Liver Disease and Periodontal Pathogens: A Bidirectional Relationship Between Liver and Oral Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- ✓
- Population (P): Individuals (children or adults) diagnosed with liver diseases (including, but not limited to, hepatic steatosis, hepatitis, cirrhosis, or liver failure).
- ✓
- Intervention/Exposure (I): Presence or detection of specific periodontal pathogens, namely Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, and Tannerella forsythia (identified by clinical, microbiological, or molecular methods).
- ✓
- Comparison (C): Individuals with liver diseases without detectable periodontal pathogens, or with a lower microbial burden, or, alternatively, healthy individuals without liver diseases.
- ✓
- Outcome (O): Primary: Prevalence and/or severity of liver disease in relation to the presence or abundance of specific periodontal pathogens: Secondary: Evidence of a correlation between periodontal infection and hepatic pathology (e.g., progression, complications, inflammatory markers, or liver-related morbidity/mortality).
2.3. Sources of Information, Search Strategy and Study Selection
- ✓
- PubMed:Search: (“Liver Diseases”[MeSH] OR “hepatic diseases” OR “hepatopathy” OR “hepatitis” OR “cirrhosis” OR “hepatic steatosis” OR “liver failure”) AND (“Periodontal Diseases”[MeSH] OR “periodontitis” OR “periodontal disease” OR “oral microbiome” OR “oral bacteria” OR “periodontal pathogens”) AND (“Porphyromonas gingivalis” OR “Aggregatibacter actinomycetemcomitans” OR “Prevotella intermedia” OR “Tannerella forsythia”) Sort by: Most RecentQuery: (“Liver Diseases”[MeSH Terms] OR “hepatic diseases”[All Fields] OR “hepatopathy”[All Fields] OR “hepatitis”[All Fields] OR “cirrhosis”[All Fields] OR “hepatic steatosis”[All Fields] OR “liver failure”[All Fields]) AND (“Periodontal Diseases”[MeSH Terms] OR “periodontitis”[All Fields] OR “periodontal disease”[All Fields] OR “oral microbiome”[All Fields] OR “oral bacteria”[All Fields] OR “periodontal pathogens”[All Fields]) AND (“Porphyromonas gingivalis”[All Fields] OR “Aggregatibacter actinomycetemcomitans”[All Fields] OR “Prevotella intermedia”[All Fields] OR “Tannerella forsythia”[All Fields])
- ✓
