooth-eruption time on one set of Danish national data. Arch. Oral Biol. 1986, 31, 815–817. [Google Scholar] [CrossRef]
- Eskeli, R.; Laine-Alava, M.T.; Hausen, H.; Pahkala, R. Standards for permanent tooth emergence in Finnish children. Angle Orthod. 1999, 69, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Leroy, R.; Bogaerts, K.; Lesaffre, E.; Declerck, D. The emergence of permanent teeth in Flemish children. Community Dent. Oral Epidemiol. 2003, 31, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Elmes, A.; Dykes, E.; Cookson, M.J. A cross-sectional survey to determine the ages of emergence of permanent teeth of Caucasian children of the Colchester area of the UK. Br. Dent. J. 2010, 209, E10. [Google Scholar] [CrossRef]
- Diamanti, J.; Townsend, G.C. New standards for permanent tooth emergence in Australian children. Aust. Dent. J. 2003, 48, 39–42. [Google Scholar] [CrossRef]
- Nizam, A.; Naing, L.; Mokhtar, N. Age and sequence of eruption of permanent teeth in Kelantan, north-eastern Malaysia. Clin. Oral Investig. 2003, 7, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Kutesa, A.; Nkamba, E.M.; Muwazi, L.; Buwembo, W.; Rwenyonyi, C.M. Weight, height and eruption times of permanent teeth of children aged 4-15 years in Kampala, Uganda. BMC Oral Health 2013, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hong, Y.; Han, D.H.; Hong, H.K.; Kim, Y.N.; Bae, K.H. A prospective cohort study on emergence of permanent teeth and caries experience in Korean children. Int. J. Paediatr. Dent. 2011, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Khan, N. Eruption time of permanent teeth in Pakistani children. Iran J. Public Health 2011, 40, 63–73. [Google Scholar]
- Demirjian, A.; Levesque, G.Y. Sexual differences in dental development and prediction of emergence. J. Dent. Res. 1980, 59, 1110–1122. [Google Scholar] [CrossRef]
- Roberts, M.W.; Li, S.H.; Cutler, G.B., Jr.; Hench, K.D.; Loriaux, D.L. Sex differences in dental development in children with precocious puberty related to central nervous system lesions. Pediatr. Dent. 1986, 8, 276–279. [Google Scholar]
