Periodontitis and Mild Cognitive Impairment Risk in Diabetic Patients: Insights from an Exploratory Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Collection and Variables
2.3.1. General Health Variables
2.3.2. Periodontal Health
2.3.3. Inflammation Status and MCI Risk
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. The Risk of MCI in High PISA Scores Groups
3.3. The Correlation Between Periodontal Status, Inflammation, and MCI Risk Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A1BG | Alpha-1-B-glycoprotein |
| A2AP | Aplha-2-antiplasmin |
| A2M | Alpha-2-macroglobulin |
| Aβ | Amyloid-beta |
| Alb | Albumin |
| ApoA1 | Apolipoprotein A1 |
| ApoC1 | Apolipoprotein C1 |
| AD | Alzheimer’s Disease |
| BBB | Blood–Brain Barrier |
| BOP | Bleeding on Probing |
| C3 | Complement component 3 |
| CRP | C-Reactive Protein |
| HPX | Hemopexin |
| IL-6 | Interleukin 6 |
| MCI | Mild Cognitive Impairment |
| PPD | Probing Pocket Depth |
| PISA | Periodontal Inflamed Surface Area |
| TNF-α | Tumor Necrosis Factor α |
| TTR | Transthyretin |
References
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 11 September 2024).
- Langa, K.M. Cognitive Aging, Dementia, and the Future of an Aging Population. In Future Directions for the Demography of Aging: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513075/ (accessed on 11 September 2024).
- Rajendran, V.; Uppoor, A.; Nayak, S.U.; Rao, S.B.; Dasson Bajaj, P. Unraveling the cognitive implications among individuals with co-occurring chronic periodontitis and type 2 diabetes mellitus: A cross-sectional study. J. Oral Biosci. 2024, 66, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, P.C.; Castro, M.M.L.; Magno, M.B.; Almeida, A.P.C.P.S.C.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. Association Between Periodontitis and Cognitive Impairment in Adults: A Systematic Review. Front. Neurol. 2019, 10, 323. [Google Scholar] [CrossRef]
- Sharma, S.; Nayak, S.U.; Uppoor, A.; Rao, S.; Pai, K.; Natarajan, S. Evaluation of Cognitive Impairment in Type 2 Diabetic Patients with Chronic Periodontitis: A Cross-sectional Study. J. Int. Soc. Prev. Community Dent. 2021, 11, 50–57. [Google Scholar] [CrossRef]
- Balasubramaniam, A. A cross-sectional investigation of cognitive impairment in type 2 diabetus mellitus patients with chronic periodontitis. Diabetes Manag. 2022, 12, 321–328. [Google Scholar]
- Miklossy, J.; McGeer, P.L. Common mechanisms involved in Alzheimer’s disease and type 2 diabates: A key role of chronic infection and inflammation. Aging 2016, 8, 575. [Google Scholar] [CrossRef]
- Onyango, E.M.; Onyango, B.M. The Rise of Noncommunicable Diseases in Kenya: An Examination of the Time Trends and Contribution of the Changes in Diet and Physical Inactivity. J. Epidemiol. Glob. Health 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Watts, A.; Crimmins, E.M.; Gatz, M. Inflammation as a potential mediator for the association between periodontal disease and Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 865–876. [Google Scholar] [CrossRef]
- Kimura, R.; Tomiyasu, H.; Takeuchi, T.; Shimizu, M.; Hayashi, Y.; Okayama, N.; Kamiya, Y.; Joh, T. Prevalence of Alzheimer’s disease with diabetes in the Japanese population. Psychogeriatrics 2008, 8, 73–78. [Google Scholar] [CrossRef]
- Morris, J.C. Mild cognitive impairment and preclinical Alzheimer’s disease. Geriatrics 2005, 60, 9–14. [Google Scholar]
- Anderson, N.D. State of the science on mild cognitive impairment (MCI). CNS Spectr. 2019, 24, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Nesse, W.; Abbas, F.; Van Der Ploeg, I.; Spijkervet, F.K.L.; Dijkstra, P.U.; Vissink, A. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef]
- Inoue, M.; Suzuki, H.; Meno, K.; Liu, S.; Korenaga, T.; Uchida, K. Identification of Plasma Proteins as Biomarkers for Mild Cognitive Impairment and Alzheimer’s Disease Using Liquid Chromatography–Tandem Mass Spectrometry. Int. J. Mol. Sci. 2023, 24, 13064. [Google Scholar] [CrossRef]
- Uchida, K.; Shan, L.; Suzuki, H.; Tabuse, Y.; Nishimura, Y.; Hirokawa, Y.; Mizukami, K.; Akatsu, H.; Meno, K.; Asada, T. Amyloid-β sequester proteins as blood-based biomarkers of cognitive decline. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2015, 1, 270–280. [Google Scholar] [CrossRef]
- MCBI Inc. Evaluation and Judgment. MCI Plus Screening. Available online: https://mci-plus.com/report/ (accessed on 12 December 2024).
