From Industry to Dentistry: A Comprehensive Review of Zeolite as a Next-Generation Multifunctional Filler for Enhanced Mechanical Reinforcement and Antimicrobial Efficacy
Abstract
1. Introduction
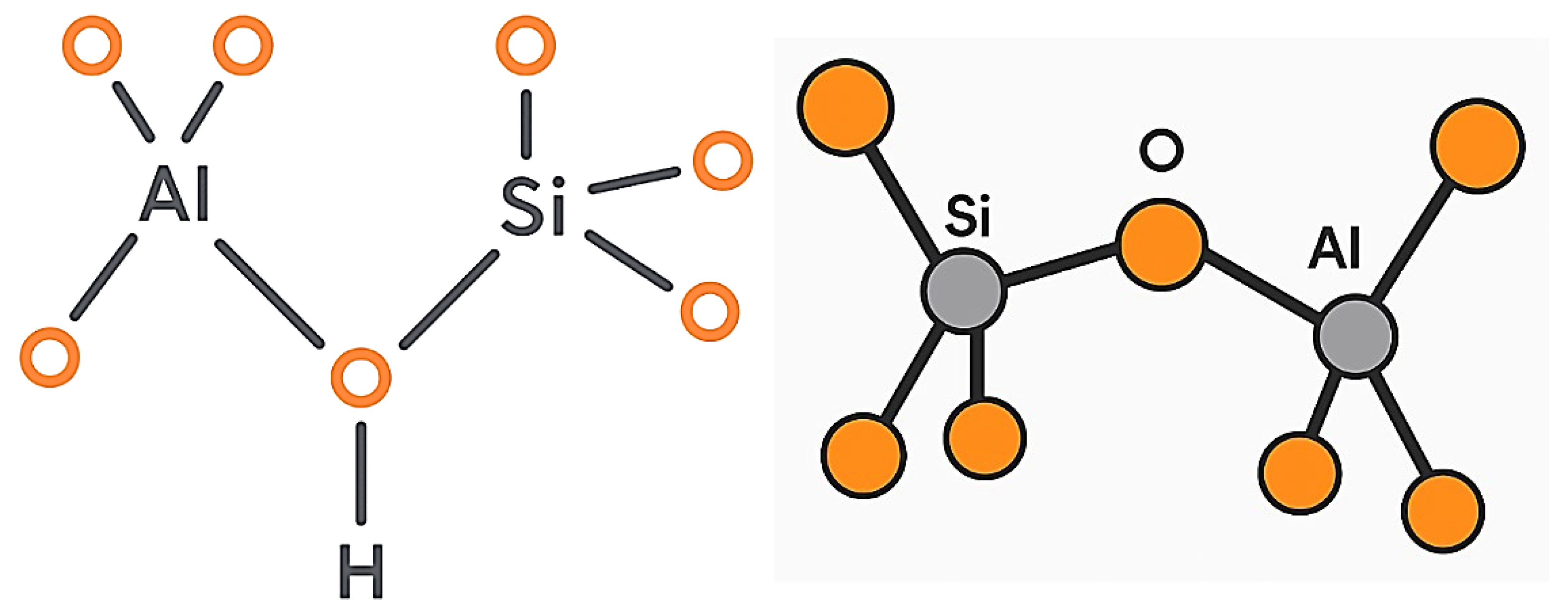
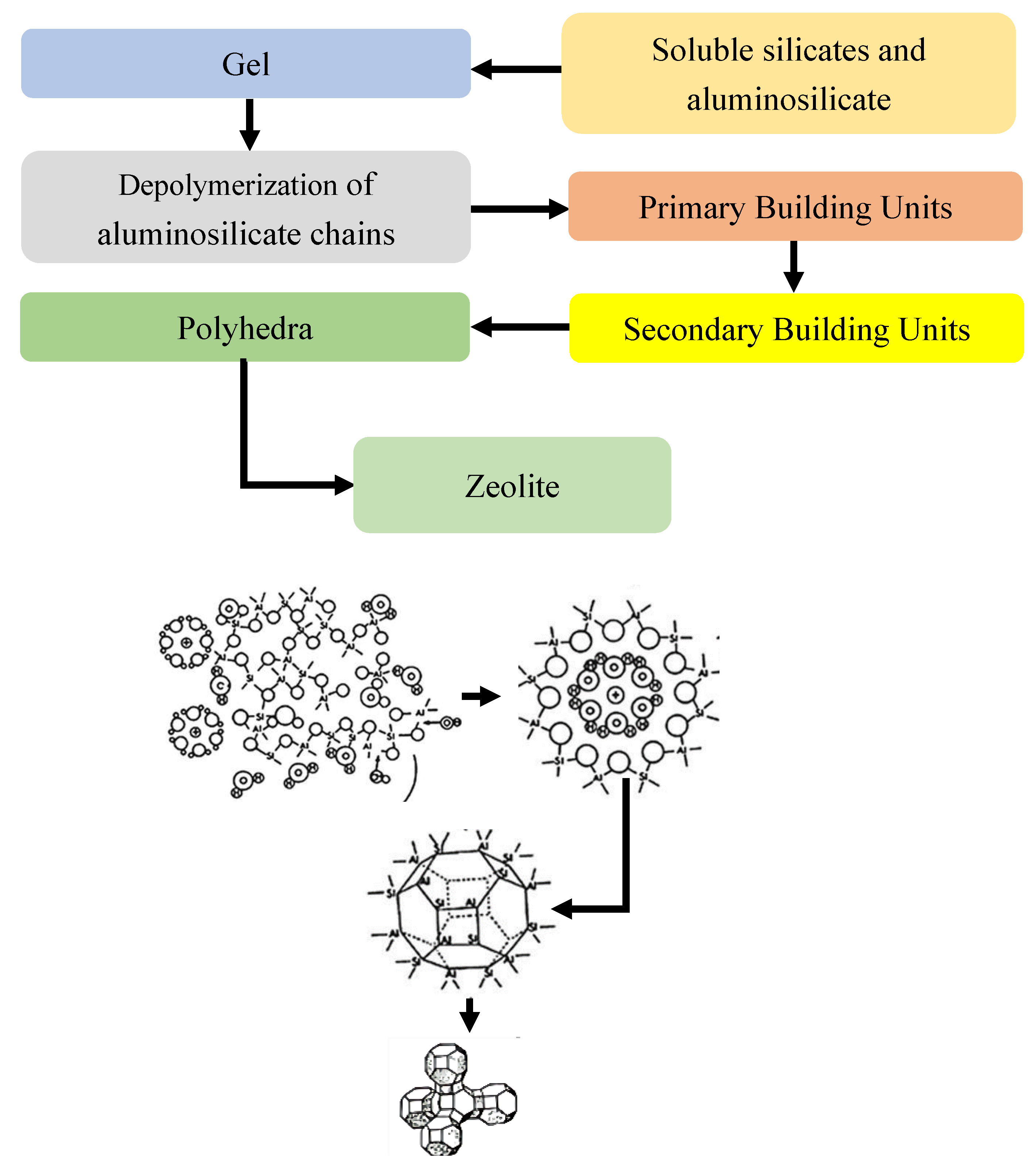
1.1. Origin, Formation, and Structure of Zeolites
1.2. Search Strategies and Data Sources
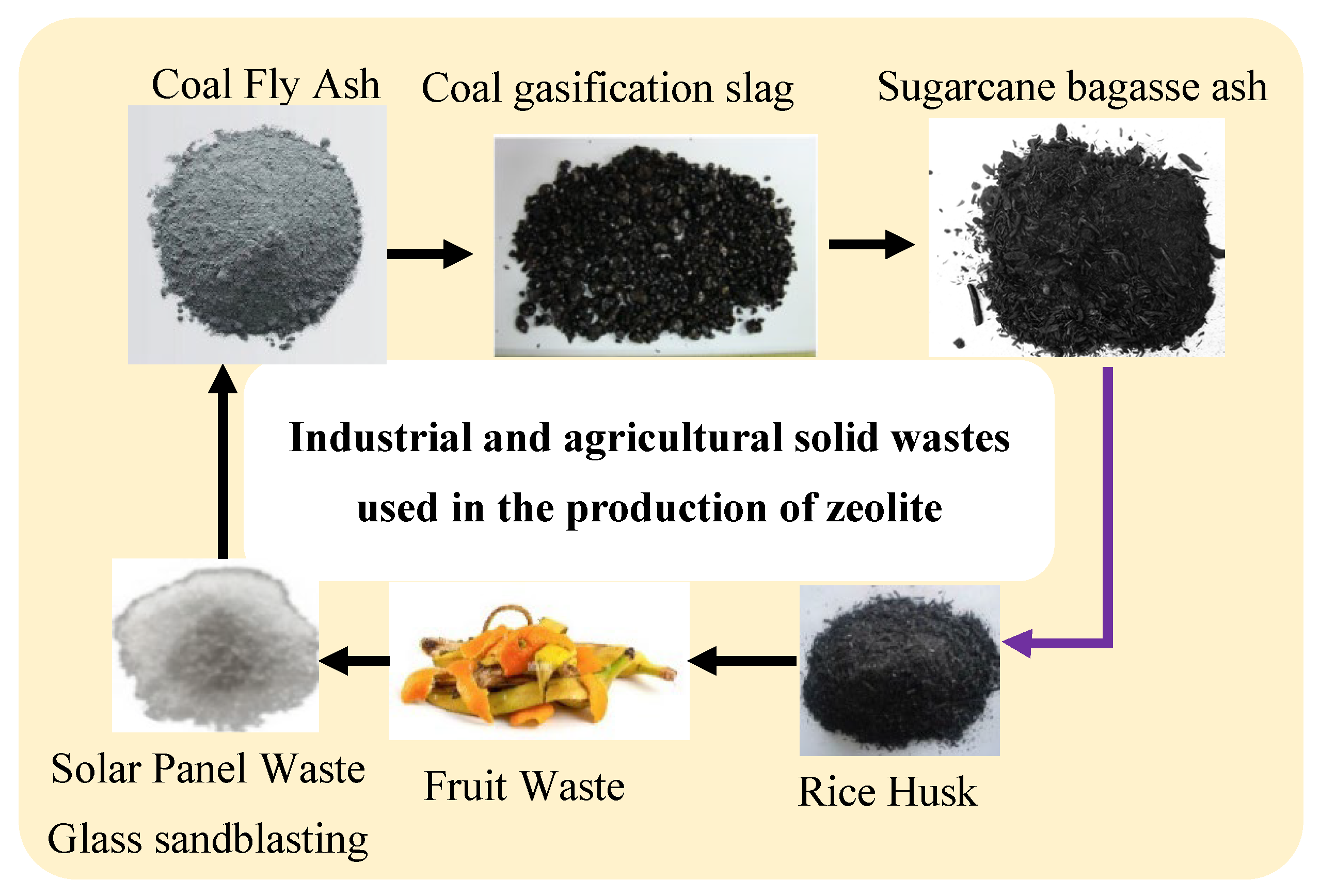
1.3. Utilization of Industrial and Agricultural Wastes Rich in Si and Al for Zeolite Synthesis
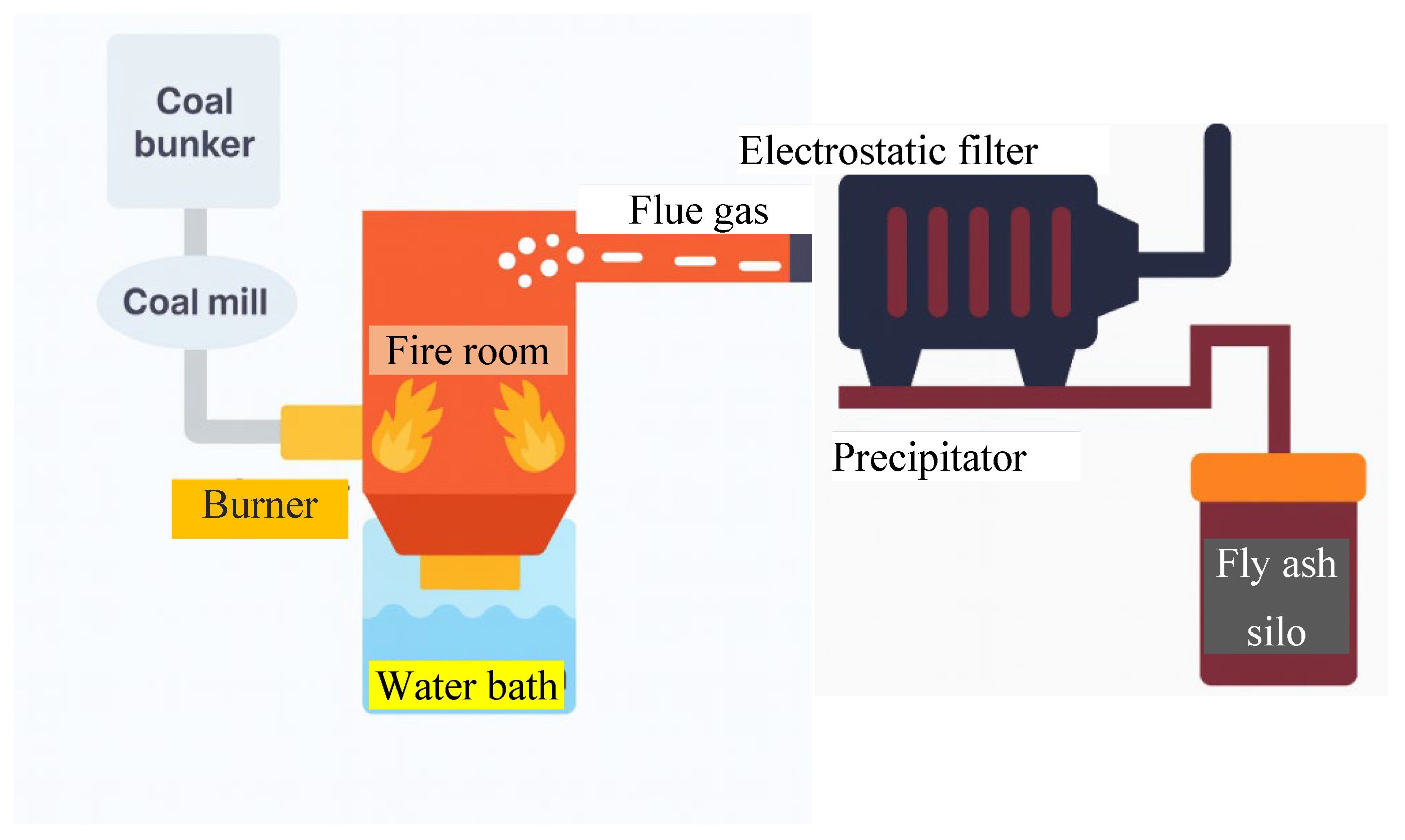
1.3.1. Coal Fly Ash (CFA)
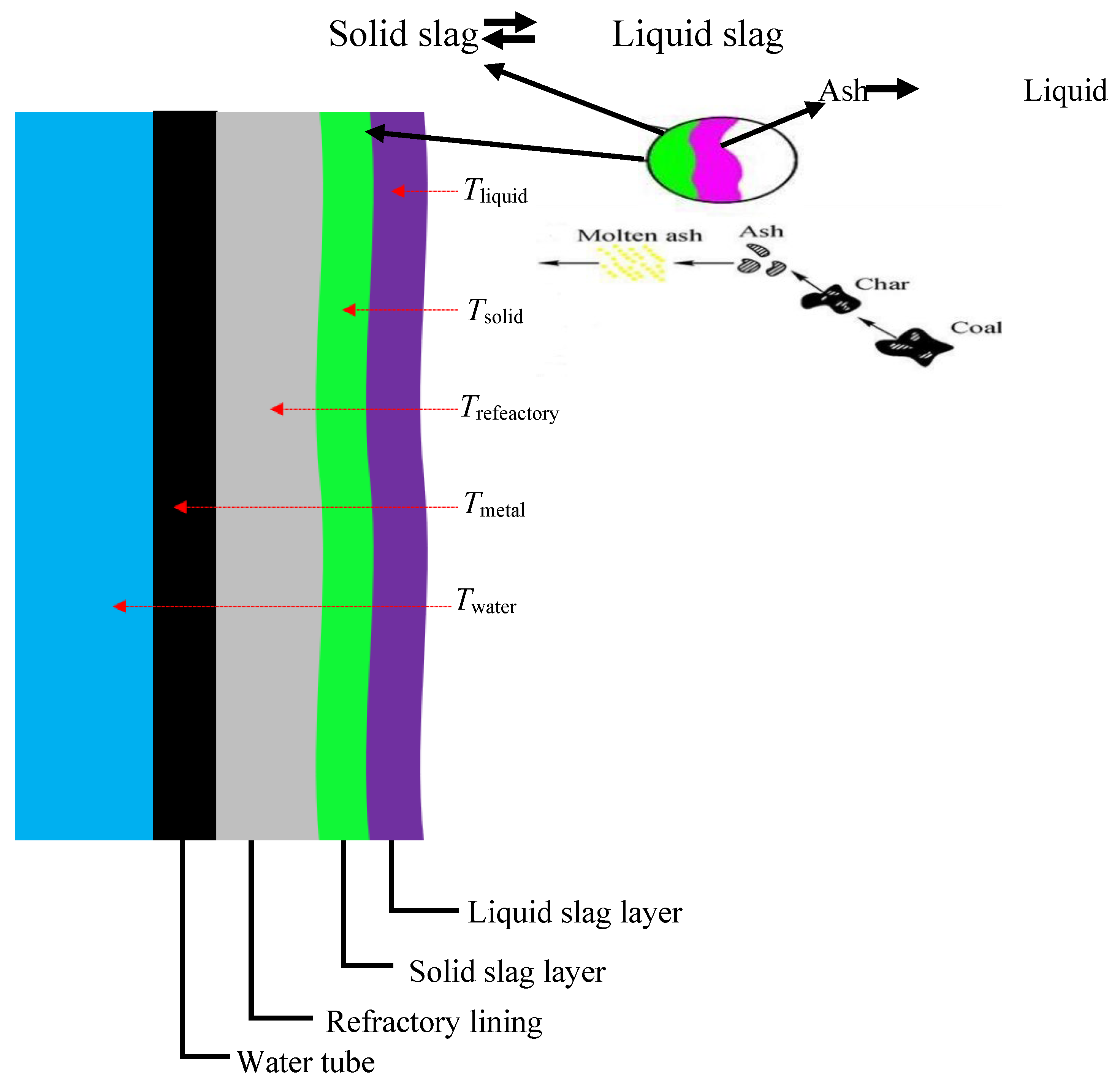
1.3.2. Coal Gasification Slag (CGS)
1.3.3. Rice Husk Ash (RHA)
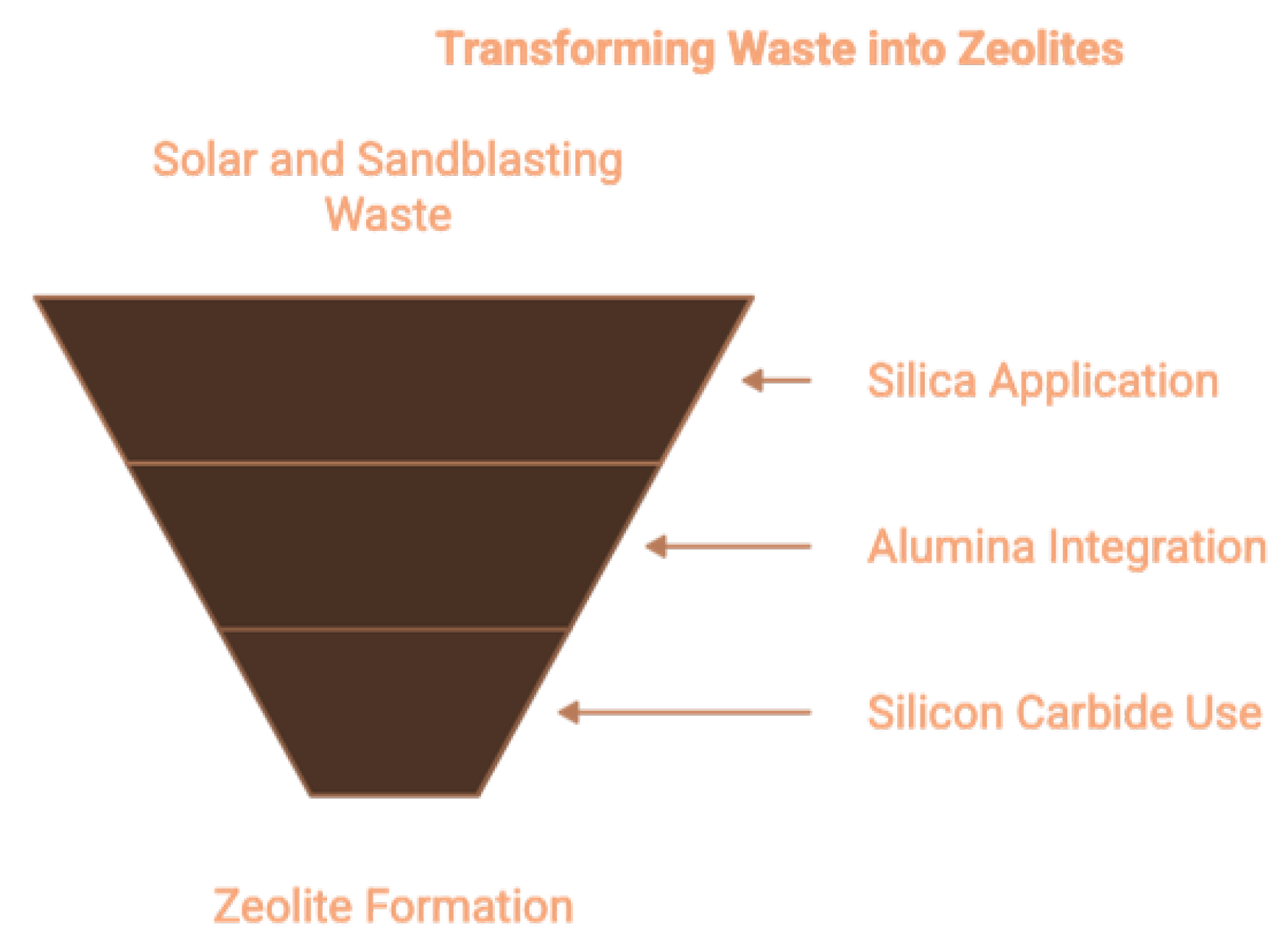
1.3.4. Solar Panel Waste Glass and Sandblasting Waste
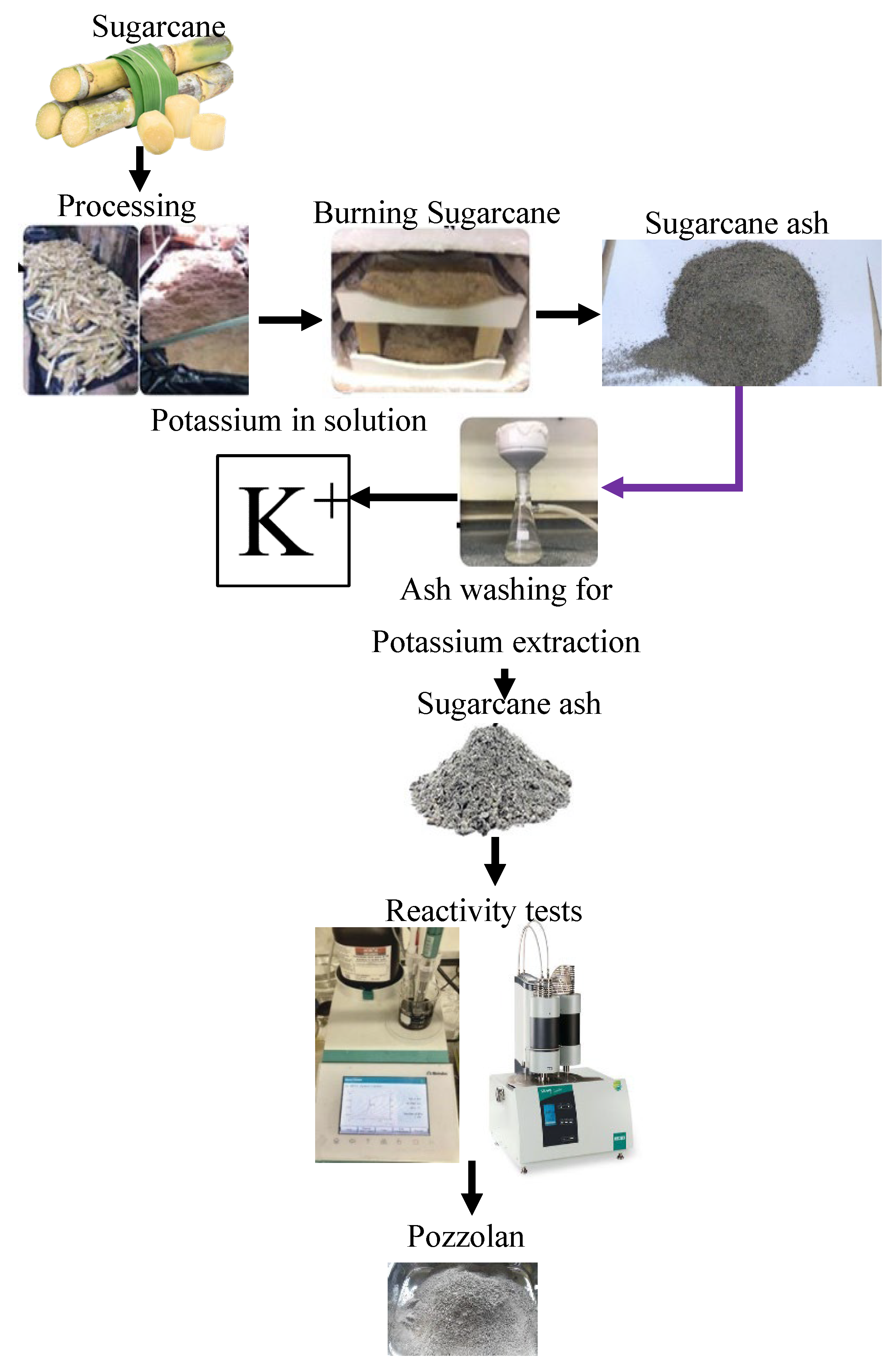
1.3.5. Sugarcane Bagasse Ash (SCBA)
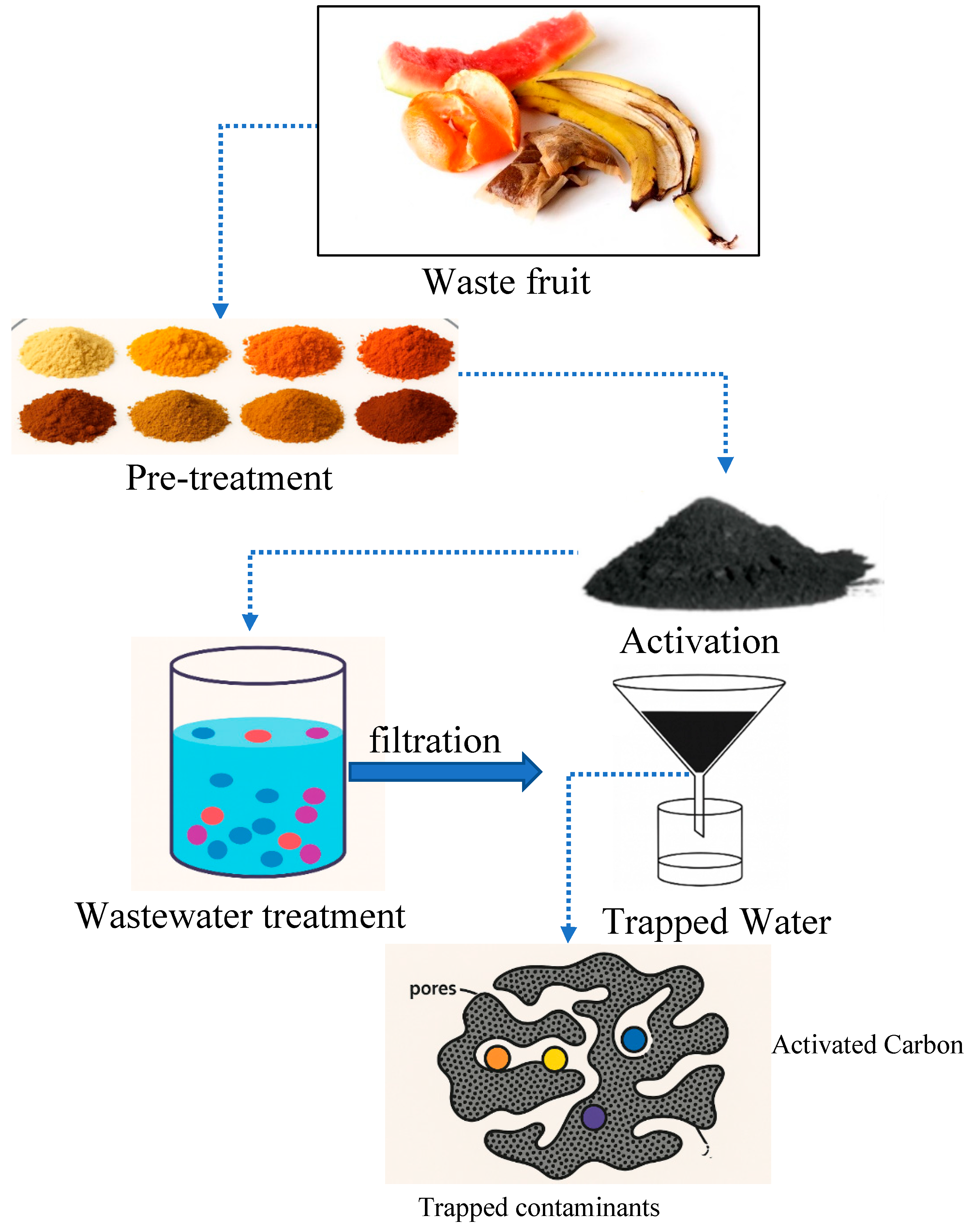
1.3.6. Fruit Waste Biomass
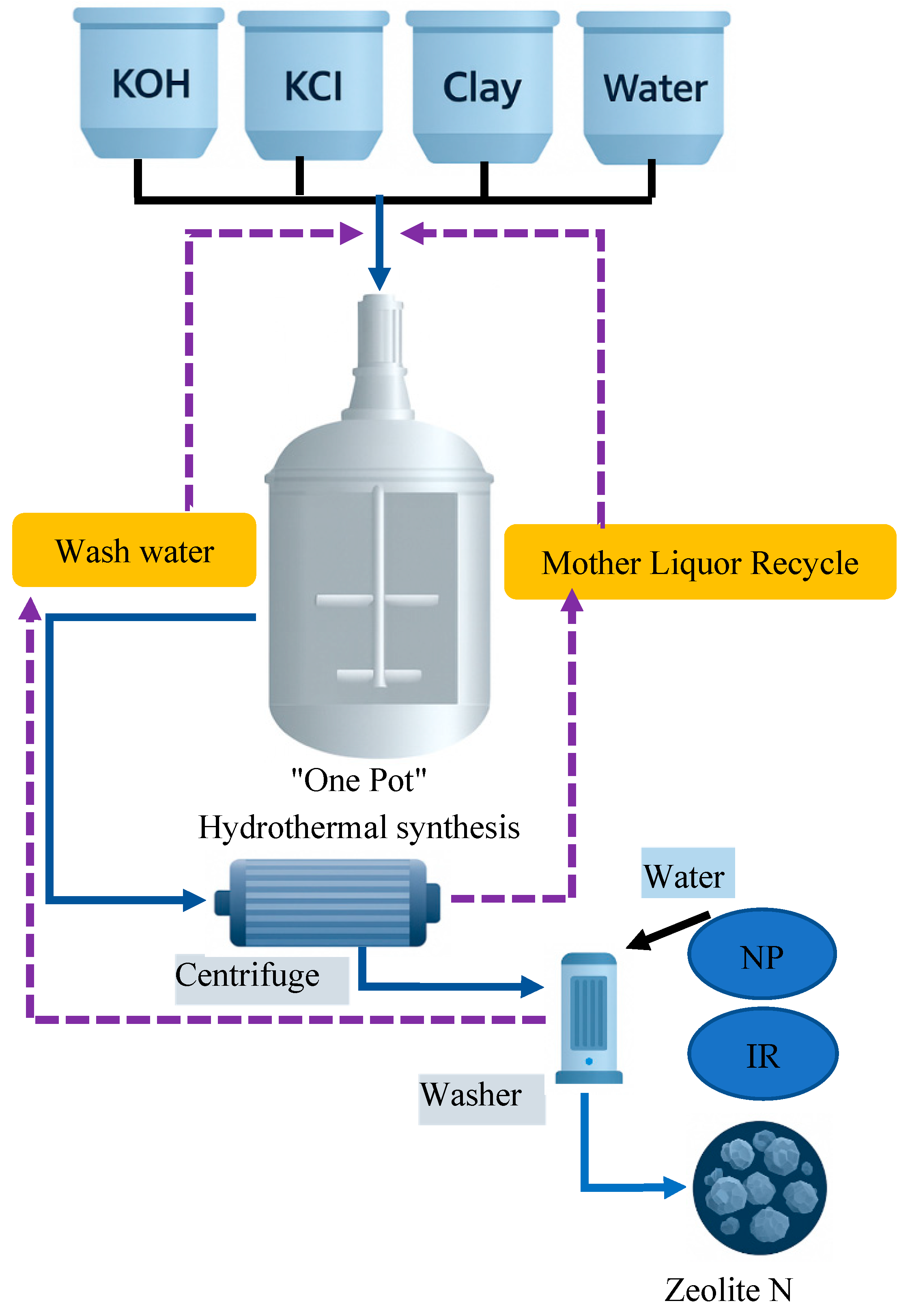
1.3.7. Alternative Water Sources for Zeolite Synthesis
2. Synthetic Zeolites
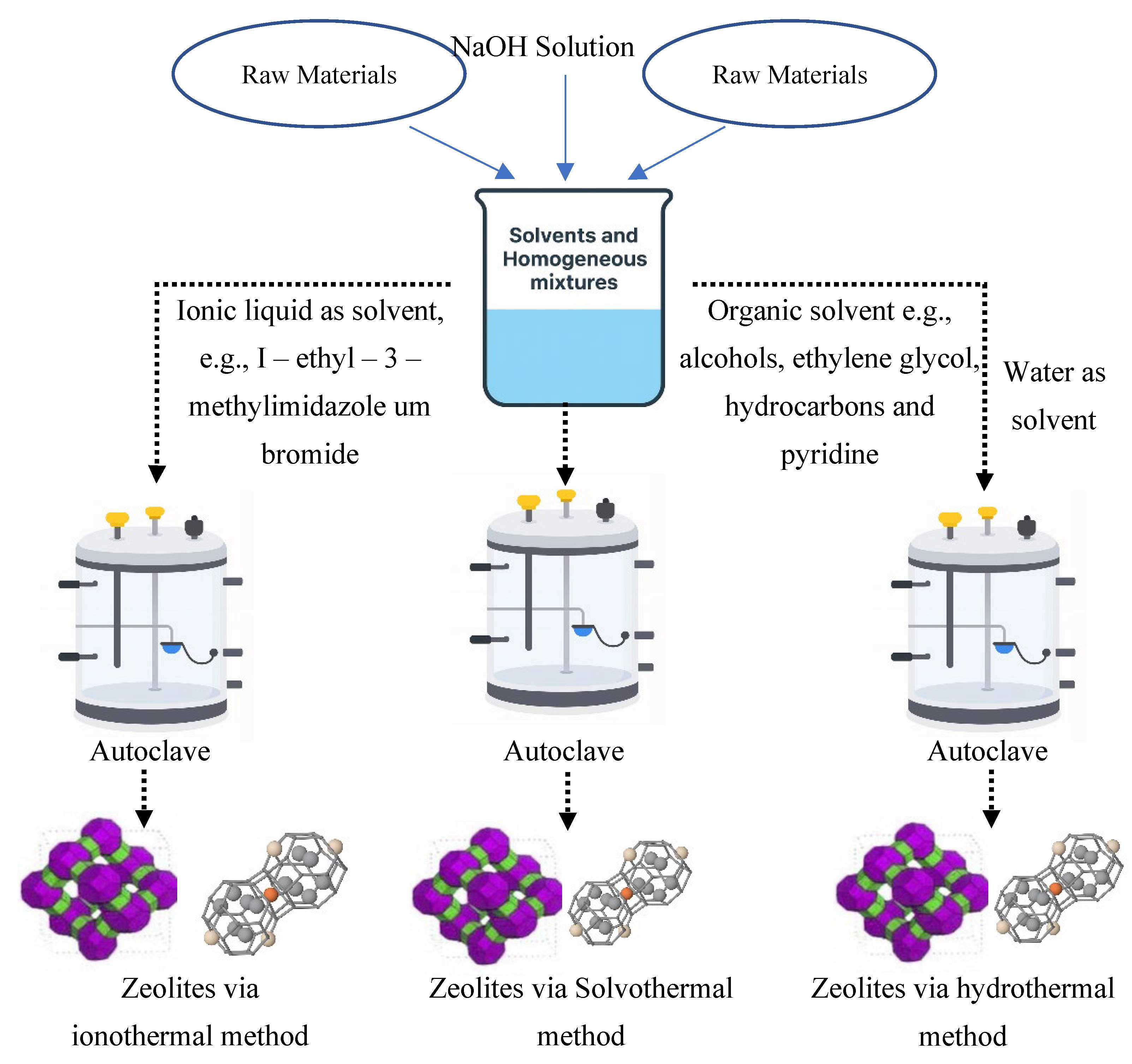
2.1. Hydrothermal Synthesis of Zeolites
2.2. Ionothermal Synthesis
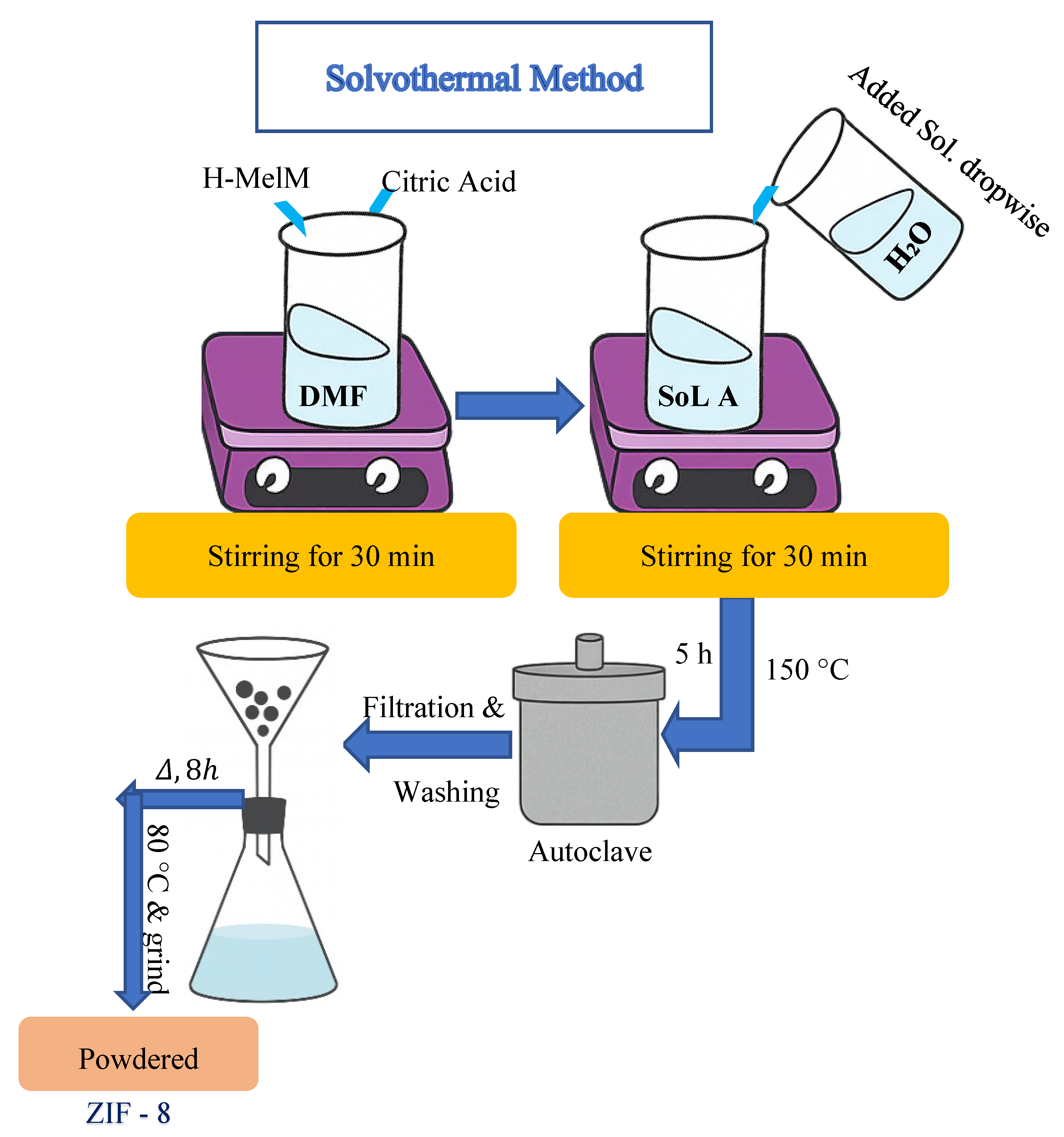
2.3. Solvothermal Method
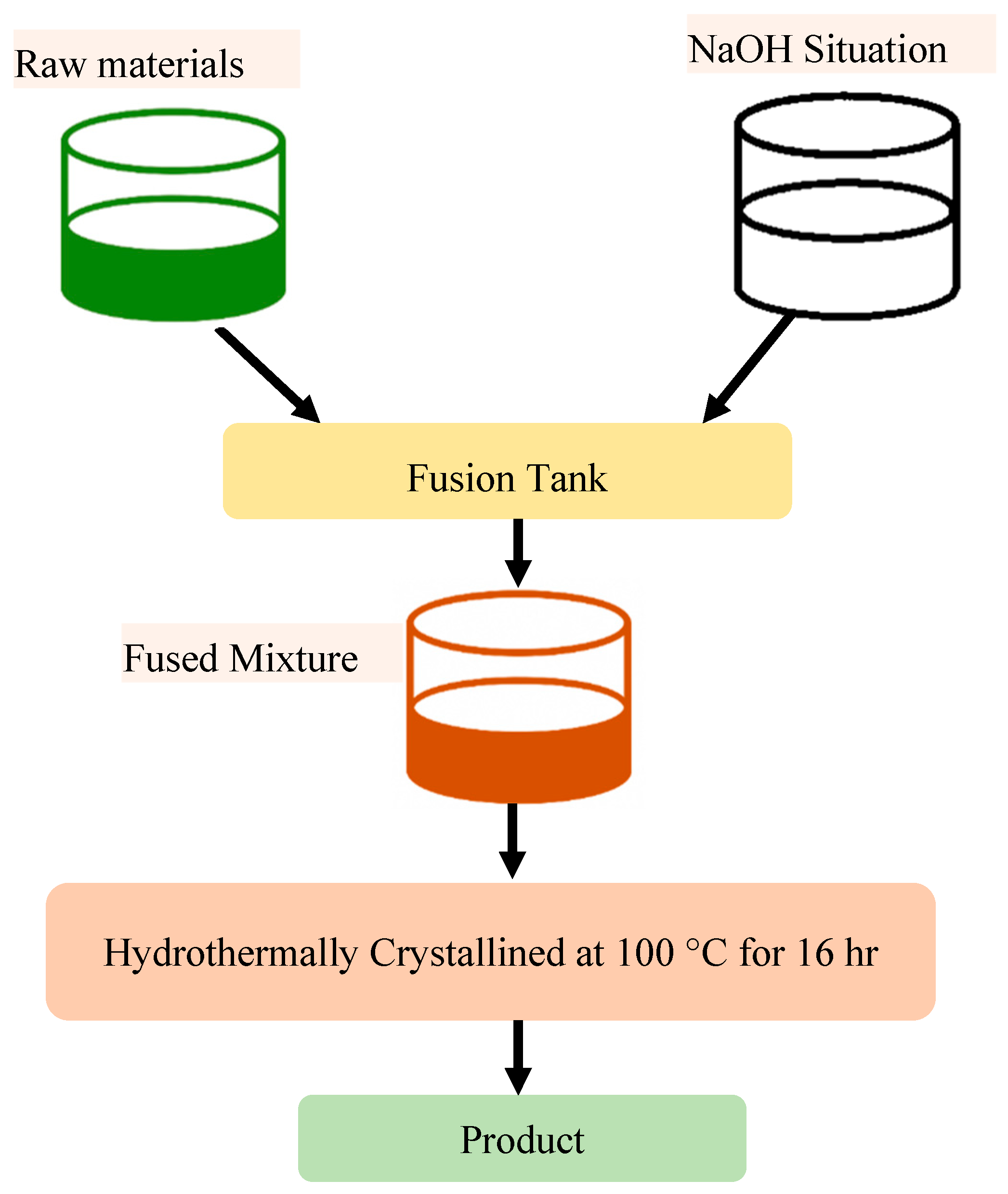
2.4. Alkali Fusion and Leaching Method
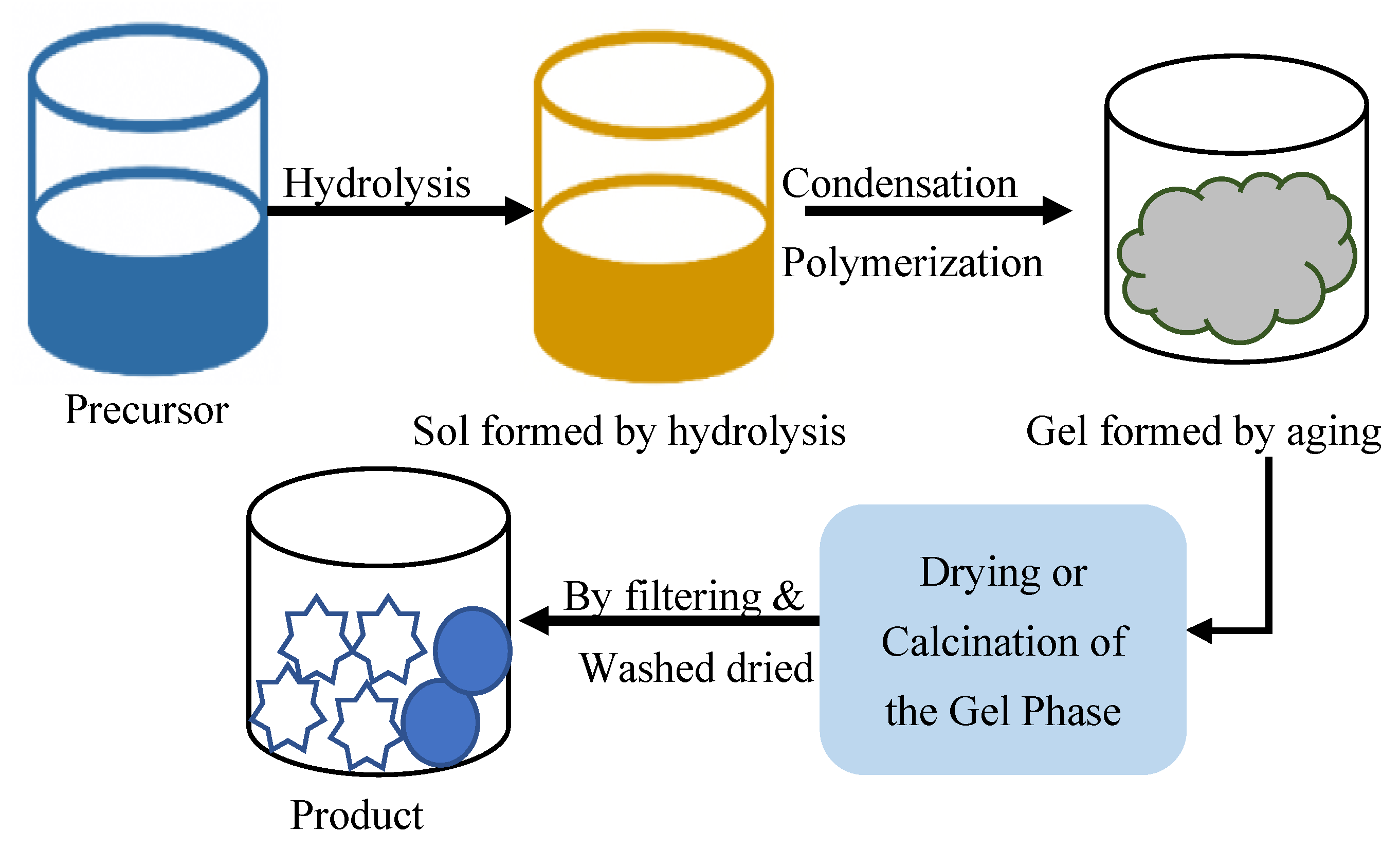
2.5. Sol–Gel Method
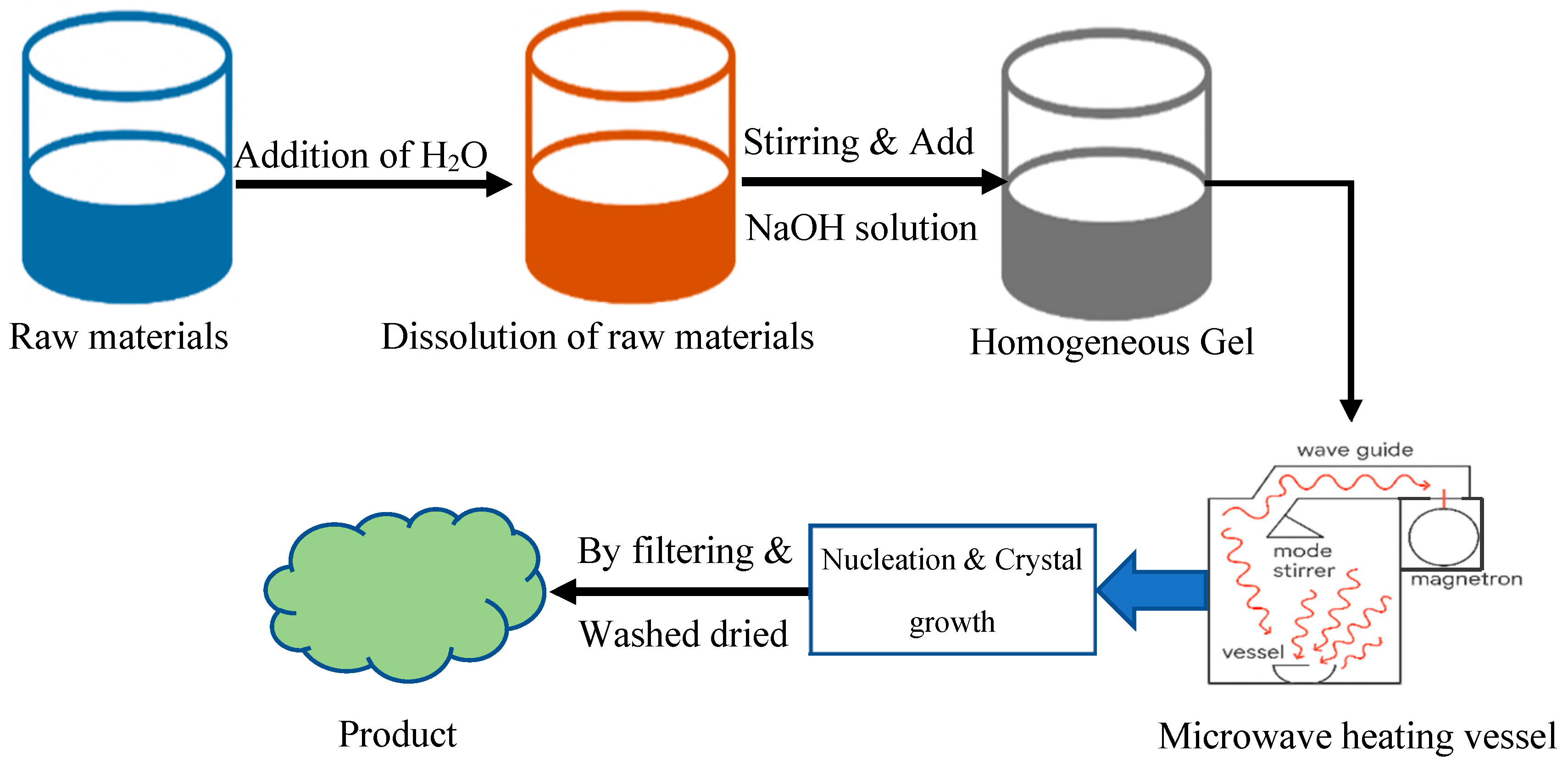
2.6. Microwave-Assisted Synthesis
2.7. Ultrasound Energy Method
3. Zeolites for Advanced Dental Applications
3.1. Functionalized Zeolite Frameworks for Dental Biomaterials
3.1.1. Silver Zeolite (AgZ): Superior Antimicrobial Functionality
3.1.2. Zinc Zeolite (ZnZ): Multifunctional Therapeutic Agent
3.1.3. Calcium Zeolite (CaZ): Remineralization Potential
3.1.4. Strontium Zeolite (SrZ): Enhanced Remineralization and Cellular Stimulation
3.1.5. Copper Zeolite (CuZ): Potent Antimicrobial and Adjunctive Agent
3.2. Clinical Applications of Zeolites in Modern Dentistry
3.2.1. Microbiological Applications: Addressing Antimicrobial Resistance
3.2.2. Dental Adhesive Systems: Enhanced Bonding and Antibacterial Properties
3.2.3. Restorative Materials: Multifunctional Zeolite-Enhanced Composites
3.2.4. Glass Ionomer Cements: Extended Antimicrobial Properties
3.2.5. Mineral Trioxide Aggregate: Enhanced Antimicrobial Properties
3.2.6. Resin Cements: Selective Antimicrobial Efficacy
3.2.7. Root Canal Irrigation Solutions: Alternative Antimicrobial Approach
3.2.8. Acrylic Resin Prosthetic Materials: Sustained Antimicrobial Protection
3.2.9. Non-Acrylic Resins and Ceramics: Enhanced Properties and Performance
3.2.10. Dental Implants: Antimicrobial Surface Modifications
3.2.11. Oral Medicine: Diagnostic Applications
3.3. Limitations, Controversies, Comparison with Other Fillers, and Future Research Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, J.; Lang, S.; Mante, F.; Pavelić, K.; Ozer, F. Antimicrobial and Mechanical Effects of Zeolite Use in Dental Materials: A Systematic Review. Acta Stomatol. Croat. 2021, 55, 76–89. [Google Scholar] [CrossRef]
- Valencia, S. Zeolitic Microporous Materials and Their Applications. Molecules 2021, 26, 730. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Mahmodi, G.; Manouchehri, S.; Mashhadzadeh, A.H.; Khodadadi, M.; Servatan, M.; Ganjali, M.R.; Azambre, B.; Kim, S.-J.; Ramsey, J.D.; et al. Zeolite in Tissue Engineering: Opportunities and Challenges. MedComm 2020, 1, 5–34. [Google Scholar] [CrossRef]
- Buchwald, Z.; Sandomierski, M.; Voelkel, A. Calcium-Rich 13X Zeolite as a Filler with Remineralizing Potential for Dental Composites. ACS Biomater. Sci. Eng. 2020, 6, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.; Araya, L.M.; Palacio, D.A.; Sánchez-Sanhueza, G.A.; Pérez, E.G.; Solís, F.J.; Meléndrez, M.F.; Medina, C. Study of the Antibacterial Capacity of a Biomaterial of Zeolites Saturated with Copper Ions (Cu2+) and Supported with Copper Oxide (CuO) Nanoparticles. Nanomaterials 2023, 13, 2140. [Google Scholar] [CrossRef]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.M. An Overview of Zeolites: From Historical Background to Diverse Applications. Molecules 2025, 30, 4036. [Google Scholar] [CrossRef]
- Weisenberger, T.B.; Selbekk, R.S. Multi-Stage Zeolite Facies Mineralization in the Hvalfjördur Area, Iceland. Int. J. Earth Sci. 2009, 98, 985–999. [Google Scholar] [CrossRef]