- Nassif, N.; Sfeir, E. Age and sequence of permanent teeth eruption in Lebanese children. Sci. World J. 2020, 2020, 9238679. [Google Scholar] [CrossRef]
- Houpt, M.I.; Adu-Aryee, S.; Grainger, R.M. Eruption times of permanent teeth in the Brong Ahafo Region of Ghana. Am. J. Orthod. 1967, 53, 95–99. [Google Scholar] [CrossRef]
- Krumholt, L.; Roed-Petersen, B.; Bindborg, J.J. Eruption times of the permanent teeth in 622 Ugandan children. Arch. Oral Biol. 1971, 16, 1281–1288. [Google Scholar] [CrossRef]
- Brown, T. Tooth emergence in Australian Aboriginals. Ann. Hum. Biol. 1978, 5, 41–54. [Google Scholar] [CrossRef]
- Lo, R.T.; Moyers, R.E. Studies in the etiology and prevention of malocclusion: I. The sequence of eruption of the permanent dentition. Am. J. Orthod. 1953, 39, 460–467. [Google Scholar] [CrossRef]
- Logan, W.H.G.; Kronfeld, R. Development of the human jaws and surrounding structures from birth to the age of fifteen years. J. Am. Dent. Assoc. 1933, 20, 379–427. [Google Scholar] [CrossRef]
- Ekstrand, K.R.; Christiansen, J.; Christiansen, M.E. Time and duration of eruption of first and second permanent molars: A longitudinal investigation. Community Dent. Oral Epidemiol. 2003, 31, 344–350. [Google Scholar] [CrossRef]

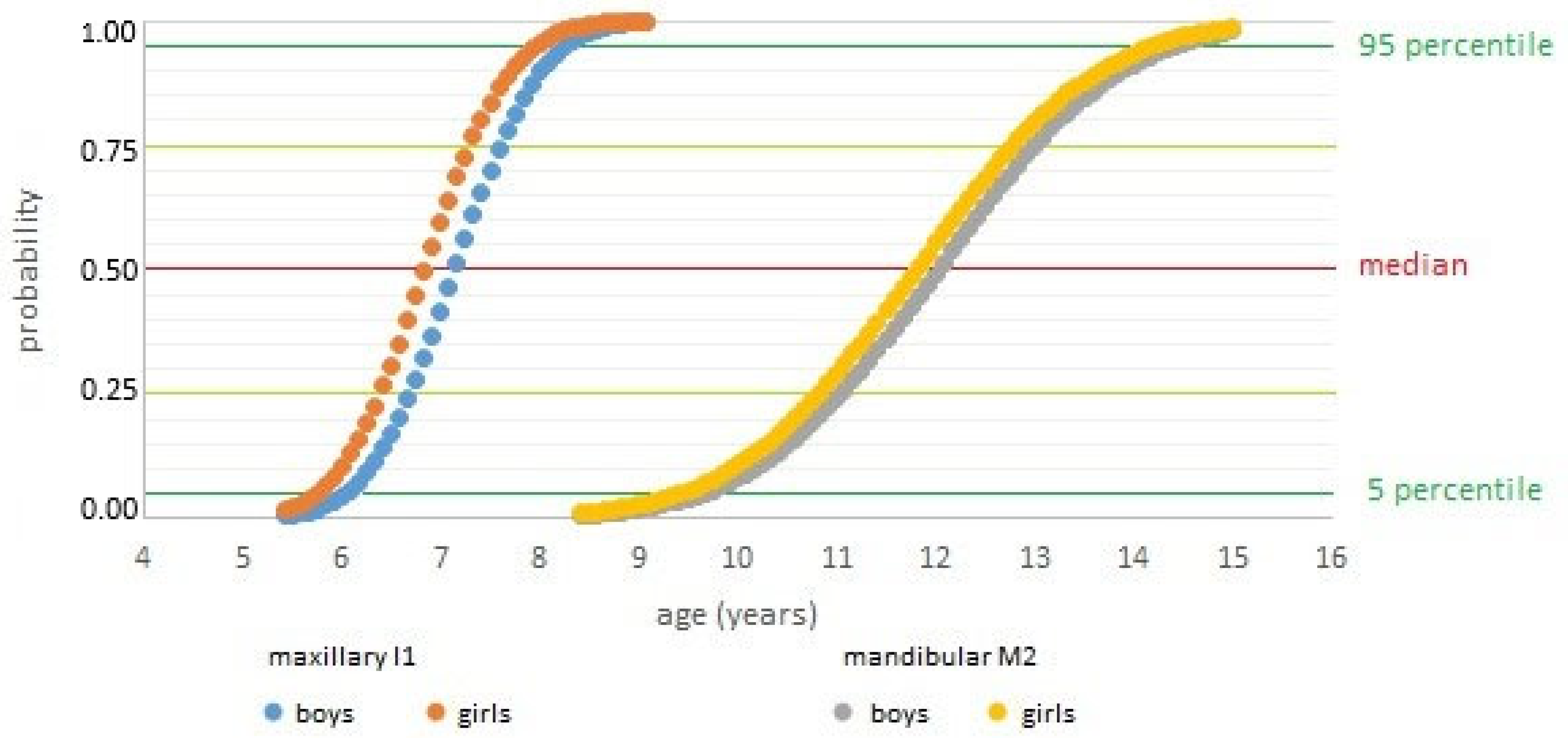
| Boys | Girls | Difference | ||||
|---|---|---|---|---|---|---|
| Median | (95% CI) | Median | (95% CI) | Months | p-Value | |
| Maxilla | ||||||
| I1 | 85.7 | (84.4–87.0) | 82.1 | (80.6–83.4) | 3.6 | <0.001 |
| I2 | 98.0 | (95.5–98.6) | 93.5 | (91.9–95.1) | 4.5 | <0.001 |
| C | 135.9 | (134.4–137.5) | 129.7 | (128.1–131.3) | 6.2 | <0.001 |
| P1 | 121.4 | (120.1–122.7) | 119.4 | (118.1–120.8) | 2.0 | 0.009 |
| P2 | 133.1 | (131.6–134.7) | 131.2 | (129.6–132.7) | 1.9 | 0.017 |
| M1 | 76.4 | (74.4–78.2) | 73.7 | (71.6–75.6) | 2.7 | 0.004 |
| M2 | 150.2 | (148.5–152.0) | 146.2 | (144.5–147.9) | 4.0 | <0.001 |
| Mandible | ||||||
| I1 | 74.8 | (72.3–76.9) | 72.6 | (69.8–74.9) | 2.2 | NS |
| I2 | 88.7 | (87.0–90.4) | 85.2 | (83.4–87.0) | 3.5 | <0.001 |
| C | 124.5 | (122.5–126.9) | 115.6 | (114.2–117.2) | 8.9 | <0.001 |