- Scopus:TITLE-ABS-KEY (“liver disease” AND “Porphyromonas”)
- ✓
- Cochrane library:Search term used in Title and Abstract: “liver periodontitis”
2.4. Data Collection Process and Data Characteristics
2.5. Risk of Bias in Individual and Cross-Sectional Studies
3. Results
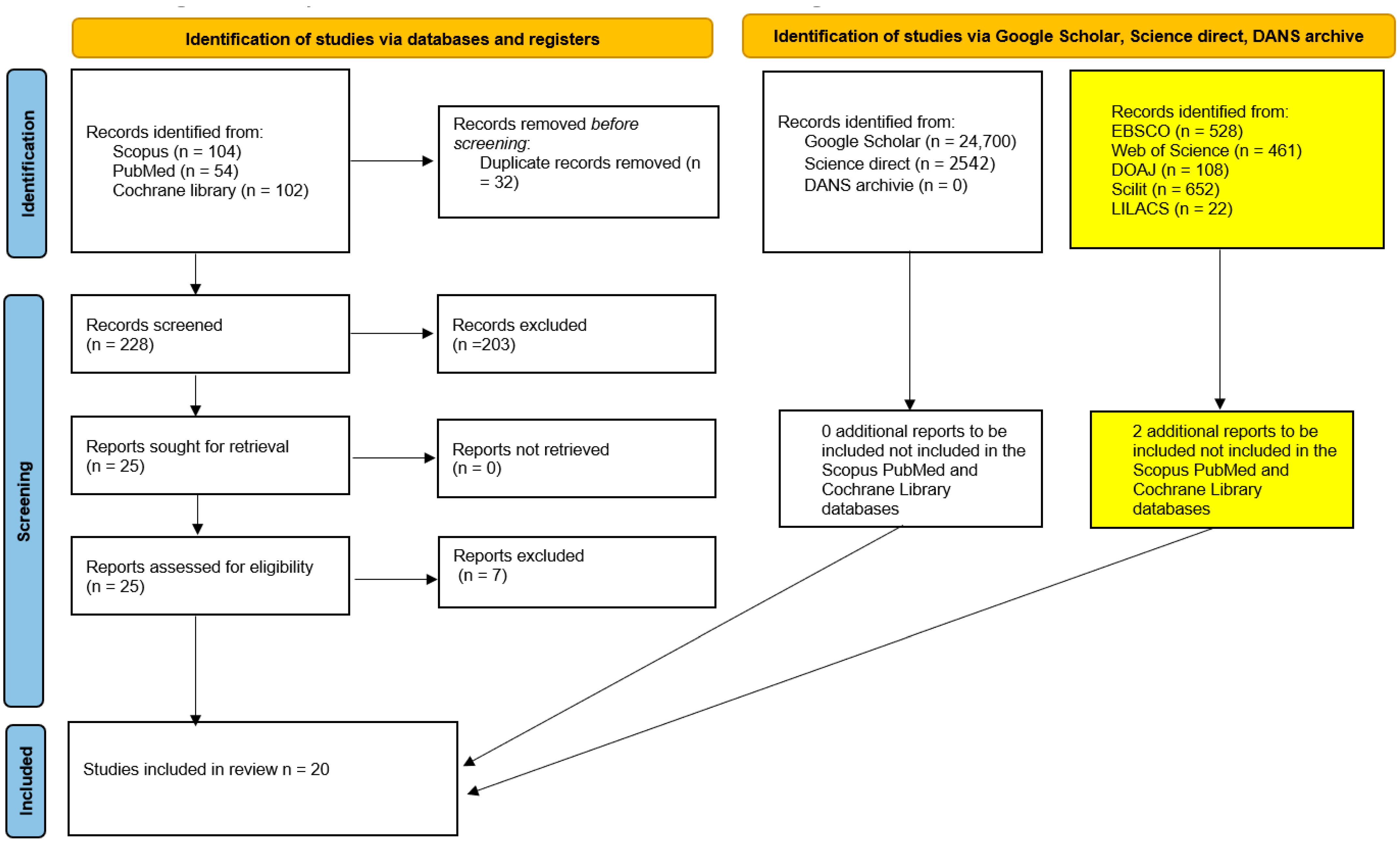
3.1. Study Selection
- ✓
- Two studies were literature reviews not explicitly identified as such in the title or abstract;
- ✓
- Two studies were in vitro studies not explicitly identified as such in the title or abstract;
- ✓
- One study was a research protocol of a randomized trial.
- ✓
- One study reported data from a set of patients already included in the review, which was excluded to avoid data overlap.
- ✓
- One study was excluded because it reported data from only five patients.
- ✓
- Kuraji et al., 2024 [22]. This study was excluded because it was an interventional animal model study (murine), with only a small translational component consisting of an observational human autopsy analysis. These details were not reported in the abstract or title. The findings indicated that mice with induced periodontitis exhibited a worsening of hepatic steatosis compared to controls. Furthermore, a correlation was observed between the severity of periodontitis (measured as tooth loss) and the severity of liver disease.
- ✓
- Kuraji et al., 2023 [23]. This study was excluded because it was a narrative review (not specified in the abstract), addressing the relationships between periodontal disease, dysbiosis, and NAFLD, as well as therapeutic strategies with a focus on the microbiome.
- ✓
- Nagao and Tsuji, 2021 [24]. This study was excluded because it was a pilot investigation involving only four cases. The main conclusion was that the elimination of HCV (Hepatitis C Virus) through direct-acting antiviral therapy in patients with hepatitis C and oral lichen planus was associated with improvements in oral lesions and a reduction in the salivary load of periodontopathogenic bacteria.
- ✓
- Kamata et al., 2020 [25]. This publication described the protocol of a prospective, multicenter, two-arm, open-label, randomized controlled trial enrolling adult patients (20–85 years, both sexes), with NAFLD and moderate periodontitis.