- Millini, R.; Garbarino, G. Zeolites: Historical Evolution and Current Relevance. In Zeolites: From Fundamentals to Emerging Applications; Royal Society of Chemistry: Cambridge, UK, 2025; pp. 1–33. [Google Scholar]
- Chudasama, C.D.; Sebastian, J.; Jasra, R.V. Pore-Size Engineering of Zeolite A for the Size/Shape Selective Molecular Separation. Ind. Eng. Chem. Res. 2005, 44, 1780–1786. [Google Scholar] [CrossRef]
- Chen, S.; Popovich, J.; Iannuzo, N.; Haydel, S.E.; Seo, D.-K. Silver-Ion-Exchanged Nanostructured Zeolite X as Antibacterial Agent with Superior Ion Release Kinetics and Efficacy against Methicillin-Resistant Staphylococcus Aureus. ACS Appl. Mater. Interfaces 2017, 9, 39271–39282. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, H.; Liu, X.; Li, R.; Xia, T.; Ma, Y.; Wang, Y.; Chen, K.; Wang, J.; Zeng, P.; et al. The Isomorphous Substitution of Si(4Al) with P in FAU Zeolite and Its Stabilization Effect. Chem. Eng. J. 2024, 486, 150422. [Google Scholar] [CrossRef]
- Zhang, H.; Samsudin, I.B.; Jaenicke, S.; Chuah, G.-K. Zeolites in Catalysis: Sustainable Synthesis and Its Impact on Properties and Applications. Catal. Sci. Technol. 2022, 12, 6024–6039. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Powers, J. Craig’s Restorative Dental Materials, 13th ed.; Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Eren, S.; Türk, F.N.; Arslanoğlu, H. Synthesis of Zeolite from Industrial Wastes: A Review on Characterization and Heavy Metal and Dye Removal. Environ. Sci. Pollut. Res. 2024, 31, 41791–41823. [Google Scholar] [CrossRef]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended Nomenclature for Zeolite Minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Mineral. Mag. 1998, 62, 533–571. [Google Scholar] [CrossRef]
- Bensafi, B.; Chouat, N.; Djafri, F. The Universal Zeolite ZSM-5: Structure and Synthesis Strategies. Coord. Chem. Rev. 2023, 496, 215397. [Google Scholar] [CrossRef]
- Darmansyah, D.; You, S.J.; Wang, Y.F. Advancements of Coal Fly Ash and Its Prospective Implications for Sustainable Materials in Southeast Asian Countries: A Review. Renew. Sustain. Energy Rev. 2023, 188, 113895. [Google Scholar] [CrossRef]
- Toniolo, N. Novel Geopolymers Incorporating Silicate Waste, Neuartige Geopolymere Aus Silikatischen Industrieabfällen. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2019. [Google Scholar]
- Ndlovu, N.Z.N.; Ameh, A.E.; Petrik, L.F.; Ojumu, T.V. Synthesis and Characterisation of Pure Phase ZSM-5 and Sodalite Zeolites from Coal Fly Ash. Mater. Today Commun. 2023, 34, 105436. [Google Scholar] [CrossRef]
- Panda, L.; Dash, S. Characterization and Utilization of Coal Fly Ash: A Review. Emerg. Mater. Res. 2020, 9, 921–934. [Google Scholar] [CrossRef]
- Cao, C.; Xuan, W.; Yan, S.; Wang, Q. Zeolites Synthesized from Industrial and Agricultural Solid Waste and Their Applications: A Review. J. Environ. Chem. Eng. 2023, 11, 110898. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Wang, Y.; Chen, H.; Liu, J. Modeling Study of the Slag Behaviors Based on Temperature in Pulverized Coal Furnace. ACS Omega 2022, 7, 2924–2934. [Google Scholar] [CrossRef]
- Song, W.; Tang, L.; Zhu, X.; Wu, Y.; Rong, Y.; Zhu, Z.; Koyama, S. Fusibility and Flow Properties of Coal Ash and Slag. Fuel 2009, 88, 297–304. [Google Scholar] [CrossRef]
- Ren, L.; Ding, L.; Guo, Q.; Gong, Y.; Yu, G.; Wang, F.C. Characterization, Carbon-Ash Separation and Resource Utilization of Coal Gasification Fine Slag: A Comprehensive Review. J. Clean. Prod. 2023, 398, 136554. [Google Scholar] [CrossRef]
- Sun, X.; Sun, R.; Liu, D.; Liu, Z.; Wang, D.; Cao, W.; Zhao, Y. Preparation of Coal Gasification Coarse Slag-Based Alkaline Activator and Its Activation Mechanism in Alkali-Activated Slag. Cem. Concr. Compos. 2024, 152, 105648. [Google Scholar] [CrossRef]
- Siddique, R.; Kunal; Mehta, A. Utilization of Industrial By-Products and Natural Ashes in Mortar and Concrete. In Nonconventional Vernacular Construction Materials: Characterisation, Properties and Applications; Elsevier: Cambridge, UK, 2020; pp. 247–303. [Google Scholar]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Silica Extraction from Rice Husk: Comprehensive Review and Applications. Hybrid Adv. 2023, 4, 100111. [Google Scholar] [CrossRef]
- Hossain, S.; Roy, P.K.; Bae, C.J. Utilization of Waste Rice Husk Ash for Sustainable Geopolymer: A Review. Constr. Build. Mater. 2021, 310, 125218. [Google Scholar] [CrossRef]
