Diagnostic Performance of Autofluorescence for Oral Lesions: A Comparison Between a Postgraduate and an Expert Clinician
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
- Pregnant women;
- Patients with pre-treated lesions (laser treatment, medical treatment, surgery);
- Patients with bioptic examination already performed.
2.2. Data Collection
2.3. Statistical Analysis
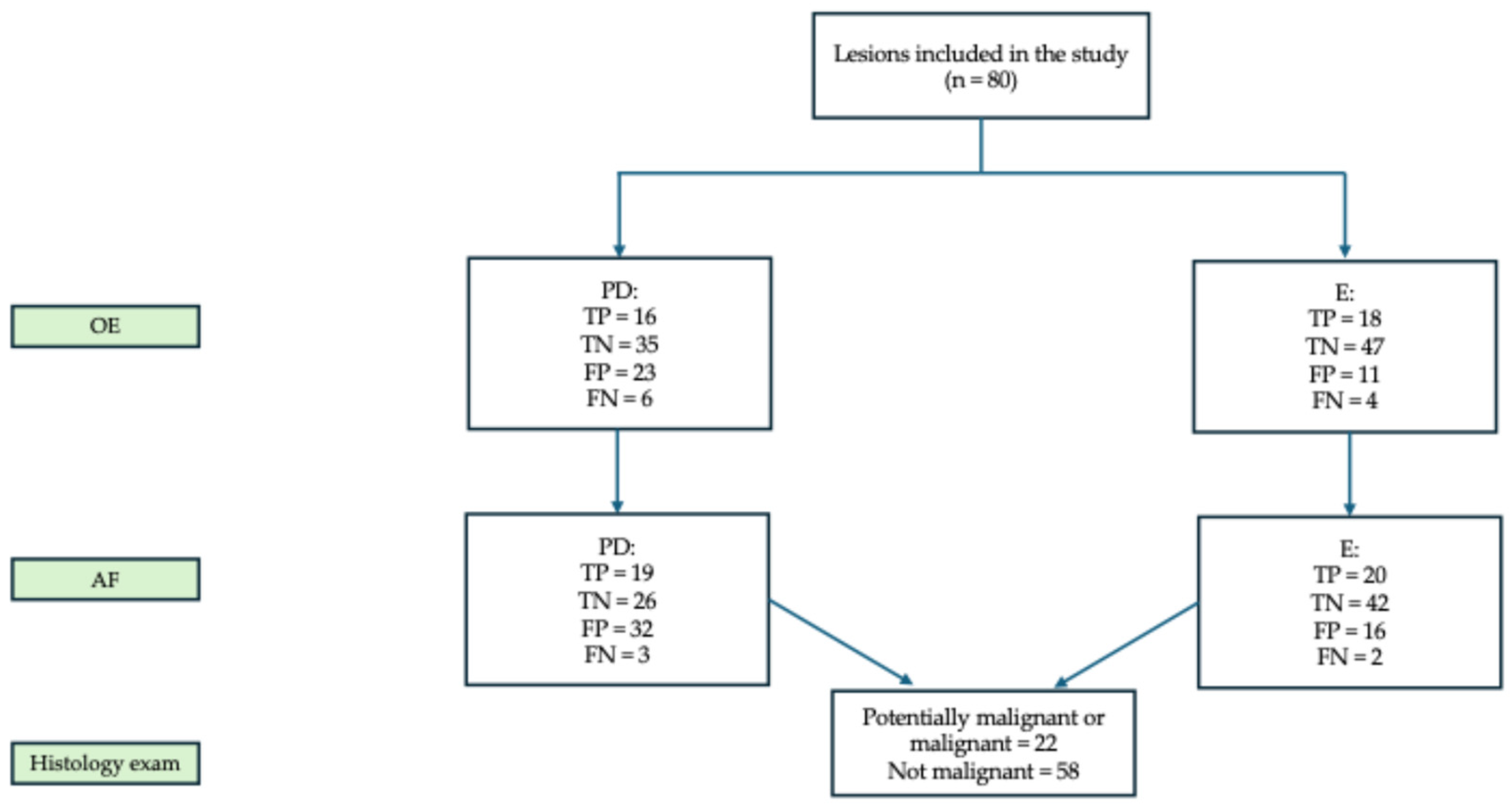
3. Results
3.1. Descriptive Analysis
3.2. Malignancy Risk Assessment and Biopsy Indications After OE
3.3. Malignancy Risk Assessment and Biopsy Indications After AF
3.4. Changes in Diagnostic Concordance Between PD and E
3.5. Clinical Predictors of RM Assessment
3.6. Inter-Operator Agreement for OE and AF
3.7. Sensitivity, Specificity, PPV, NPV, and Accuracy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAF | Alterations of Autofluorescence |
| AF | Autofluorescence |
| CI | Confidence Intervals |
| E | Expert |
| FAD | Flavin Adenine Dinucleotide |
| FN | False Negative |
| FP | False Positive |
| LAF | Loss of Autofluorescence |
| NADH | Nicotinamide Adenine Dinucleotide |
| NPV | Negative Predictive Value |
| NRM | Non-Risk of Malignancy |
| OE | Oral Examination |
| OLP | Oral Lichen Planus |
| OPMDs | Oral Potentially Malignant Disorders |
| OSCC | Oral Squamous Cell Carcinoma |
| PD | Postgraduate Dentist |
| PGCG | Peripheral Giant Cell Granuloma |
| PPV | Positive Predictive Value |
| RM | Risk of Malignancy |
| SD | Standard Deviation |
| TN | True Negative |
| TP | True Positive |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today (Version 1.1); International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 24 June 2025).