- ✓
- Wu et al., 2018 [26]. This was an in vitro study and was excluded (not stated in the title or abstract).
- ✓
- Lê et al., 2024 [28]. This study was excluded because it was a narrative review (not stated in the title or abstract), addressing the topic of liver diseases and the oral microbiota.
- ✓
- Emelyanov and Emelyanova, 2022 [27]. This study addressed data that had already been reported.
3.2. Data Characteristics
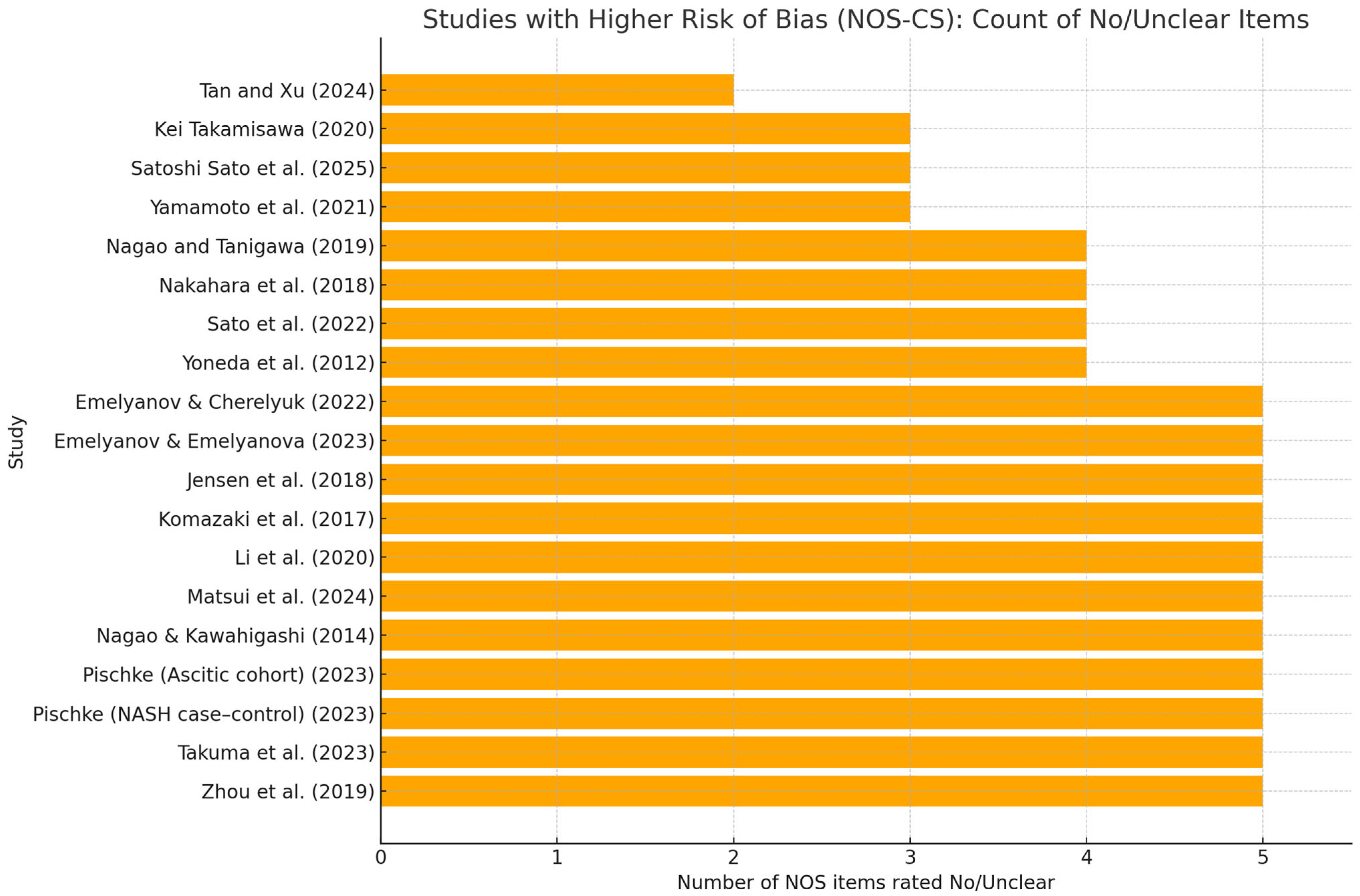
3.3. Risk of Bias
3.4. Study-Level Synthesis of Periodontal Exposures and Liver Outcomes
3.4.1. MASLD/NAFLD
- Tan and Xu, 2024 [29]. In a nationally representative sample of U.S. adults (NHANES III; n = 6330), the authors reported that there was no evidence of an association between circulating antibody responses to key periodontal pathogens (including Porphyromonas gingivalis) and ultrasound-defined NAFLD after survey-weighted adjustment; (OR 0.996; 95% CI 0.963–1.030), consistent with null effect.