- Prasad, D.S.; Sanjana, B.; Kiran, D.S.; Kumar, P.P.S.; Ratheesh, R. A Unique Sustainable Chemical Method for the Recovery of Pure Silicon from Waste Crystalline Silicon Solar Panels. Sustain. Mater. Technol. 2023, 37, e00671. [Google Scholar] [CrossRef]
- Lee, W.H.; Lin, Y.W.; Lin, K.L. Optimization of Synthesis Parameters for the Preparation of Zeolite by Reusing Industrial Waste as Raw Material: Sandblasting Waste and Solar Panel Waste Glass. Solid State Sci. 2023, 143, 107277. [Google Scholar] [CrossRef]
- Pazouki, G.; Tao, Z.; Saeed, N.; Kang, W.H. Using Artificial Intelligence Methods to Predict the Compressive Strength of Concrete Containing Sugarcane Bagasse Ash. Constr. Build. Mater. 2023, 409, 134047. [Google Scholar] [CrossRef]
- Dewajani, H.; Zamrudy, W.; Irfin, Z.; Ningtyas, D.; Ridlo, N.M. Utilization of Indonesian Sugarcane Bagasse into Bio Asphalt through Pyrolysis Process Using Zeolite-Based Catalyst. Mater. Today Proc. 2023, 87, 383–389. [Google Scholar] [CrossRef]
- Mambetova, M.; Dossumov, K.; Baikhamurova, M.; Yergaziyeva, G. Sorbents Based on Natural Zeolites for Carbon Dioxide Capture and Removal of Heavy Metals from Wastewater: Current Progress and Future Opportunities. Processes 2024, 12, 2071. [Google Scholar] [CrossRef]
- Lyra, G.; Borrachero, M.V.; Soriano, L.; Paya, J.J.; Rossignolo, J.A. Comparison of Original and Washed Pure Sugar Cane Bagasse Ashes as Supplementary Cementing Materials. Constr. Build. Mater. 2021, 272, 122001. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Solangi, N.H.; Kumar, J.; Mazari, S.A.; Ahmed, S.; Fatima, N.; Mubarak, N.M. Development of Fruit Waste Derived Bio-Adsorbents for Wastewater Treatment: A Review. J. Hazard. Mater. 2021, 416, 125848. [Google Scholar] [CrossRef]
- Behin, J.; Bukhari, S.S.; Dehnavi, V.; Kazemian, H.; Rohani, S. Using Coal Fly Ash and Wastewater for Microwave Synthesis of LTA Zeolite. Chem. Eng. Technol. 2014, 37, 1532–1540. [Google Scholar] [CrossRef]
- Kordala, N.; Wyszkowski, M. Zeolite Properties, Methods of Synthesis, and Selected Applications. Molecules 2024, 29, 1069. [Google Scholar] [CrossRef]
- Probst, J.; Couperthwaite, S.J.; Millar, G.J.; Kaparaju, P. Ammoniacal Nitrogen Removal and Reuse: Process Engineering Design and Technoeconomics of Zeolite N Synthesis. J. Environ. Chem. Eng. 2022, 10, 107942. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Zeolite Synthesis from Coal Fly Ash by Hydrothermal Reaction Using Various Alkali Sources. J. Chem. Technol. Biotechnol. 2002, 77, 280–286. [Google Scholar] [CrossRef]
- Visa, M.; Enesca, A. Opportunities for Recycling PV Glass and Coal Fly Ash into Zeolite Materials Used for Removal of Heavy Metals (Cd, Cu, Pb) from Wastewater. Materials 2023, 16, 239. [Google Scholar] [CrossRef]
- Kim, N.S.; Numan, M.; Nam, S.C.; Park, S.E.; Jo, C. Dynamic Adsorption/Desorption of p-Xylene on Nanomorphic MFI Zeolites: Effect of Zeolite Crystal Thickness and Mesopore Architecture. J. Hazard. Mater. 2021, 403, 123659. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Grand, J.; Čaučo, O.; Mintova, S.; Patarin, J. Mechanism of Zeolite Crystal Growth: New Findings and Open Questions. CrystEngComm 2016, 18, 650–664. [Google Scholar] [CrossRef]
- Chen, Q.; Long, L.; Liu, X.; Liu, X.; Jiang, Y.; Chi, J.; Yan, X.; Zhao, L.; Kong, L. Low-Toxic Zeolite Fabricated from Municipal Solid Waste Incineration Fly Ash via Microwave-Assisted Hydrothermal Process with Fusion Pretreatment. J. Mater. Cycles Waste Manag. 2020, 22, 1196–1207. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Guo, Z.; Liu, Y. The Application of Power Ultrasound to Reaction Crystallization. Ultrason. Sonochem. 2006, 13, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of Coal Fly Ash to Zeolite Utilizing Microwave and Ultrasound Energies: A Review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Chon, S.K.; Ihm, Y.S.; Uh, W. Progress in Zeolite and Microporous Materials; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Jusoh, N.; Yeong, Y.F.; Mohamad, M.; Lau, K.K.; Shariff, A.M. Rapid-Synthesis of Zeolite T via Sonochemical-Assisted Hydrothermal Growth Method. Ultrason. Sonochem. 2016, 34, 273–280. [Google Scholar] [CrossRef]