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially Malignant Disorders of the Oral Cavity and Oral Dysplasia: A Systematic Review and Meta-Analysis of Malignant Transformation Rate by Subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.M.; Ogden, G.R. Oral Cancer Awareness of General Medical and General Dental Practitioners. Br. Dent. J. 2007, 203, E10. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Aguilar-Ruiz, M.; Ramos-García, P. Challenges in the Early Diagnosis of Oral Cancer, Evidence Gaps and Strategies for Improvement: A Scoping Review of Systematic Reviews. Cancers 2022, 14, 4967. [Google Scholar] [CrossRef]
- Güneri, P.; Epstein, J.B. Late Stage Diagnosis of Oral Cancer: Components and Possible Solutions. Oral Oncol. 2014, 50, 1131–1136. [Google Scholar] [CrossRef]
- Saka-Herrán, C.; Jané-Salas, E.; Mari-Roig, A.; Estrugo-Devesa, A.; López-López, J. Time-to-Treatment in Oral Cancer: Causes and Implications for Survival. Cancers 2021, 13, 1321. [Google Scholar] [CrossRef]
- Poudel, P.; Srii, R.; Marla, V. Oral Cancer Awareness among Undergraduate Dental Students and Dental Surgeons: A Descriptive Cross-Sectional Study. J. Nepal Med. Assoc. 2020, 58, 102–107. [Google Scholar] [CrossRef]
- Greenwood, M.; Lowry, R.J. Primary Care Clinicians’ Knowledge of Oral Cancer: A Study of Dentists and Doctors in the North East of England. Br. Dent. J. 2001, 191, 510–512. [Google Scholar] [CrossRef]
- Strey, J.R.; Roxo-Gonçalves, M.; Guzenski, B.D.; Martins, M.A.T.; Romanini, J.; de Figueiredo, M.A.Z.; D’Ávila, O.P.; Gonçalves, M.R.; Umpierre, R.N.; Harzheim, E.; et al. Oral Medicine Experience and Attitudes Toward Oral Cancer: An Evaluation of Dentists Working in Primary Health Care. J. Cancer Educ. 2022, 37, 1621–1628. [Google Scholar] [CrossRef]
- Shubayr, M.A.; Moaleem, M.M.A.; Hakami, S.A.; Khalufi, K.N.; Daghriri, R.M.; Bokhari, A.M.; Alhazmi, A.S.; Farsi, A.H.; Adawi, M.A.; Nahari, H.H.; et al. Factors Influencing Engagement in Oral Cancer Prevention Activities among Dental Students and Professionals in Saudi Arabia. BMC Oral Health 2024, 24, 1465. [Google Scholar] [CrossRef]
- Pentenero, M.; Chiecchio, A.; Gandolfo, S. Impact of Academic and Continuing Education on Oral Cancer Knowledge, Attitude and Practice among Dentists in North-Western Italy. J. Cancer Educ. 2014, 29, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Shadid, R.M.; Abu Ali, M.A.; Kujan, O. Knowledge, Attitudes, and Practices of Oral Cancer Prevention among Dental Students and Interns: An Online Cross-sectional Questionnaire in Palestine. BMC Oral Health 2022, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Duffy, N.G.; Walters, K.C.; Day, T.A. Oral Cancer Knowledge and Experience: A Survey of South Carolina Medical Students in 2002. J. Cancer Educ. 2005, 20, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.F.A.; Oliveira, M.C.M.; Leite, A.C.; Bruzinga, F.F.B.; Mendes, P.A.; Grossmann, S.D.M.C.; De Araújo, V.E.; Souto, G.R. Assessment of Screening Programs as a Strategy for Early Detection of Oral Cancer: A Systematic Review. Oral Oncol. 2022, 130, 105936. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Su, Y.-F.; Chen, Y.-J.; Tsai, F.-T.; Li, W.-C.; Hsu, M.-L.; Wang, D.-H.; Yang, C.-C. Current Insights into Oral Cancer Diagnostics. Diagnostics 2021, 11, 1287. [Google Scholar] [CrossRef]