| P1 | 123.5 | (121.4–126.2) | 119.4 | (117.6–121.7) | 4.1 | <0.001 |
| P2 | 135.4 | (133.9–136.9) | 131.3 | (129.9–132.8) | 4.1 | <0.001 |
| M1 | 75.2 | (72.8–77.3) | 72.3 | (69.8–74.5) | 2.9 | 0.042 |
| M2 | 144.3 | (142.2–146.5) | 141.6 | (139.5–143.7) | 2.7 | 0.002 |
| Boys | Girls | Total Sample | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentile | Percentile | Percentile | Difference | ||||||||||||||
| 5th | 25th | 50th Median | 75th | 95th | 5th | 25th | 50th Median | 75th | 95th | 5th | 25th | 50th Median | 75th | 95th | 5–95 | 25–75 | |
| Maxilla | |||||||||||||||||
| I1 | 6.03 | 6.69 | 7.14 | 7.60 | 8.25 | 5.73 | 6.38 | 6.84 | 7.30 | 7.95 | 5.84 | 6.52 | 6.99 | 7.46 | 8.14 | 2.30 | 0.94 |
| I2 | 6.67 | 7.51 | 8.09 | 8.67 | 9.51 | 6.37 | 7.21 | 7.79 | 8.38 | 9.21 | 6.49 | 7.35 | 7.94 | 8.54 | 9.40 | 2.91 | 1.19 |
| C | 9.37 | 10.52 | 11.33 | 12.13 | 13.28 | 8.85 | 10.01 | 10.81 | 11.61 | 12.76 | 9.10 | 10.27 | 11.07 | 11.88 | 13.05 | 3.95 | 1.61 |
| P1 | 8.08 | 9.28 | 10.12 | 10.95 | 12.16 | 7.91 | 9.12 | 9.95 | 10.79 | 11.99 | 8.00 | 9.20 | 10.04 | 10.88 | 12.08 | 4.08 | 1.68 |
| P2 | 8.82 | 10.16 | 11.10 | 12.03 | 13.37 | 8.65 | 10.00 | 10.93 | 11.86 | 13.21 | 8.74 | 10.08 | 11.02 | 11.95 | 13.29 | 4.55 | 1.87 |
| M1 | 4.92 | 5.77 | 6.37 | 6.96 | 7.81 | 4.70 | 5.55 | 6.14 | 6.73 | 7.58 | 4.80 | 5.66 | 6.25 | 6.85 | 7.70 | 2.90 | 1.19 |
| M2 | 10.18 | 11.56 | 12.52 | 13.48 | 14.86 | 9.85 | 11.23 | 12.18 | 13.14 | 14.52 | 10.01 | 11.39 | 12.35 | 13.30 | 14.68 | 4.67 | 1.91 |
| Mandible | |||||||||||||||||
| I1 | 4.90 | 5.69 | 6.24 | 6.78 | 7.57 | 4.72 | 5.51 | 6.05 | 6.60 | 7.39 | 4.81 | 5.60 | 6.15 | 6.69 | 7.48 | 2.67 | 1.09 |
| I2 | 6.06 | 6.85 | 7.40 | 7.94 | 8.73 | 5.77 | 6.56 | 7.10 | 7.65 | 8.44 | 5.88 | 6.69 | 7.25 | 7.81 | 8.62 | 2.74 | 1.12 |
| C | 8.86 | 9.75 | 10.37 | 11.00 | 11.89 | 8.12 | 9.01 | 9.63 | 10.26 | 11.15 | 8.40 | 9.36 | 10.03 | 10.70 | 11.67 | 3.27 | 1.34 |
| P1 | 8.47 | 9.55 | 10.30 | 11.04 | 12.12 | 8.13 | 9.21 | 9.95 | 10.70 | 11.78 | 8.29 | 9.38 | 10.14 | 10.90 | 11.99 | 3.70 | 1.52 |