- Barrer, R.M. Hydrothermal Chemistry of Zeolites; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Parnham, E.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic Liquids and Eutectic Mixtures as Solvent and Template in Synthesis of Zeolite Analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Alam, M.; Hoque, M.; Mondal, S.; Haider, J.B.; Xu, B.; Johir, M.A.H.; Kris, A.; Moni, M.A.; Zhou, J.L.; et al. Zeolite Synthesis from Low-Cost Materials and Environmental Applications: A Review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Guo, K.; Lin, X.; Meng, P.; Huang, Y.; Wei, R.; Zhang, R. Ionothermal Synthesis of Germanosilicate Zeolites Constructed with Double-Four-Ring Structure-Building Units in the Presence of Organic Base. Chem.-Asian J. 2019, 14, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, C.; Baskar, P. Zeolite and Its Potential Uses in Agriculture: A Critical Review. Agric. Rev. 2016, 37, 94–104. [Google Scholar] [CrossRef]
- Johnson, E.B.G.; Arshad, S.E.B. Hydrothermally Synthesized Zeolites Based on Kaolinite: A Review. Appl. Clay Sci. 2014, 97–98, 215–221. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Effect of Calcination Temperature on Electrochemical Behavior of Pristine Zeolitic Imidazolate Framework-8. Ionics 2022, 28, 4265–4274. [Google Scholar] [CrossRef]
- Hart, A.; Wood, J. Methodological Review of Zeolite Synthesis from Industrial Waste and Natural Clays and the Fabrication of Hierarchical Pore Structures. Mater 2025, 9, 101113. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S. Solvothermal Synthesis of Porous Hydrangea-like Zeolitic Imidazole Framework-8 (ZIF-8) Crystals. J. Solid State Chem. 2019, 276, 123–129. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Niceforo, G.; Lettino, A. Sodalite, Faujasite and A-Type Zeolite from 2:1 Dioctahedral and 2:1:1 Trioctahedral Clay Minerals. A Singular Review of Synthesis Methods through Laboratory Trials at a Low Incubation Temperature. Powder Technol. 2017, 320, 69–85. [Google Scholar] [CrossRef]
- Christidis, G.E.; Papantoni, H. Synthesis of FAU Type Zeolite Y from Natural Raw Materials: Hydrothermal SiO2-Sinter and Perlite Glass. Open Mineral. J. 2008, 2, 1–5. [Google Scholar] [CrossRef]
- Ma, H.; Yao, Q.; Fu, Y.; Dong, X. Synthesis of Zeolite of Type A from Bentonite by Alkali Fusion Activation Using Na2CO3. Ind. Eng. Chem. Res. 2010, 49, 454–458. [Google Scholar] [CrossRef]
- Yoldi, M.; Fuentes-Ordoñez, E.G.; Korili, S.A.; Gil, A. Zeolite Synthesis from Industrial Wastes. Microporous Mesoporous Mater. 2019, 284, 139–155. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–Gel Based Materials for Biomedical Applications. Prog. Mater. Sci. 2016, 81, 1–79. [Google Scholar] [CrossRef]
- Brinker, C.J.; Sehgal, R.; Hietala, S.L.; Deshpande, R.; Smith, D.M.; Loy, D.; Ashley, C.S. Sol-Gel Strategies for Controlled Porosity Inorganic Materials. J. Membr. Sci. 1994, 94, 85–102. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Rao, K.J.; Vaidhyanathan, B.; Ganguli, M.; Ramakrishnan, P. Synthesis of Inorganic Solids Using Microwaves. Chem. Mater. 1999, 11, 882–895. [Google Scholar] [CrossRef]
- Xu, X.; Yang, W.; Liu, J.; Lin, L. Synthesis of a High-Permeance NaA Zeolite Membrane by Microwave Heating. Adv. Mater. 2000, 12, 195–198. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W. Microwave Synthesis of Zeolite Membranes: A Review. J. Membr. Sci. 2008, 316, 3–17. [Google Scholar] [CrossRef]
- Kim, D.S.; Chang, J.-S.; Hwang, J.-S.; Park, S.-E.; Kim, J.M. Synthesis of Zeolite Beta in Fluoride Media under Microwave Irradiation. Microporous Mesoporous Mater. 2004, 70, 29–37. [Google Scholar] [CrossRef]
- Ou, X.; Xu, S.; Warnett, J.M.; Holmes, S.M.; Zaheer, A.; Garforth, A.A.; Williams, M.A.; Jiao, Y.; Fan, X. Creating Hierarchies Promptly: Microwave-Accelerated Synthesis of ZSM-5 Zeolites on Macrocellular Silicon Carbide (SiC) Foams. Chem. Eng. J. 2017, 313, 954–964. [Google Scholar] [CrossRef]
- Ren, X.; Ghazani, M.S.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and Opportunities in Microwave-Assisted Catalytic Pyrolysis of Biomass: A Review. Appl. Energy 2022, 323, 118970. [Google Scholar] [CrossRef]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications, 2nd ed.; Woodhead Publishing: Sawston, UK, 2002. [Google Scholar]
- Boels, L.; Wagterveld, R.M.; Mayer, M.J.; Witkamp, G.J. Seeded Calcite Sonocrystallization. J. Cryst. Growth 2010, 312, 961–966. [Google Scholar] [CrossRef]
- Suslick, K.S.; Doktycz, S.J.; Flint, E.B. On the Origin of Sonoluminescence and Sonochemistry. Ultrason. Sonochem. 1990, 1, S1–S3. [Google Scholar] [CrossRef]