- Giovannacci, I.; Vescovi, P.; Manfredi, M.; Meleti, M. Non-Invasive Visual Tools for Diagnosis of Oral Cancer and Dysplasia: A Systematic Review. Med. Oral Patol. Oral Bucal 2016, 21, e305–e315. [Google Scholar] [CrossRef]
- Cheng, Y.-S.L.; Jordan, L.; Rees, T.; Chen, H.-S.; Oxford, L.; Brinkmann, O.; Wong, D. Levels of Potential Oral Cancer Salivary mRNA Biomarkers in Oral Cancer Patients in Remission and Oral Lichen Planus Patients. Clin. Oral Investig. 2014, 18, 985–993. [Google Scholar] [CrossRef]
- Warin, K.; Suebnukarn, S. Deep Learning in Oral Cancer- a Systematic Review. BMC Oral Health 2024, 24, 212. [Google Scholar] [CrossRef]
- Lingen, M.W.; Kalmar, J.R.; Karrison, T.; Speight, P.M. Critical Evaluation of Diagnostic Aids for the Detection of Oral Cancer. Oral Oncol. 2008, 44, 10–22. [Google Scholar] [CrossRef]
- Antonelli, A.; Battaglia, A.M.; Sacco, A.; Petriaggi, L.; Giorgio, E.; Barone, S.; Biamonte, F.; Giudice, A. Ferroptosis and Oral Squamous Cell Carcinoma: Connecting the Dots to Move Forward. Front. Oral Health 2024, 5, 1461022. [Google Scholar] [CrossRef]
- Sacco, A.; Battaglia, A.M.; Santamaria, G.; Buffone, C.; Barone, S.; Procopio, A.; Lavecchia, A.M.; Aversa, I.; Giorgio, E.; Petriaggi, L.; et al. SOX2 Promotes a Cancer Stem Cell-like Phenotype and Local Spreading in Oral Squamous Cell Carcinoma. PLoS ONE 2023, 18, e0293475. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Warnakulasuriya, S. The Use of Light-Based (Optical) Detection Systems as Adjuncts in the Detection of Oral Cancer and Oral Potentially Malignant Disorders: A Systematic Review. J. Oral Pathol. Med. 2015, 44, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Petriaggi, L.; Giorgio, E.; Bulotta, S.; Antonelli, A.; Bonacci, S.; Frisina, M.; Procopio, A.; Prestagiacomo, L.E.; Giuliano, A.; Gaspari, M.; et al. Acute Exposure to Cadmium Triggers NCOA4-Mediated Ferritinophagy and Ferroptosis in Never-Smokers Oral Cancer Cells. Int. J. Biol. Sci. 2025, 21, 4131–4152. [Google Scholar] [CrossRef] [PubMed]
- Giovannacci, I.; Magnoni, C.; Vescovi, P.; Painelli, A.; Tarentini, E.; Meleti, M. Which Are the Main Fluorophores in Skin and Oral Mucosa? A Review with Emphasis on Clinical Applications of Tissue Autofluorescence. Arch. Oral Biol. 2019, 105, 89–98. [Google Scholar] [CrossRef]
- Nagi, R.; Reddy-Kantharaj, Y.-B.; Rakesh, N.; Janardhan-Reddy, S.; Sahu, S. Efficacy of Light Based Detection Systems for Early Detection of Oral Cancer and Oral Potentially Malignant Disorders: Systematic Review. Med. Oral Patol. Oral Bucal 2016, 21, e447–e455. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Fiorillo, L.; D’Amico, C.; Oteri, G.; Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Herford, A.S.; Crimi, S.; et al. Early Diagnosis on Oral and Potentially Oral Malignant Lesions: A Systematic Review on the VELscope® Fluorescence Method. Dent. J. 2019, 7, 93. [Google Scholar] [CrossRef]
- Leuci, S.; Coppola, N.; Turkina, A.; Bizzoca, M.E.; Favia, G.; Spagnuolo, G.; Mignogna, M.D. May VelScope Be Deemed an Opportunistic Oral Cancer Screening by General Dentists? A Pilot Study. J. Clin. Med. 2020, 9, 1754. [Google Scholar] [CrossRef]