- Pischke et al., 2023 [33]. NASH severity (prospective), focusing on P. gingivalis and Aggregatibacter actinomycetemcomitans as principal periodontal pathogens, the study reported a close correlation between periodontal disease and more severe NASH, supporting the hypothesis that poor oral health may contribute to systemic inflammation and liver-disease progression (observational association; no proof of causality).
- Sato et al., 2022 [35]. Periodontitis, particularly when associated with P. gingivalis, was linked to a higher risk of advanced hepatic fibrosis in NAFLD (salivary P. gingivalis proportion ≥ 0.01% by qPCR associated with increased liver stiffness); findings support periodontitis as a potential additional risk factor for fibrosis progression; (OR 4.05; 95% CI 1.056–15.52), but precision is low (very wide CI).
- Yamamoto et al., 2021 [36]. Toothbrushing three times daily was associated with a significantly lower risk of NAFLD compared with less frequent brushing, independent of age, sex, BMI and lifestyle factors; the association was most apparent in individuals with obesity. The study assessed behaviors (brushing frequency) rather than specific oral bacteria.
- Nakahara et al., 2018 [41]. Biopsy-proven NAFLD/P. gingivalis infection, specifically seropositivity to fimA type 4, was independently associated with advanced hepatic fibrosis in NAFLD after multivariable adjustment; (OR 2081; 95% CI 1098–3943) moderate effect, but with non-negligible uncertainty.
- Yoneda et al., 2012 [43]. NAFLD vs. controls. P. gingivalis detection frequency was significantly higher in NAFLD than in non-NAFLD controls; invasive fimA genotypes were more often represented in NAFLD; (OR 2615; 95% CI 1001–6832), borderline and imprecise estimate for CI very close to unity.
- Emelyanov and Emelyanova, 2023 [45]. NAFLD vs. healthy and cohabiting relatives. NAFLD was associated with oral dysbiosis, with increased second-order periodontopathogens (e.g., Porphyromonas endodontalis, Prevotella intermedia, Fusobacterium nucleatum), altered salivary parameters (reduced flow, higher viscosity, more acidic pH), and higher oral endotoxin levels.
- Takamisawa et al. [37]. Higher anti-P. gingivalis IgG levels were associated with an increased risk of liver enzyme abnormalities, particularly alanine aminotransferase (ALT); effects were often sex-stratified (e.g., stronger associations in women), (OR 2.80; 95% CI 1.22–6.44); enzyme/sex-specific model, limited generalizability.
- Sato et al., 2025 [30]. Population-based cohort (MASLD). In a large Japanese population sample, salivary 16S-based community profiles (e.g., clusters dominated by Neisseria, Streptococcus, Fusobacterium, Veillonella) were associated with MASLD phenotypes defined by CAP and cardiometabolic criteria. Pg-specific signals were not dominant; models included key behavioral and metabolic confounders. Interpretation: supports a community-level oral signal for MASLD rather than a single-pathogen effect.
- Emelyanov & Cherelyuk, 2022 [44]. NAFLD vs. healthy (Ukraine). Using qRT-PCR for P. gingivalis and gingipain-K ELISA, alongside measurements of salivary physicochemical properties, NAFLD participants showed higher periodontal pathogen burden, increased oral endotoxin, and poorer salivary function, consistent with an oral–systemic inflammatory axis.