- Andaç, Ö.; Tatlıer, M.; Sirkecioğlu, A.; Ece, I.; Erdem-Şenatalar, A. Effects of Ultrasound on Zeolite A Synthesis. Microporous Mesoporous Mater. 2005, 79, 75–83. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, Y.; Fan, J.; Guo, C. Effect of Ultrasound Pretreatment on the Hydrothermal Synthesis of SSZ-13 Zeolite. Ultrason. Sonochem. 2017, 38, 476–481. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, P.; Arandiyan, H.; Guan, Y.; Liu, J.; Wang, N.; Jiao, Y.; Fan, X. Using Ultrasound to Improve the Sequential Post-Synthesis Modification Method for Making Mesoporous Y Zeolites. Front. Chem. Sci. Eng. 2020, 14, 1101–1110. [Google Scholar] [CrossRef]
- Li, L.J.; Lung, C.Y.-K.; Ge, K.X.; Song, K.; Chu, C.-H.; Yu, O.Y. Developing a Novel Calcium Silver Zeolite for Caries Management. BMC Oral Health 2024, 24, 1098. [Google Scholar] [CrossRef]
- Tan, L.; Yuan, G.; Wang, P.; Feng, S.; Tong, Y.; Wang, C. pH-Responsive Ag-Phy@ZIF-8 Nanoparticles Modified by Hyaluronate for Efficient Synergistic Bacteria Disinfection. Int. J. Biol. Macromol. 2022, 206, 605–613. [Google Scholar] [CrossRef]
- Chebbi, M.; Azambre, B.; Cantrel, L.; Huvé, M.; Albiol, T. Influence of Structural, Textural and Chemical Parameters of Silver Zeolites on the Retention of Methyl Iodide. Microporous Mesoporous Mater. 2017, 244, 137–150. [Google Scholar] [CrossRef]
- Saengmee-anupharb, S.; Srikhirin, T.; Thaweboon, B.; Thaweboon, S.; Amornsakchai, T.; Dechkunakorn, S.; Suddhasthira, T. Antimicrobial Effects of Silver Zeolite, Silver Zirconium Phosphate Silicate and Silver Zirconium Phosphate against Oral Microorganisms. Asian Pac. J. Trop. Biomed. 2013, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, J.; Hrenovic, J.; Matijasevic, D.; Niksic, M.; Rajic, N. Bactericidal Activity of Cu-, Zn-, and Ag-Containing Zeolites toward Escherichia Coli Isolates. Environ. Sci. Pollut. Res. 2017, 24, 20273–20281. [Google Scholar] [CrossRef]
- Mallette, A.J.; Hong, S.; Freeman, E.E.; Saslow, S.A.; Mergelsberg, S.; Motkuri, R.K.; Neeway, J.J.; Mpourmpakis, G.; Rimer, J.D. Heteroatom Manipulation of Zeolite Crystallization: Stabilizing Zn-FAU against Interzeolite Transformation. JACS Au 2022, 2, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Zinc Oxide Nanoparticles Impacts: Cytotoxicity, Genotoxicity, Developmental Toxicity, and Neurotoxicity. Crit. Rev. Toxicol. 2019, 49, 300–311. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Okulus, Z.; Sandomierski, M.; Zielińska, M.; Buchwald, T.; Voelkel, A. Zeolite Fillers for Resin-Based Composites with Remineralizing Potential. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Dotta, T.C.; Hayann, L.; de P. A. Almeida, L.; Nogueira, L.F.B.; Arnez, M.M.; Castelo, R.; Cassiano, A.F.B.; Faria, G.; Martelli-Tosi, M.; Bottini, M.; et al. Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization. J. Funct. Biomater. 2022, 13, 250. [Google Scholar] [CrossRef]
- Cao, Z.; Cai, X.; Feltrin, A.C.; Feng, P.; Kaiser, A.; Akhtar, F. Calcium/Strontium Chloride Impregnated Zeolite A and X Granules as Optimized Ammonia Sorbents. RSC Adv. 2022, 12, 35115–35122. [Google Scholar] [CrossRef]
- Rieger, K.A.; Cho, H.J.; Yeung, H.F.; Fan, W.; Schiffman, J.D. Antimicrobial Activity of Silver Ions Released from Zeolites Immobilized on Cellulose Nanofiber Mats. ACS Appl. Mater. Interfaces 2016, 8, 3032–3040. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric Materials with Antimicrobial Activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of Bactericidal Action of Silver Zeolite and Its Comparison with That of Silver Nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef]
- Sabbani, S.; Gallego-Perez, D.; Nagy, A.; Waldman, W.J.; Hansford, D.; Dutta, P.K. Synthesis of Silver-Zeolite Films on Micropatterned Porous Alumina and Its Application as an Antimicrobial Substrate. Microporous Mesoporous Mater. 2010, 135, 131–136. [Google Scholar] [CrossRef]
- Fox, S.; Wilkinson, T.S.; Wheatley, P.S.; Xiao, B.; Morris, R.E.; Sutherland, A.; Simpson, A.J.; Barlow, P.G.; Butler, A.R.; Megson, I.L.; et al. NO-Loaded Zn2+-Exchanged Zeolite Materials: A Potential Bifunctional Anti-Bacterial Strategy. Acta Biomater. 2010, 6, 1515–1521. [Google Scholar] [CrossRef]
- Li, W.; Qi, M.; Sun, X.; Chi, M.; Wan, Y.; Zheng, X.; Li, C.; Wang, L.; Dong, B. Novel Dental Adhesive Containing Silver Exchanged EMT Zeolites against Cariogenic Biofilms to Combat Dental Caries. Microporous Mesoporous Mater. 2020, 298, 110113. [Google Scholar] [CrossRef]