- Simonato, L.E.; Tomo, S.; Miyahara, G.I.; Navarro, R.S.; Villaverde, A.G.J.B. Fluorescence Visualization Efficacy for Detecting Oral Lesions More Prone to Be Dysplastic and Potentially Malignant Disorders: A Pilot Study. Photodiagn. Photodyn. Ther. 2017, 17, 1–4. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Kujan, O.; Oliver, R.J.; Khattab, A.; Roberts, S.A.; Thakker, N.; Sloan, P. Evaluation of a New Binary System of Grading Oral Epithelial Dysplasia for Prediction of Malignant Transformation. Oral Oncol. 2006, 42, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Reibel, J.; Bouquot, J.; Dabelsteen, E. Oral Epithelial Dysplasia Classification Systems: Predictive Value, Utility, Weaknesses and Scope for Improvement. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2008, 37, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral Potentially Malignant Disorders: Risk of Progression to Malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Dost, F.; Lê Cao, K.-A.; Ford, P.J.; Farah, C.S. A Retrospective Analysis of Clinical Features of Oral Malignant and Potentially Malignant Disorders with and without Oral Epithelial Dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 725–733. [Google Scholar] [CrossRef]
- Jeng, P.-Y.; Chang, M.-C.; Chiang, C.-P.; Lee, C.-F.; Chen, C.-F.; Jeng, J.-H. Oral Soft Tissue Biopsy Surgery: Current Principles and Key Tissue Stabilization Techniques. J. Dent. Sci. 2024, 19, 11–20. [Google Scholar] [CrossRef]
- Shanti, R.M.; Tanaka, T.; Stanton, D.C. Oral Biopsy Techniques. Dermatol. Clin. 2020, 38, 421–427. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P. An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 608. [Google Scholar] [CrossRef]
- Roberts, S.L.; Bhamra, R.; Ilankovan, V. Malignant Transformation Rate of Erosive Oral Lichen Planus: A Retrospective Study. Br. J. Oral Maxillofac. Surg. 2024, 62, 788–793. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ruiz-Ávila, I.; González-Ruiz, L.; Ayén, Á.; Gil-Montoya, J.A.; Ramos-García, P. Malignant Transformation Risk of Oral Lichen Planus: A Systematic Review and Comprehensive Meta-Analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Lim, K.; Moles, D.R.; Downer, M.C.; Speight, P.M. Opportunistic Screening for Oral Cancer and Precancer in General Dental Practice: Results of a Demonstration Study. Br. Dent. J. 2003, 194, 497–502; discussion 493. [Google Scholar] [CrossRef]
- Lingen, M.W.; Abt, E.; Agrawal, N.; Chaturvedi, A.K.; Cohen, E.; D’Souza, G.; Gurenlian, J.; Kalmar, J.R.; Kerr, A.R.; Lambert, P.M.; et al. Evidence-Based Clinical Practice Guideline for the Evaluation of Potentially Malignant Disorders in the Oral Cavity: A Report of the American Dental Association. J. Am. Dent. Assoc. 1939 2017, 148, 712–727.e10. [Google Scholar] [CrossRef]
- Awadallah, M.; Idle, M.; Patel, K.; Kademani, D. Management Update of Potentially Premalignant Oral Epithelial Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Tarakji, B. Dentists’ Perception of Oral Potentially Malignant Disorders. Int. Dent. J. 2022, 72, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, D.W.V.N.; Wijeratne, K.M.S.L.; Amarasinghe, K.A.D.K.D.; Jayasinghe, R.D.; Jayasooriya, P.R.; Mendis, B.R.R.N.; Lombardi, T. A Preliminary Study on Early Detection of Oral Cancer with Opportunistic Screening: Insights from Dental Surgeons in Sri Lanka. Cancers 2023, 15, 5511. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Halimi, R.; Gadiraju, S.; Frydrych, A.M.; Kujan, O. Clinical Competency of Dental Health Professionals and Students in Diagnosing Oral Mucosal Lesions. Oral Dis. 2024, 30, 3108–3116. [Google Scholar] [CrossRef]