- Kostanek, J.; Karolczak, K.; Kuliczkowski, W.; Watala, C. Bootstrap Method as a Tool for Analyzing Data with Atypical Distributions Deviating from Parametric Assumptions: Critique and Effectiveness Evaluation. Data 2024, 9, 95. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Milward, M.R.; Dietrich, T. The Prevalence of Inflammatory Periodontitis Is Negatively Associated with Serum Antioxidant Concentrations. J. Nutr. 2007, 137, 657–664. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Li, A.; Du, M.; Chen, Y.; Marks, L.A.M.; Visser, A.; Xu, S.; Tjakkes, G.E. Periodontitis and cognitive impairment in older adults: The mediating role of mitochondrial dysfunction. J. Periodontol. 2022, 93, 1302–1313. [Google Scholar] [CrossRef]
- Sharma, S.; Uppoor, A.; Natarajan, S. Diabetes and Periodontitis—Role in Cognitive Impairment. Dent. Hypotheses 2018, 9, 20. [Google Scholar] [CrossRef]
- Saedi, E.; Gheini, M.R.; Faiz, F.; Arami, M.A. Diabetes mellitus and cognitive impairments. World J. Diabetes 2016, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.; Stephen, R.; Mäntylä, P.; Suominen, A.L.; Solomon, A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J. Am. Geriatr. Soc. 2022, 70, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Desta, N.T. Pathophysiological association between periodontal disease and Alzheimer’s disease: Importance of periodontal health in the elderly. J. Oral Biosci. 2021, 63, 351–359. [Google Scholar] [CrossRef]
- Lee, K.S.; Chung, J.H.; Choi, T.K.; Suh, S.Y.; Oh, B.H.; Hong, C.H. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2009, 28, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Abbayya, K.; Puthanakar, N.Y.; Naduwinmani, S.; Chidambar, Y.S. Association between Periodontitis and Alzheimer’s Disease. N. Am. J. Med. Sci. 2015, 7, 241–246. [Google Scholar] [CrossRef]
- Leira, Y.; Martin-Lanharro, P.; Blanco, J. Periodontal inflamed surface area and periodontal case definition classification. Acta Odontol. Scand. 2018, 76, 195–198. [Google Scholar] [CrossRef]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. C-Reactive Protein (CRP) and its Association with Periodontal Disease: A Brief Review. J. Clin. Diagn. Res. 2014, 8, ZE21. [Google Scholar] [CrossRef]