- Reddy, Y.N.; De, A.; Paul, S.; Pujari, A.K.; Bhaumik, J. In Situ Nanoarchitectonics of a MOF Hydrogel: A Self-Adhesive and pH-Responsive Smart Platform for Phototherapeutic Delivery. Biomacromolecules 2023, 24, 1717–1730. [Google Scholar] [CrossRef]
- Bim-Júnior, O.; Gaglieri, C.; Bedran-Russo, A.K.; Bueno-Silva, B.; Bannach, G.; Frem, R.; Ximenes, V.F.; Lisboa-Filho, P.N. MOF-Based Erodible System for On-Demand Release of Bioactive Flavonoid at the Polymer–Tissue Interface. ACS Biomater. Sci. Eng. 2020, 6, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- El-Guindy, J.; Selim, M.; El-Agroudi, M. Alternative Pretreatment Modalities with a Self-Adhesive System to Promote Dentin/Alloy Shear Bond Strength. J. Prosthodont. 2010, 19, 205–211. [Google Scholar] [CrossRef]
- Bim-Junior, O.; Alania, Y.; Tabatabaei, F.S.; Frem, R.; Bedran-Russo, A.K.; Lisboa-Filho, P.N. Biomimetic Growth of Metal–Organic Frameworks for the Stabilization of the Dentin Matrix and Control of Collagenolysis. Langmuir 2022, 38, 1600–1610. [Google Scholar] [CrossRef]
- Rüttermann, S.; Trellenkamp, T.; Bergmann, N.; Raab, W.H.-M.; Ritter, H.; Janda, R. A New Approach to Influence Contact Angle and Surface Free Energy of Resin-Based Dental Restorative Materials. Acta Biomater. 2011, 7, 1160–1165. [Google Scholar] [CrossRef]
- Sandomierski, M.; Buchwald, Z.; Koczorowski, W.; Voelkel, A. Calcium Forms of Zeolites A and X as Fillers in Dental Restorative Materials with Remineralizing Potential. Microporous Mesoporous Mater. 2019, 284, 109899. [Google Scholar] [CrossRef]
- Hotta, M.; Nakajima, H.; Yamamoto, K.; Aono, M. Antibacterial Temporary Filling Materials: The Effect of Adding Various Ratios of Ag-Zn-Zeolite. J. Oral Rehabil. 1998, 25, 485–489. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.; Kim, K.N.; Lee, Y.K. Antibacterial Effect of Silver-Zeolites in Glass-Ionomer Cements. Key Eng. Mater. 2007, 330–332, 831–834. [Google Scholar] [CrossRef]
- Kim, H.-J.; Son, J.S.; Kim, K.-H.; Kwon, T.-Y. Antimicrobial Activity of Glass Ionomer Cement Incorporated with Chlorhexidine-Loaded Zeolite Nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 1450–1453. [Google Scholar] [CrossRef]
- McDougall, I.G.; Patel, V.; Santerre, P.; Friedman, S. Resistance of Experimental Glass Ionomer Cement Sealers to Bacterial Penetration in Vitro. J. Endod. 1999, 25, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Ghatole, K.; Patil, A.; Giriyappa, R.H.; Singh, T.V.; Jyotsna, S.V.; Rairam, S. Evaluation of Antibacterial Efficacy of MTA with and without Additives like Silver Zeolite and Chlorhexidine. J. Clin. Diagn. Res. 2016, 10, ZC11–ZC14. [Google Scholar] [CrossRef]
- Odabaş, M.E.; Çinar, C.; Akça, G.; Araz, İ.; Ulusu, T.; Yücel, H. Short-Term Antimicrobial Properties of Mineral Trioxide Aggregate with Incorporated Silver-Zeolite. Int. Endod. J. 2011, 44, 510–515. [Google Scholar] [CrossRef]
- Çinar, C.; Odabaş, M.; Güler, M.A.; Baldağ, I. The Effects of Incorporation of Silver-Zeolite on Selected Properties of Mineral Trioxide Aggregate. Dent. Mater. J. 2013, 32, 872–876. [Google Scholar] [CrossRef]
- Ghasemi, N.; Rahimi, S.; Samiei, M.; Mohamadi, M.; Rezaei, Y.; Divband, B.; Farhangi, N. Effect of the Zeolite Containing Silver-Zinc Nanoparticles on the Push-out Bond Strength of Mineral Trioxide Aggregate in Simulated Furcation Perforation. J. Dent. Shiraz 2019, 20, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Ghasemi, N.; Asl-Aminabadi, N.; Divband, B.; Golparvar-Dashti, Y.; Shirazi, S. Zeolite-Silver-Zinc Nanoparticles: Biocompatibility and Their Effect on the Compressive Strength of Mineral Trioxide Aggregate. J. Clin. Exp. Dent. 2017, 9, e356–e360. [Google Scholar] [CrossRef] [PubMed]
- Ghivari, S.; Bhattacharya, H.; Bhat, K.; Pujar, M.A. Antimicrobial Activity of Root Canal Irrigants against Biofilm Forming Pathogens—An in Vitro Study. J. Conserv. Dent. 2017, 20, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, K.; Hayashi, T.; Sato, K.; Asai, T.; Okano, M.; Kominami, Y.; Takahashi, Y.; Kawai, T. Effect of Self-Cured Acrylic Resin Added with an Inorganic Antibacterial Agent on Streptococcus Mutans. Dent. Mater. J. 2010, 29, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Malic, S.; Rai, S.; Redfern, J.; Pritchett, J.; Liauw, C.M.; Verran, J.; Tosheva, L. Zeolite-Embedded Silver Extends Antimicrobial Activity of Dental Acrylics. Colloids Surf. B Biointerfaces 2019, 173, 719–726. [Google Scholar] [CrossRef]