- Sari, E.F.; Hidayat, W.; Dewi, T.S.; Rezeki, S.; Krimadi, R.; McCullough, M.J.; Cirillo, N. General Dentists’ Knowledge, Perceptions, and Practices Regarding Oral Potentially Malignant Disorders and Oral Cancer in Indonesia. Clin. Exp. Dent. Res. 2024, 10, e807. [Google Scholar] [CrossRef]
- Najim, A.M.; Bruers, J.J.M.; De Visscher, J.G. Knowledge, Attitude, and Practice of Dutch Dentists on Oral Leukoplakia and Their Possible Role in Its Follow-Up. Int. Dent. J. 2025, 75, 1029–1035. [Google Scholar] [CrossRef]
- Colella, G.; Gaeta, G.M.; Moscariello, A.; Angelillo, I.F. Oral Cancer and Dentists: Knowledge, Attitudes, and Practices in Italy. Oral Oncol. 2008, 44, 393–399. [Google Scholar] [CrossRef]
- Chaurasia, A.; Alam, S.I.; Singh, N. Oral Cancer Diagnostics: An Overview. Natl. J. Maxillofac. Surg. 2021, 12, 324–332. [Google Scholar] [CrossRef]
- Jitender, S.; Sarika, G.; Varada, H.R.; Omprakash, Y.; Mohsin, K. Screening for Oral Cancer. J. Exp. Ther. Oncol. 2016, 11, 303–307. [Google Scholar] [PubMed]
- Buenahora, M.R.; Peraza-L, A.; Díaz-Báez, D.; Bustillo, J.; Santacruz, I.; Trujillo, T.G.; Lafaurie, G.I.; Chambrone, L. Diagnostic Accuracy of Clinical Visualization and Light-Based Tests in Precancerous and Cancerous Lesions of the Oral Cavity and Oropharynx: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.; Toratani, S.; Matsui, K.; Hayashi, S.; Eboshida, N.; Hamada, A.; Ito, N.; Obayashi, F.; Kimura, N.; Yanamoto, S. Evaluation of Oral Mucosal Lesions Using the IllumiScan® Fluorescence Visualisation Device: Distinguishing Squamous Cell Carcinoma. Int. J. Environ. Res. Public Health 2022, 19, 10414. [Google Scholar] [CrossRef]
- Moro, A.; De Waure, C.; Di Nardo, F.; Spadari, F.; Mignogna, M.D.; Giuliani, M.; Califano, L.; Giannì, A.B.; Cardarelli, L.; Celentano, A.; et al. The GOCCLES® Medical Device Is Effective in Detecting Oral Cancer and Dysplasia in Dental Clinical Setting. Results from a Multicentre Clinical Trial. Acta Otorhinolaryngol. Ital. 2015, 35, 449–454. [Google Scholar] [CrossRef]
- Lajolo, C.; Tranfa, M.; Patini, R.; Fiorino, A.; Musarra, T.; Boniello, R.; Moro, A. Clinical Evaluation of the Optical Filter for Autofluorescence Glasses for Oral Cancer Curing Light Exposed (GOCCLES®) in the Management of Potentially Premalignant Disorders: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 5579. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, V.; Sullivan, M.; Merzianu, M.; Rigual, N.R.; Loree, T.R.; Popat, S.R.; Moysich, K.B.; Ramananda, S.; Johnson, T.; Marshall, J.R.; et al. Autofluorescence-Guided Surveillance for Oral Cancer. Cancer Prev. Res. 2009, 2, 966–974. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Autofluorescence Imaging to Identify Oral Malignant or Premalignant Lesions: Systematic Review and Meta-Analysis. Head Neck 2020, 42, 3735–3743. [Google Scholar] [CrossRef]
- Awan, K.H.; Morgan, P.R.; Warnakulasuriya, S. Evaluation of an Autofluorescence Based Imaging System (VELscopeTM) in the Detection of Oral Potentially Malignant Disorders and Benign Keratoses. Oral Oncol. 2011, 47, 274–277. [Google Scholar] [CrossRef]
- Ganga, R.S.; Gundre, D.; Bansal, S.; Shirsat, P.M.; Prasad, P.; Desai, R.S. Evaluation of the Diagnostic Efficacy and Spectrum of Autofluorescence of Benign, Dysplastic and Malignant Lesions of the Oral Cavity Using VELscope. Oral Oncol. 2017, 75, 67–74. [Google Scholar] [CrossRef]