- Bozluolcay, M.; Andican, G.; Fırtına, S.; Erkol, G.; Konukoglu, D. Inflammatory hypothesis as a link between A lzheimer’s disease and diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 1161–1166. [Google Scholar] [CrossRef]
- Montgomery, S.L.; Bowers, W.J. Tumor Necrosis Factor-alpha and the Roles it Plays in Homeostatic and Degenerative Processes Within the Central Nervous System. J. Neuroimmune Pharmacol. 2012, 7, 42–59. [Google Scholar] [CrossRef]
- Park, K.M.; Bowers, W.J. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell. Signal. 2010, 22, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Roselli, F.; Tartaglione, B.; Federico, F.; Lepore, V.; Defazio, G.; Livrea, P. Rate of MMSE score change in Alzheimer’s disease: Influence of education and vascular risk factors. Clin. Neurol. Neurosurg. 2009, 111, 327–330. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Engelhart, M.J.; Geerlings, M.I.; Meijer, J. Inflammatory Proteins in Plasma and the Risk of Dementia: The Rotterdam Study. Arch. Neurol. 2004, 61, 668–672. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased Clearance of CNS β-Amyloid in Alzheimer’s Disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef] [PubMed]
- Bi, B.-T.; Lin, H.-B.; Cheng, Y.-F.; Zhou, H.; Lin, T.; Zhang, M.-Z.; Li, T.-J.; Xu, J.-P. Promotion of β-amyloid production by C-reactive protein and its implications in the early pathogenesis of Alzheimer’s disease. Neurochem. Int. 2012, 60, 257–266. [Google Scholar] [CrossRef]
- Nikitina, L.; Paidi, R.; Furuoka, F. Using bootstrapped quantile regression analysis for small sample research in applied linguistics: Some methodological considerations. PLoS ONE 2019, 14, e0210668. [Google Scholar] [CrossRef] [PubMed]
- Fuior, E.V.; Gafencu, A.V. Apolipoprotein C1: Its Pleiotropic Effects in Lipid Metabolism and Beyond. Int. J. Mol. Sci. 2019, 20, 5939. [Google Scholar] [CrossRef]