- Casemiro, L.A.; Martins, C.H.G.; Pires-de-Souza, F.C.P.; Panzeri, H. Antimicrobial and Mechanical Properties of Acrylic Resins with Incorporated Silve–Zinc Zeolite–Part I. Gerodontology 2008, 25, 187–194. [Google Scholar] [CrossRef]
- Yadav, N.S.; Saraf, S.; Mishra, S.K.; Hazari, P. Effects of Fluconazole, Chlorhexidine Gluconate, and Silver-Zinc Zeolite on Flexural Strength of Heat-Cured Polymethyl Methacrylate Resin. J. Nat. Sci. Biol. Med. 2015, 6, 340–342. [Google Scholar] [CrossRef]
- Nakanoda, S.; Nikawa, H.; Hamada, T.; Nakamoto, K. The Material and Antifungal Properties of Antibiotic Zeolite Incorporated Acrylic Resin. Nihon Hotetsu Shika Gakkai Zasshi J. Jpn. Prosthodont. Soc. 1995, 39, 919–926. [Google Scholar] [CrossRef]
- Saravanan, M.; Anandkumar, V.; Padmanabhan, T.V.; Banu, F. Viscoelastic Properties and Antimicrobial Effects of Soft Liners with Silver Zeolite in Complete Dental Prosthesis Wearers: An in Vivo Study. Int. J. Prosthodont. 2015, 28, 265–269. [Google Scholar] [CrossRef][Green Version]
- Naji, G.A.-H.; Omar, R.A.; Yahya, R. Effect of Sintering Temperature on the Microstructures and Mechanical Properties of Sodalite Infiltrate All-Ceramic Material for Dental Restorations. J. Mech. Behav. Mater. 2018, 117, 71–76. [Google Scholar] [CrossRef]
- Naji, G.A.-H.; Omar, R.A.; Yahya, R. The Effect of Sodalite Zeolite Infiltrated Material on the Fracture Toughness, Elastic Modulus and Optical Properties of All-Ceramic Dental Prostheses. Ceram. Int. 2017, 43, 1741–1747. [Google Scholar] [CrossRef]
- Naji, G.A.-H.; Omar, R.A.; Yahya, R.; Dabbagh, A. Sodalite Zeolite as an Alternative All-Ceramic Infiltrating Material for Alumina and Zirconia Toughened Alumina Frameworks. Ceram. Int. 2016, 42, 11861–11866. [Google Scholar] [CrossRef]
- Naji, G.A.-H.; Omar, R.A.; Yahya, R. Influence of Sodalite Zeolite Infiltration on the Coefficient of Thermal Expansion and Bond Strength of All-Ceramic Dental Prostheses. J. Mech. Behav. Biomed. Mater. 2017, 67, 20–26. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Guo, S.; Zhang, J.; Song, Y.; Dong, X.; Wang, X.; Yu, J. Antibacterial and Anti-Adhesive Zeolite Coatings on Titanium Alloy Surface. Microporous Mesoporous Mater. 2011, 145, 97–103. [Google Scholar] [CrossRef]
- Shigeyama, H.; Wang, T.; Ichinose, M.; Ansai, T.; Lee, S.-W. Identification of Volatile Metabolites in Human Saliva from Patients with Oral Squamous Cell Carcinoma via Zeolite-Based Thin-Film Microextraction Coupled with GC–MS. J. Chromatogr. B 2019, 1104, 49–58. [Google Scholar] [CrossRef]
- Mochalski, P.; Sponring, A.; King, J.; Amann, A. Release and Uptake of Volatile Organic Compounds by Human Hepatocellular Carcinoma Cells (HepG2) in Vitro. Cancer Cell Int. 2013, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Goda, S.; Selyanchyn, R.; Wang, T.; Nakazawa, K.; Hirano, T.; Matsui, H.; Lee, S.-W. In Vitro Detection of Small Molecule Metabolites Excreted from Cancer Cells Using a Tenax TA Thin-Film Microextraction Device. J. Chromatogr. B 2015, 1006, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Milašin, I.S.; Eken, Z.B.; Mravak-Stipetić, M.; Pavelić, K.; Ozer, F. Effects of Zeolite as a Drug Delivery System on Cancer Therapy: A Systematic Review. Molecules 2021, 26, 6196. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart Dental Materials for Antimicrobial Applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; Jun, Z. Influence of Zeolite Addition on Mechanical Performance and Shrinkage of High Strength Engineered Cementitious Composites. J. Build. Eng. 2021, 36, 102124. [Google Scholar] [CrossRef]
- Pandya, T.; Patel, S.; Kulkarni, M.; Singh, Y.R.; Khodakiya, A.; Bhattacharya, S.; Prajapati, B.G. Zeolite-Based Nanoparticles Drug Delivery Systems in Modern Pharmaceutical Research and Environmental Remediation. Heliyon 2024, 10, e36417. [Google Scholar] [CrossRef]
- Vogelsang, C.; Umar, M. Municipal Solid Waste Fly Ash-Derived Zeolites as Adsorbents for the Recovery of Nutrients and Heavy Metals—A Review. Water 2023, 15, 3817. [Google Scholar] [CrossRef]
- Al-Haddad, A.; Alarami, N.; Alshammari, A.F.; Madfa, A.A. Efficacy of Bioactive Glass-Based Desensitizer Compared to Other Desensitizing Agents or Techniques in Dentin Hypersensitivity: A Systematic Review. BMC Oral Health 2025, 25, 899. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Berakdar, G.A.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Sarvaiya, B.B.; Kumar, S.; Pathan, M.S.H.; Patel, S.; Gupta, V.; Haque, M. The Impact of Implant Surface Modifications on the Osseointegration Process: An Overview. Cureus 2025, 17, e81576. [Google Scholar] [CrossRef] [PubMed]