- Bhatia, N.; Lalla, Y.; Vu, A.N.; Farah, C.S. Advances in Optical Adjunctive Aids for Visualisation and Detection of Oral Malignant and Potentially Malignant Lesions. Int. J. Dent. 2013, 2013, 194029. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; McIntosh, L.; Georgiou, A.; McCullough, M.J. Efficacy of Tissue Autofluorescence Imaging (Velscope) in the Visualization of Oral Mucosal Lesions. Head Neck 2012, 34, 856–862. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.K.; Martin, B.D.; Evans, E.W.; Kalmar, J.R. The Role of Direct Visual Fluorescent Examination (VELscope) in Routine Screening for Potentially Malignant Oral Mucosal Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Moffa, A.; Giorgi, L.; Costantino, A.; De Benedetto, L.; Cassano, M.; Spriano, G.; Mercante, G.; De Virgilio, A.; Casale, M. Accuracy of Autofluorescence and Chemiluminescence in the Diagnosis of Oral Dysplasia and Carcinoma: A Systematic Review and Meta-Analysis. Oral Oncol. 2021, 121, 105482. [Google Scholar] [CrossRef]
- Shi, L.; Li, C.; Shen, X.; Zhou, Z.; Liu, W.; Tang, G. Potential Role of Autofluorescence Imaging in Determining Biopsy of Oral Potentially Malignant Disorders: A Large Prospective Diagnostic Study. Oral Oncol. 2019, 98, 176–179. [Google Scholar] [CrossRef]
- Flores Dos Santos, L.C.; Fernandes, J.R.; Lima, I.F.P.; Bittencourt, L.D.S.; Martins, M.D.; Lamers, M.L. Applicability of Autofluorescence and Fluorescent Probes in Early Detection of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Data Analysis. Photodiagn. Photodyn. Ther. 2022, 38, 102764. [Google Scholar] [CrossRef]
- Poh, C.F.; Ng, S.P.; Williams, P.M.; Zhang, L.; Laronde, D.M.; Lane, P.; Macaulay, C.; Rosin, M.P. Direct Fluorescence Visualization of Clinically Occult High-Risk Oral Premalignant Disease Using a Simple Hand-Held Device. Head Neck 2007, 29, 71–76. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can Autofluorescence Guide Surgeons in the Treatment of Medication-Related Osteonecrosis of the Jaw? A Prospective Feasibility Study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef]
- Biamonte, F.; Buffone, C.; Santamaria, G.; Battaglia, A.M.; Mignogna, C.; Fortunato, L.; Costanzo, F.S.; Giudice, A. Gene Expression Analysis of Autofluorescence Margins in Leukoplakia and Oral Carcinoma: A Pilot Study. Oral Dis. 2021, 27, 193–203. [Google Scholar] [CrossRef]
- Petruzzi, M.; Della Vella, F.; Squicciarini, N.; Lilli, D.; Campus, G.; Piazzolla, G.; Lucchese, A.; van der Waal, I. Diagnostic Delay in Autoimmune Oral Diseases. Oral Dis. 2023, 29, 2614–2623. [Google Scholar] [CrossRef]
- Villa, A.; Stock, S.; Aboalela, A.; Lerman, M.A.; Woo, S.-B.; Sonis, S.T.; Treister, N.S. Oral Medicine Referrals at a Hospital-Based Practice in the United States. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 423–429. [Google Scholar] [CrossRef]
- Arduino, P.G.; Broccoletti, R.; Carbone, M.; Gambino, A.; Sciannameo, V.; Conrotto, D.; Cabras, M.; Sciascia, S.; Ricceri, F.; Baldovino, S.; et al. Long-term Evaluation of Pemphigus Vulgaris: A Retrospective Consideration of 98 Patients Treated in an Oral Medicine Unit in North-west Italy. J. Oral Pathol. Med. 2019, 48, 406–412. [Google Scholar] [CrossRef]
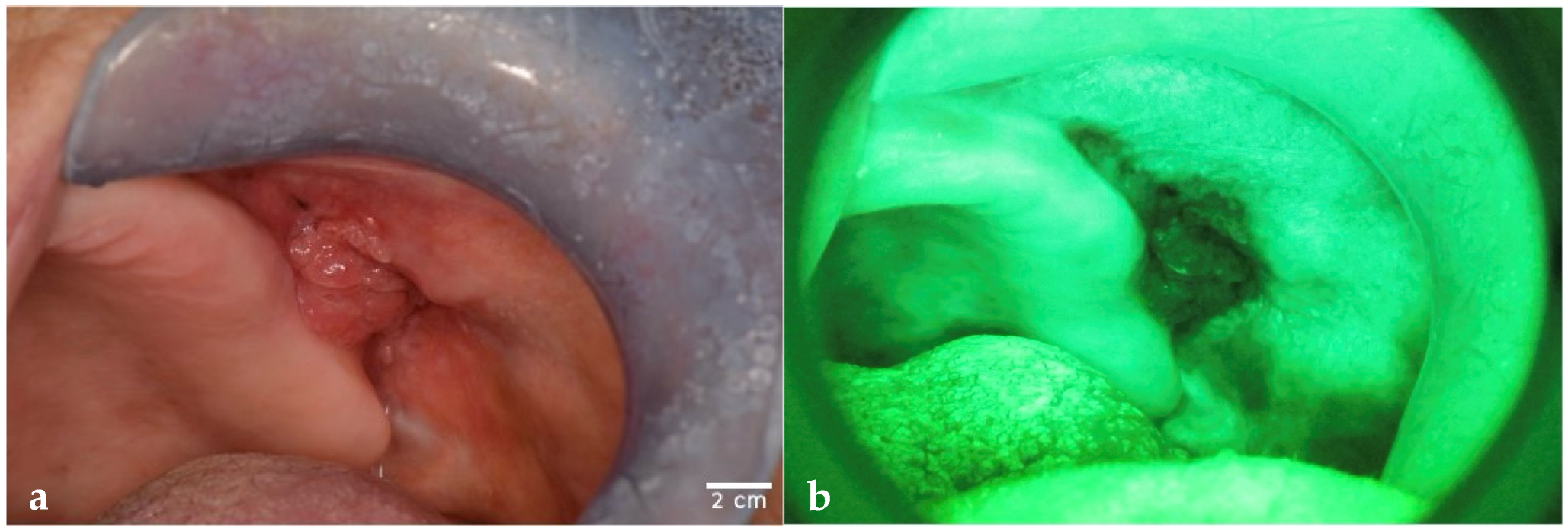
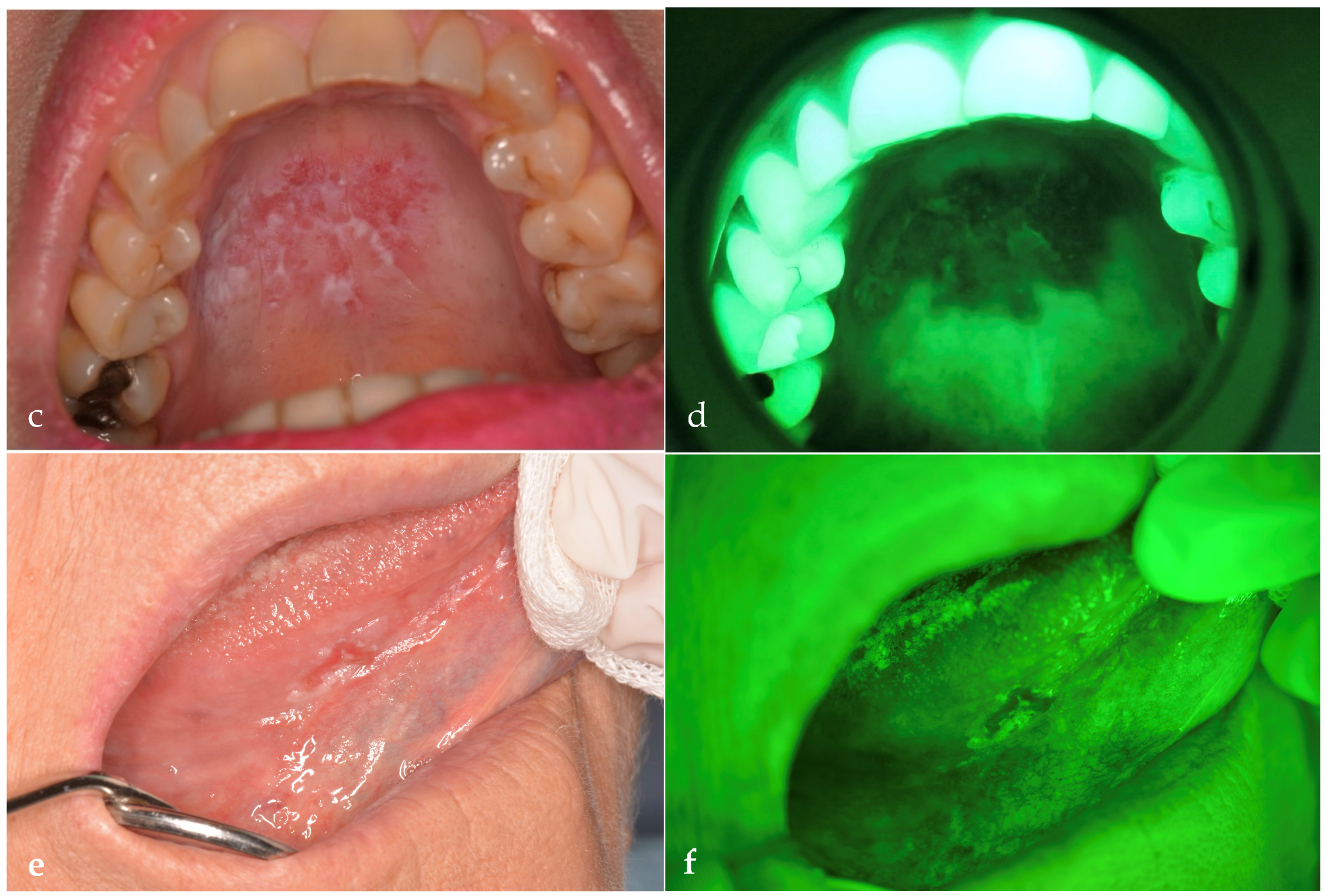
| Demographic Variables | Patients (n = 80) |
|---|---|
| Gender | Frequency (%) |
| Male | 38 (47.5) |
| Female | 42 (52.5) |
| Age (in years) | Mean ± SD |
| 54 ± 14.6 | |
| Smoking | Frequency (%) |
| Yes | 41(51.2) |
| No | 39 (48.7) |
| Site and Characteristics of the Lesions | Frequency (%) |