| P2 | 8.97 | 10.33 | 11.28 | 12.23 | 13.60 | 8.63 | 10.00 | 10.95 | 11.90 | 13.26 | 8.80 | 10.17 | 11.12 | 12.07 | 13.43 | 4.63 | 1.90 |
| M1 | 4.87 | 5.69 | 6.27 | 6.84 | 7.66 | 4.63 | 5.45 | 6.02 | 6.59 | 7.41 | 4.74 | 5.57 | 6.14 | 6.72 | 7.55 | 2.81 | 1.15 |
| M2 | 9.63 | 11.05 | 12.03 | 13.01 | 14.42 | 9.41 | 10.82 | 11.80 | 12.78 | 14.19 | 9.53 | 10.93 | 11.91 | 12.89 | 14.29 | 4.76 | 1.96 |
| Caucasian | Asian | African | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HUN | CRO | LTU | CZE | FIN | BEL a | GBR | JOR | AUS c | MAS | KOR a | UGA b | |
| Present | [23] | [24] | [25] | [29] | [30] | [31] | [22] | [32] | [33] | [35] | [34] | |
| Maxilla | ||||||||||||
| I1 | 7.1 | 7.5 | 6.9 | 7.0 | 6.8 | 7.1 | 7.4 | 7.3 | 7.4 | 7.2 | 7.4 | 6.3 |
| I2 | 8.2 | 8.5 | 8.0 | 8.0 | 8.1 | 8.3 | 8.7 | 8.5 | 8.6 | 8.6 | 8.5 | 8.5 |
| C | 11.3 | 11.6 | 11.1 | 11.3 | 11.3 | 11.5 | 12.0 | 11.6 | 11.8 | 11.0 | 10.9 | 10.8 |
| P1 | 10.1 | 10.3 | 9.9 | 9.5 | 10.9 | 10.7 | 11.2 | 10.5 | 11.3 | 9.5 | 9.7 | 9.6 |
| P2 | 11.1 | 10.8 | 10.8 | 11.0 | 11.7 | 11.6 | 12.3 | 11.4 | 12.1 | 10.4 | 10.5 | 9.5 |
| M1 | 6.4 | 6.8 | 6.4 | 6.9 | 6.3 | 6.3 | 6.8 | 6.4 | 6.7 | 6.4 | 6.6 | 6.4 |
| M2 | 12.5 | 12.6 | 12.3 | 12.7 | 12.4 | 12.3 | 12.8 | 12.6 | 12.7 | 12.2 | 12.8 | 11.0 |
| Mandible | ||||||||||||
| I1 | 6.2 | 6.6 | 6.1 | 6.4 | 6.0 | 6.3 | 6.6 | 6.5 | 6.6 | 6.4 | 6.5 | 6.5 |
| I2 | 7.4 | 7.7 | 7.2 | 7.3 | 7.1 | 7.4 | 7.8 | 7.5 | 7.8 | 7.5 | 7.5 | 5.8 |
| C | 10.4 | 10.9 | 10.4 | 9.4 | 10.5 | 10.6 | 11.0 | 10.6 | 11.0 | 10.2 | 10.2 | 10.1 |
| P1 | 10.3 | 10.6 | 10.1 | 10.0 | 10.7 | 10.7 | 11.2 | 10.5 | 11.2 | 9.9 | 10.0 | 10.0 |
| P2 | 11.3 | 10.9 | 11.1 | 10.9 | 11.6 | 11.7 | 12.2 | 11.7 | 12.1 | 10.9 | 10.9 | 10.8 |
| M1 | 6.3 | 6.6 | 6.2 | 6.5 | 6.2 | 6.3 | 6.8 | 6.2 | 6.6 | 6.0 | 6.1 | 6.0 |
| M2 | 12.0 | 11.9 | 11.7 | 12.4 | 12.0 | 11.8 | 12.3 | 12.2 | 12.2 | 11.4 | 11.7 | 11.5 |
| Caucasian | Asian | African | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HUN | CRO | LTU | CZE | FIN | BEL a | GBR | JOR | AUS c | MAS | KOR a | UGA b | |
| Present | [23] | [24] | [25] | [29] | [30] | [31] | [22] | [31] | [33] | [35] | [34] | |
| Maxilla | ||||||||||||