- Guntermann, L.; Rohrbach, A.; Schäfer, E.; Dammaschke, T. Remineralization and Protection from Demineralization: Effects of a Hydroxyapatite-Containing, a Fluoride-Containing and a Fluoride- and Hydroxyapatite-Free Toothpaste on Human Enamel In Vitro. Head Face Med. 2022, 18, 26. [Google Scholar] [CrossRef] [PubMed]
| Ion | Zeolite Frameworks Used | Dental Applications | Main Benefits | References |
|---|---|---|---|---|
| Silver (Ag+) | FAU (Zeolite X/Y), LTA, Na-A | Restorative composites, glass ionomer cements (GICs), mineral trioxide aggregate (MTA), resin cements, prosthetic acrylic polymers, and implant coatings | Sustained ion release prevents secondary caries; efficacious against S. mutans, E. faecalis, and C. albicans; broad-spectrum antimicrobial activity | [1,5,6,11] |
| Zinc (Zn2+) | LTA, Na-A, FAU | Dental adhesives, restorative composites, GICs, and implant coatings | inhibits dentin collagen degradation (MMP inhibition); exhibits antimicrobial, anti-inflammatory, and osteogenic properties | [2,3,4] |
| Calcium (Ca2+) | CAU, Na-A, LTA | Remineralizing additives in cements, adhesives, and composites | Enhances the remineralization of enamel/dentin, promotes hydroxyapatite regeneration, and aids in the prevention of caries | [16,40,90] |
| Strontium (Sr2+) | CAU, LTA | Restorative composites and regenerative materials | It serves a dual function by stimulating stem cells and remineralizing teeth, thereby improving the acid resistance of dental tissues. | [11,81,91] |
| Copper (Cu2+) | FAU, LTA | Restorative materials and antibacterial coatings | Strong antimicrobial effects, including those against resistant pathogens; can be combined with CuO nanoparticles to enhance their effectiveness |
| Attribute | Zeolites | Bioactive Glass (BAG) | Hydroxyapatite Nanoparticles (nHA) | References |
|---|---|---|---|---|
| Primary function(s) | Drug delivery via ion exchange, remineralization (Ca, Sr), antimicrobial (Ag, Zn) | Hydroxycarbonate apatite layer formation; desensitization; Ca/Si/P release | “Biomimetic remineralization; osteointegration (coatings)” | [1,128,131,132,133,134,135] |
| Release profile | Risk of chronic exposure if uncontrolled; tunable/sustained | Frequently, the initial explosion is followed by a reduction. | Low unless functionalized or enhanced | [128,131,132,133,134] |
| Antimicrobial activity | Broad spectrum; high with Ag/Cu exchange | Mostly pH/ionic, moderate | Modest unless altered | [1,129,131,135] |
| Mechanical properties | Interface Key; load/dispersion-sensitive | High pressures may cause brittleness. | Aggregation may deteriorate; compatibility is necessary. | [129,131,135] |
| Clinical maturity | Predominantly preclinical/bench; limited clinical | Implemented in desensitizers; select restoratives | Early clinical application in coatings and pastes; a smaller number of dental products that have been approved | [131,135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, S.F.; Yhaya, M.F.; Nongman, A.F.; Al-Rawas, M.; Arbilei, M.N.; Noorani, T.Y. From Industry to Dentistry: A Comprehensive Review of Zeolite as a Next-Generation Multifunctional Filler for Enhanced Mechanical Reinforcement and Antimicrobial Efficacy. Dent. J. 2025, 13, 540. https://doi.org/10.3390/dj13110540
Mohammed SF, Yhaya MF, Nongman AF, Al-Rawas M, Arbilei MN, Noorani TY. From Industry to Dentistry: A Comprehensive Review of Zeolite as a Next-Generation Multifunctional Filler for Enhanced Mechanical Reinforcement and Antimicrobial Efficacy. Dentistry Journal. 2025; 13(11):540. https://doi.org/10.3390/dj13110540
Chicago/Turabian StyleMohammed, Sohaib Fadhil, Mohd Firdaus Yhaya, Abdul Fattah Nongman, Matheel Al-Rawas, Marwan N. Arbilei, and Tahir Yusuf Noorani. 2025. "From Industry to Dentistry: A Comprehensive Review of Zeolite as a Next-Generation Multifunctional Filler for Enhanced Mechanical Reinforcement and Antimicrobial Efficacy" Dentistry Journal 13, no. 11: 540. https://doi.org/10.3390/dj13110540
APA StyleMohammed, S. F., Yhaya, M. F., Nongman, A. F., Al-Rawas, M., Arbilei, M. N., & Noorani, T. Y. (2025). From Industry to Dentistry: A Comprehensive Review of Zeolite as a Next-Generation Multifunctional Filler for Enhanced Mechanical Reinforcement and Antimicrobial Efficacy. Dentistry Journal, 13(11), 540. https://doi.org/10.3390/dj13110540