- Garedow, A.W.; Jemaneh, T.M.; Hailemariam, A.G.; Tesfaye, G.T. Lifestyle modification and medication use among diabetes mellitus patients attending Jimma University Medical Center, Jimma zone, south west Ethiopia. Sci. Rep. 2023, 13, 4956. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Madureira, P.; Leal, E.C.; Fonseca, A.C.; Carvalho, E. Immune aging in diabetes and its implications in wound healing. Clin. Immunol. 2019, 200, 43–54. [Google Scholar] [CrossRef]
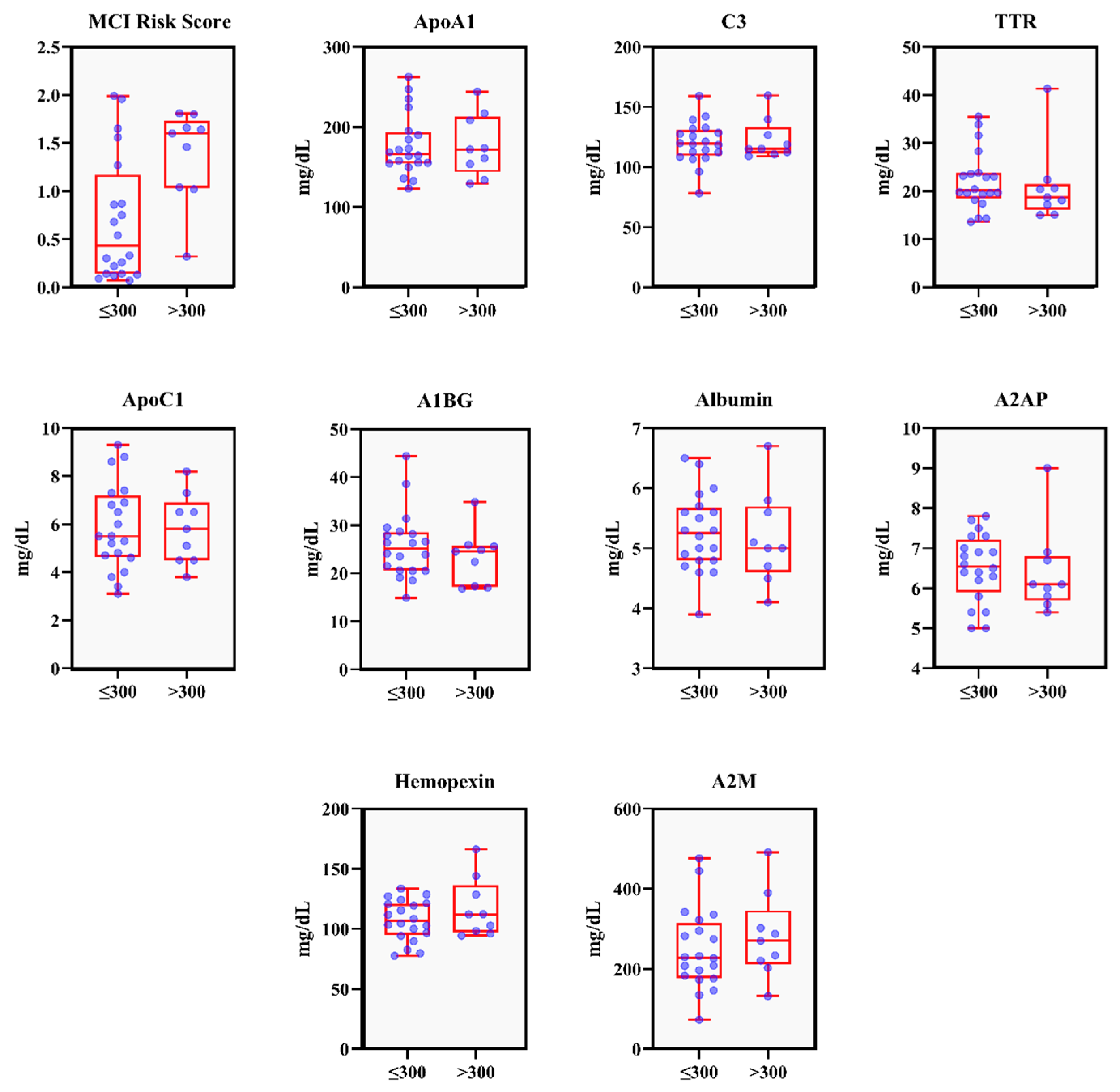
| Variable | Pisa Score Group | p-Value | |
|---|---|---|---|
| ≤300 mm2 (n = 20) | >300 mm2 (n = 9) | ||
| Age | 61.60 ± 11.63 | 67.11 ± 13.54 | 0.18 |
| Gender | |||
| Male | 13 (81.3) | 3 (18.8) | |
| Female | 7 (53.8) | 6 (46.2) | |
| No of teeth | 25.30 ± 4.25 | 25.44 ± 4.44 | 0.87 |
| BMI (kg/m2) | 25.69 ± 4.06 | 25.94 ± 2.96 | 0.90 |
| HbA1c (%) | 7.09 ± 0.67 | 7.32 ± 0.88 | 0.50 |
| Periodontal Parameters | |||
| PISA (mm2) | 147.66 ± 82.52 | 451.98 ± 172.37 | <0.01 * |
| PPD ≥ 4 mm (%) | 6.73 ± 4.72 | 20.60 ± 18.37 | 0.03 * |
| BOP (%) | 9.84 ± 4.64 | 28.87 ± 10.82 | <0.01 * |
| Inflammation Parameters | |||
| CRP (mg/dL) | 0.09 ± 0.14 | 0.22 ± 0.31 | 0.25 |
| IL-6 (pg/mL) | 18.59 ± 74.81 | 26.28 ± 70.19 | 0.29 |
| TNF-α (pg/mL) | 1.68 ± 1.70 | 2.54 ± 3.63 | 0.53 |
| MCI Risk Parameters | |||
| ApoA1 (mg/dL) | 177.30 ± 38.37 | 177.10 ± 38.82 | 1.00 |
| C3 (mg/dL) | 120.11 ± 17.47 | 123.022 ± 16.71 | 0.98 |
| TTR (mg/dL) | 22.10 ± 6.15 | 20.97 ± 8.01 | 0.36 |
| ApoC1 (mg/dL) | 5.87 ± 1.79 | 5.80 ± 1.45 | 0.87 |
| A1BG (mg/dL) | 25.75 ± 6.90 | 23.23 ± 5.77 | 0.31 |
| Albumin (mg/dL) | 5.26 ± 0.64 | 5.16 ± 0.77 | 0.66 |
| A2AP (mg/dL) | 6.51 ± 0.84 | 6.40 ± 1.08 | 0.36 |
| Hemopexin (mg/dL) | 107.08 ± 16.82 | 117.21 ± 24.57 | 0.50 |
| A2M (mg/dL) | 248.03 ± 100.69 | 281.22 ± 106.50 | 0.41 |
| MCI Risk score | 0.69 ± 0.65 | 1.37 ± 0.49 | 0.01 * |
| No risk—Low risk | 15 (75.0) | 1 (11.1) | |
| At risk—High risk | 5 (25.0) | 8 (88.9) | |