| I1 | 6.8 | 7.2 | 6.8 | 6.9 | 6.8 | 6.9 | 7.2 | 7.1 | 7.2 | 7.1 | 7.2 | 6.2 |
| I2 | 7.8 | 8.3 | 7.6 | 7.6 | 7.6 | 7.9 | 8.2 | 8.1 | 8.2 | 8.5 | 8.0 | 7.2 |
| C | 10.8 | 11.1 | 10.5 | 10.4 | 10.8 | 11.0 | 11.4 | 11.1 | 11.2 | 10.5 | 10.0 | 9.3 |
| P1 | 10.0 | 10.1 | 9.5 | 9.4 | 10.3 | 10.4 | 10.9 | 10.0 | 10.8 | 9.2 | 9.2 | 9.3 |
| P2 | 10.9 | 10.7 | 10.6 | 10.9 | 11.6 | 11.4 | 11.8 | 11.0 | 11.7 | 10.2 | 10.1 | 10.1 |
| M1 | 6.1 | 6.9 | 6.3 | 6.6 | 6.1 | 6.2 | 6.5 | 6.2 | 6.5 | 6.2 | 6.4 | 5.3 |
| M2 | 12.2 | 12.4 | 12.1 | 12.5 | 11.9 | 12.0 | 12.4 | 12.3 | 12.3 | 12.0 | 12.1 | 10.7 |
| Mandible | ||||||||||||
| I1 | 6.1 | 7.2 | 5.9 | 6.2 | 5.9 | 6.2 | 6.4 | 6.3 | 6.3 | 6.3 | 6.1 | 5.6 |
| I2 | 7.1 | 7.2 | 6.9 | 7.2 | 6.8 | 7.1 | 7.4 | 7.3 | 7.4 | 7.3 | 7.2 | 6.8 |
| C | 9.6 | 10.0 | 9.6 | 9.1 | 9.7 | 9.7 | 10.3 | 9.8 | 10.1 | 9.5 | 9.4 | 9.7 |
| P1 | 10.0 | 10.4 | 9.7 | 9.7 | 10.3 | 10.3 | 10.7 | 10.1 | 10.6 | 9.7 | 9.6 | 9.2 |
| P2 | 10.9 | 10.9 | 10.6 | 10.6 | 11.3 | 11.4 | 11.9 | 11.2 | 11.7 | 10.6 | 10.5 | 10.2 |
| M1 | 6.0 | 7.0 | 6.0 | 6.2 | 6.1 | 6.2 | 6.5 | 6.1 | 6.3 | 6.0 | 6.1 | 5.2 |
| M2 | 11.8 | 11.8 | 11.3 | 11.8 | 11.6 | 11.6 | 12.0 | 11.7 | 11.8 | 11.0 | 11.3 | 10.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapusi-Papp, Z.; Máth, J.; Nemes, J.Á. Reference Values for Permanent-Tooth Emergence in Hungarian Children: A Cross-Sectional Study. Dent. J. 2025, 13, 542. https://doi.org/10.3390/dj13110542
Kapusi-Papp Z, Máth J, Nemes JÁ. Reference Values for Permanent-Tooth Emergence in Hungarian Children: A Cross-Sectional Study. Dentistry Journal. 2025; 13(11):542. https://doi.org/10.3390/dj13110542
Chicago/Turabian StyleKapusi-Papp, Zsuzsa, János Máth, and Judit Ágnes Nemes. 2025. "Reference Values for Permanent-Tooth Emergence in Hungarian Children: A Cross-Sectional Study" Dentistry Journal 13, no. 11: 542. https://doi.org/10.3390/dj13110542
APA StyleKapusi-Papp, Z., Máth, J., & Nemes, J. Á. (2025). Reference Values for Permanent-Tooth Emergence in Hungarian Children: A Cross-Sectional Study. Dentistry Journal, 13(11), 542. https://doi.org/10.3390/dj13110542