|---|---|
| Site | |
| Lower lip | 2 (1.9) |
| Buccal mucosa | 50 (48.1) |
| Upper vestibule | 1 (1) |
| Gingiva | 6 (5.8) |
| Tongue | 26 (25) |
| Floor of the mouth | 1 (1) |
| Hard palate | 12 (11.5) |
| Soft palate | 5 (4.8) |
| Tonsillar pillar | 1 (1) |
| Color | |
| Erythema | 22 (21.2) |
| Keratotic | 29 (27.9) |
| Erythema and keratotic | 47 (45.2) |
| Pigmented | 6 (5.8) |
| Appearance | |
| Flat | 88 (84.6) |
| Exophytic | 16 (15.4) |
| Phenotype | |
| Homogeneous | 51 (49.1) |
| Dishomogeneous | 53 (50.9) |
| PD | E | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OE | AF | OE | AF | ||||||
| Histology | Frequency (%) | RM (%) | NRM (%) | RM (%) | NRM (%) | RM (%) | NRM (%) | RM (%) | NRM (%) |
| Malignant | |||||||||
| Actinic cheilitis | 2 (2.5) | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 |
| Adenosquamous carcinoma | 1 (1.2) | 0 | 1 (100) | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 |
| Mucoepidermoid carcinoma | 1 (1.2) | 0 | 1 (100) | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 |
| OSCC | 5 (6.2) | 5 (100) | 0 | 5 (100) | 0 | 5 (100) | 0 | 5 (100) | 0 |
| Carcinoma in situ | 2 (2.5) | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 |
| Leukoplakia | 2 (2.5) | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 |
| Erosive OLP | 9 (11.2) | 5 (55.5) | 4 (44.4) | 6 (66.6) | 3 (33.3) | 5 (55.5) | 4 (44.4) | 7 (77.7) | 2 (22.2) |
| PD | E | ||||||||
| OE | AF | OE | AF | ||||||
| Not Malignant | Frequency (%) | RM (%) | NRM (%) | RM (%) | NRM (%) | RM (%) | NRM (%) | RM (%) | NRM (%) |
| Capillary subepithelial hemangioma | 1 (1.2) | 1 (100) | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 (100) | 0 |
| Fibroma | 9 (11.2) | 4 (44.4) | 5 (55.5) | 4 (44.4) | 5 (55.9) | 1 (11.1) | 8 (88.8) | 0 | 9 (100) |
| PGCG | 5 (6.2) | 0 | 5 (100) | 5 (100) | 0 | 0 | 5 (100) | 2 (40) | 3 (60) |
| Keratotic OLP | 13 (16.2) | 8 (61.5) | 5 (38.5) | 9 (69.2) | 4 (30.8) | 6 (46.2) | 7 (53.8) | 7 (53.2) | 6 (46.1) |
| Melanocytic nevus | 5 (6.2) | 0 | 5 (100) | 1 (20) | 4 (80) | 0 | 5 (100) | 1 (20) | 4 (80) |
| Pemphigus vulgaris | 6 (7.5) | 2 (33.3) | 4 (66.7) | 1 (16.6) | 5 (83.3) | 0 | 6 (100) | 0 | 6 (100) |
| Traumatic keratosis | 5 (6.2) | 4 (80) | 1 (20) | 2 (40) | 3 (60) | 2 (40) | 3 (60) | 2 (40) | 3 (60) |
| Traumatic ulcer | 14 (17.5) | 4 (28.6) | 10 (71.4) | 9 (64.3) | 5 (35.7) | 2 (14.3) | 12 (85.7) | 3 (21.5) | 11 (78.5) |
| PD | E | ||||||
|---|---|---|---|---|---|---|---|
| Histology | Frequency (%) | Normal (%) | AAF (%) | LAF (%) | Normal (%) | AAF (%) | LAF (%) |
| Potentially malignant or malignant | |||||||
| Actinic cheilitis | 2 (2.5) | 0 | 0 | 2 (100) | 0 | 0 | 2 (100) |
| Adenosquamous carcinoma | 1 (1.2) | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) |
| Mucoepidermoid carcinoma | 1 (1.2) | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) |
| OSCC | 5 (6.2) | 0 | 0 | 5 (100) | 0 | 0 | 5 (100) |
| Carcinoma in situ | 2 (2.5) | 0 | 1 (50) | 1 (50) | 0 | 1 (50) | 1 (50) |
| Leukoplakia | 2 (2.5) | 0 | 0 | 2 (100) | 0 | 0 | 2 (100) |
| Erosive OLP | 9 (11.2) | 3 (33.3) | 1 (11.1) | 5 (55.5) | 2 (22.2) | 3 (33.3) | 4 (44.4) |
| PD | E | ||||||
| Not Malignant | Frequency (%) | Normal (%) | AAF (%) | LAF (%) | Normal (%) | AAF (%) | LAF (%) |
| Capillary subepithelial hemangioma | 1 (1.2) | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) |
| Fibroma | 9 (11.2) | 2 (22.2) | 3 (33.3) | 4 (44.4) | 4 (44.4) | 5 (55.5) | 0 |