| Variable | N | “At risk” to “High risk” of MCI | OR (95% CI) | p-Value |
|---|---|---|---|---|
| PISA Score | 24.00 (1.338–30.606) | <0.01 * | ||
| ≤300 mm2 (Ref) | 20 | 5 (25.0%) | ||
| >300 mm2 | 9 | 8 (88.9%) |
| Variable 1 | Variable 2 | Spearman’s Correlation (ρ) | p-Value | Bootstrapped 95% Confidence Interval |
|---|---|---|---|---|
| PISA | MCI risk | 0.373 | 0.046 | [0.013, 0.657] |
| ApoC1 | −0.429 | 0.020 | [−0.736, −0.073] | |
| MCI risk | PISA | 0.373 | 0.046 | [0.013, 0.657] |
| TTR | −0.515 | 0.004 | [−0.777, −0.146] | |
| CRP | ApoA1 | −0.423 | 0.022 | [−0.676, −0.074] |
| HPX | 0.384 | 0.040 | [−0.033, 0.677] | |
| C3 | 0.428 | 0.020 | [0.085, 0.708] | |
| IL-6 | ApoA1 | −0.435 | 0.018 | [−0.695, −0.109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadhani, A.; Minagawa, K.; Takehara, S.; Kaneko, N.; Yamada, T.; Kitazawa, M.; Sone, H.; Fitrah, Y.A.; Nohno, K.; Ogawa, H. Periodontitis and Mild Cognitive Impairment Risk in Diabetic Patients: Insights from an Exploratory Analysis. Dent. J. 2025, 13, 505. https://doi.org/10.3390/dj13110505
Ramadhani A, Minagawa K, Takehara S, Kaneko N, Yamada T, Kitazawa M, Sone H, Fitrah YA, Nohno K, Ogawa H. Periodontitis and Mild Cognitive Impairment Risk in Diabetic Patients: Insights from an Exploratory Analysis. Dentistry Journal. 2025; 13(11):505. https://doi.org/10.3390/dj13110505
Chicago/Turabian StyleRamadhani, Aulia, Kumiko Minagawa, Sachiko Takehara, Noboru Kaneko, Takaho Yamada, Masaru Kitazawa, Hirohito Sone, Yusran Ady Fitrah, Kaname Nohno, and Hiroshi Ogawa. 2025. "Periodontitis and Mild Cognitive Impairment Risk in Diabetic Patients: Insights from an Exploratory Analysis" Dentistry Journal 13, no. 11: 505. https://doi.org/10.3390/dj13110505
APA StyleRamadhani, A., Minagawa, K., Takehara, S., Kaneko, N., Yamada, T., Kitazawa, M., Sone, H., Fitrah, Y. A., Nohno, K., & Ogawa, H. (2025). Periodontitis and Mild Cognitive Impairment Risk in Diabetic Patients: Insights from an Exploratory Analysis. Dentistry Journal, 13(11), 505. https://doi.org/10.3390/dj13110505