| PGCG | 5 (6.2) | 0 | 0 | 5 (100) | 0 | 3 (60) | 2 (40) |
| Keratotic OLP | 13 (16.2) | 1 (7.6) | 7 (53.8) | 6 (46.1) | 0 | 8 (61.5) | 6 (46.1) |
| Melanocytic nevus | 5 (6.2) | 0 | 4 (80) | 1 (20) | 4 (80) | 1 (20) | 0 |
| Pemphigus vulgaris | 6 (7.5) | 2 (33.3) | 3 (50) | 1 (16.6) | 4 (66.6) | 0 | 1 (16.6) |
| Traumatic keratosis | 5 (6.2) | 1 (20) | 2 (40) | 2 (40) | 3 (60) | 2 (40) | 0 |
| Traumatic ulcer | 14 (17.5) | 1 (7.1) | 6 (42.8) | 8 (57.1) | 2 (14.2) | 10 (71.4) | 1 (7.1) |
| PD | E | |||||||
|---|---|---|---|---|---|---|---|---|
| OE | AF | OE | AF | |||||
| Predictor | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Dishomogeous phenoype | 3.06 (1.170–8.03) | 0.0231 * | 1.38 (0.502–3.81) | 0.530 | 4.3 (1.51–12.2) | 0.006 ** | 2.77 (1.02–7.51) | 0.046 * |
| Keratotic | <0.001 (0-inf) † | 0.996 | 7.80 (0-inf.) † | 0.996 | <0.001 (0-inf) † | 0.994 | <0.001 (0-inf) † | 0.994 |
| Keratotic + erythema | 0.930 (0.275–3.14) | 0.907 | 8.16 (0.173–3.85) | 0.798 | 0.947 (0.271–3.30) | 0.932 | 2.02 (0.527–7.74) | 0.305 |
| Pigmented | <0.001 (0-inf) | 0.993 | 1.24 | 0.993 | 0.631 (0.047–8.52) | 0.729 | 0.723 (0.05–11.30) | 0.8170 |
| Erythema | 1.14 (0.312–4.15) | 0.846 | 4.96 (0.092–2.68) | 0.415 | 0.469 (0.12–1.83) | 0.276 | 0.585 (0.13–2.64) | 0.487 |
| Examination | κ (CI 95%) |
|---|---|
| EO | 0.494 (0.303–0.686) |
| AF | 0.284 (0.081–0.4856) |
| PD | OE | AF | Combined |
|---|---|---|---|
| Sensitivity | 68.2% | 86.4% | 90.9% |
| Specificity | 60.3% | 43.1% | 37.9% |
| PPV | 39.5% | 36.5% | 35.7% |
| NPV | 83.3% | 89.3% | 91.7% |
| Accuracy | 62.5% | 55% | 52.5% |
| E | EO | AF | Combined |
| Sensitivity | 81.8% | 95.4% | 95.4% |
| Specificity | 79.3% | 74.1% | 70.7% |
| PPV | 60% | 58.3% | 55.3% |
| NPV | 92% | 97.7% | 97.6% |
| Accuracy | 81.25% | 80% | 77.1% |
| Operator | χ2 (Continuity-Corrected) | p-Value |
|---|---|---|
| PD | 10.240 | 0.001 ** |
| E | 1.778 | 0.182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonelli, A.; D’Antonio, C.; Battaglia, A.M.; Finamore, R.; Madonna, A.; Greco, V.; Cosentino, V.; Barone, S.; Biamonte, F.; Giudice, A.; et al. Diagnostic Performance of Autofluorescence for Oral Lesions: A Comparison Between a Postgraduate and an Expert Clinician. Dent. J. 2025, 13, 512. https://doi.org/10.3390/dj13110512
Antonelli A, D’Antonio C, Battaglia AM, Finamore R, Madonna A, Greco V, Cosentino V, Barone S, Biamonte F, Giudice A, et al. Diagnostic Performance of Autofluorescence for Oral Lesions: A Comparison Between a Postgraduate and an Expert Clinician. Dentistry Journal. 2025; 13(11):512. https://doi.org/10.3390/dj13110512
Chicago/Turabian StyleAntonelli, Alessandro, Cristina D’Antonio, Anna Martina Battaglia, Riccardo Finamore, Antonio Madonna, Vincenzo Greco, Vincenzo Cosentino, Selene Barone, Flavia Biamonte, Amerigo Giudice, and et al. 2025. "Diagnostic Performance of Autofluorescence for Oral Lesions: A Comparison Between a Postgraduate and an Expert Clinician" Dentistry Journal 13, no. 11: 512. https://doi.org/10.3390/dj13110512
APA StyleAntonelli, A., D’Antonio, C., Battaglia, A. M., Finamore, R., Madonna, A., Greco, V., Cosentino, V., Barone, S., Biamonte, F., Giudice, A., & Bennardo, F. (2025). Diagnostic Performance of Autofluorescence for Oral Lesions: A Comparison Between a Postgraduate and an Expert Clinician. Dentistry Journal, 13(11), 512. https://doi.org/10.3390/dj13110512