- Komazaki et al., 2017 [48]. Oral signal centered on Aggregatibacter actinomycetemcomitans: higher anti-Aa IgG, as well as weakly higher anti-Fusubacterium nucleatum, were linked to greater visceral/total adiposity, higher fasting insulin and HOMA-IR, and a lower CT liver–spleen ratio (more steatosis). No relevant association for P. gingivalis. Overall, Aa-specific serology is the most consistent oral marker of a worse hepatic/metabolic phenotype in NAFLD, though evidence is correlational and unadjusted.
3.4.2. Viral Hepatitis (HBV/HCV)
- 12.
- Li et al. [46]. HBV → cirrhosis → HCC (small 16S study). Across HBV, HBV cirrhosis, and HCC, shifts in salivary bacterial diversity and taxa were observed, suggesting exploratory biomarker potential (sample sizes per group were small; findings are preliminary).
- 13.
- Nagao and Tanigawa, 2019 [38]. Chronic viral hepatitis. Patients with chronic viral hepatitis exhibited greater clinical attachment loss and poorer periodontal status than comparators, indicating a heavier periodontal burden in this setting; (OR 4.08; 95% CI 1.12–15.96), limited extrapolability to a non-hepatic outcome.
- 14.
- Nagao et al., 2014 [42]. HBV/HCV liver disease. Periodontitis was associated with fibrosis progression in viral liver disease (HBV and/or HCV). Several systemic and behavioral factors (low platelet count, older age, obesity, poor oral hygiene, interferon therapy) were identified as risk factors for periodontitis.
3.4.3. Alcohol-Related Disease/AAH
- 15.
- Zhou et al., 2019 [39]. Acute alcoholic hepatitis (AAH). Patients with AAH had significantly higher plasma antibodies (IgG/IgA/IgM) to P. gingivalis than healthy controls, especially in severe AAH and certain subgroups (e.g., women). In AAH, an active IgM response to P. gingivalis correlated with indices of liver injury (AST/ALT ratio, MELD), whereas long-standing alcohol exposure appeared to attenuate IgM responses.
3.4.4. Fibrosis/Cirrhosis/Ascites
- 16.
- Pischke et al., 2023 [32]. Decompensated cirrhosis with ascites (prospective cohort). Using real-time PCR, P. gingivalis was detected in periodontal pockets in 26% and in feces in 7% of patients, but not in ascitic fluid; Aggregatibacter (Actinobacillus) actinomycetemcomitans was found in pockets in 7% and not in feces or ascites. There was no evidence of direct translocation into ascites.
- 17.
- Jensen et al., 2018 [40]. Cirrhosis with periodontitis. Among patients with cirrhosis, periodontitis was associated with a distinct subgingival microbiota compared with typical periodontitis, characterized by low abundance of classical red-complex pathogens and relative predominance of bacteria usually deemed commensals, consistent with cirrhosis-related immune dysfunction enabling altered pathogenicity.
3.4.5. HCC/Malignant Progression
- 18.
- Takuma et al., 2023 [47]. Oral P. gingivalis and Fusubacterium nucleatum was found to be likely associated with the pathogenesis of NASH-HCC. Furthermore, Fusubacterium nucleatum was found to be negatively correlated with salivary IgA levels.
- 19.
- Matsui et al., 2024 [31]. Salivary levels of P. gingivalis, Tannerella forsythia and Prevotella intermedia tended to be higher in MASH-HCC than in MASH alone, but these differences did not consistently reach statistical significance. Overall salivary community structure was broadly similar between groups at the phylum and genus levels; however, Fusobacterium nucleatum was significantly enriched in MASH-HCC.
3.4.6. Interventional Evidence
- 20.
- Kamata et al., 2022 [34]. Randomized controlled trial. Intensive periodontal therapy (scaling and root planing, SRP) in NAFLD patients with moderate periodontitis led to greater improvements in liver enzymes, steatosis indices, and endotoxin, as well as reductions in anti-P. gingivalis responses and improved periodontal parameters, compared with home care alone, suggesting periodontal treatment as a potential adjunctive strategy in NAFLD with periodontitis.
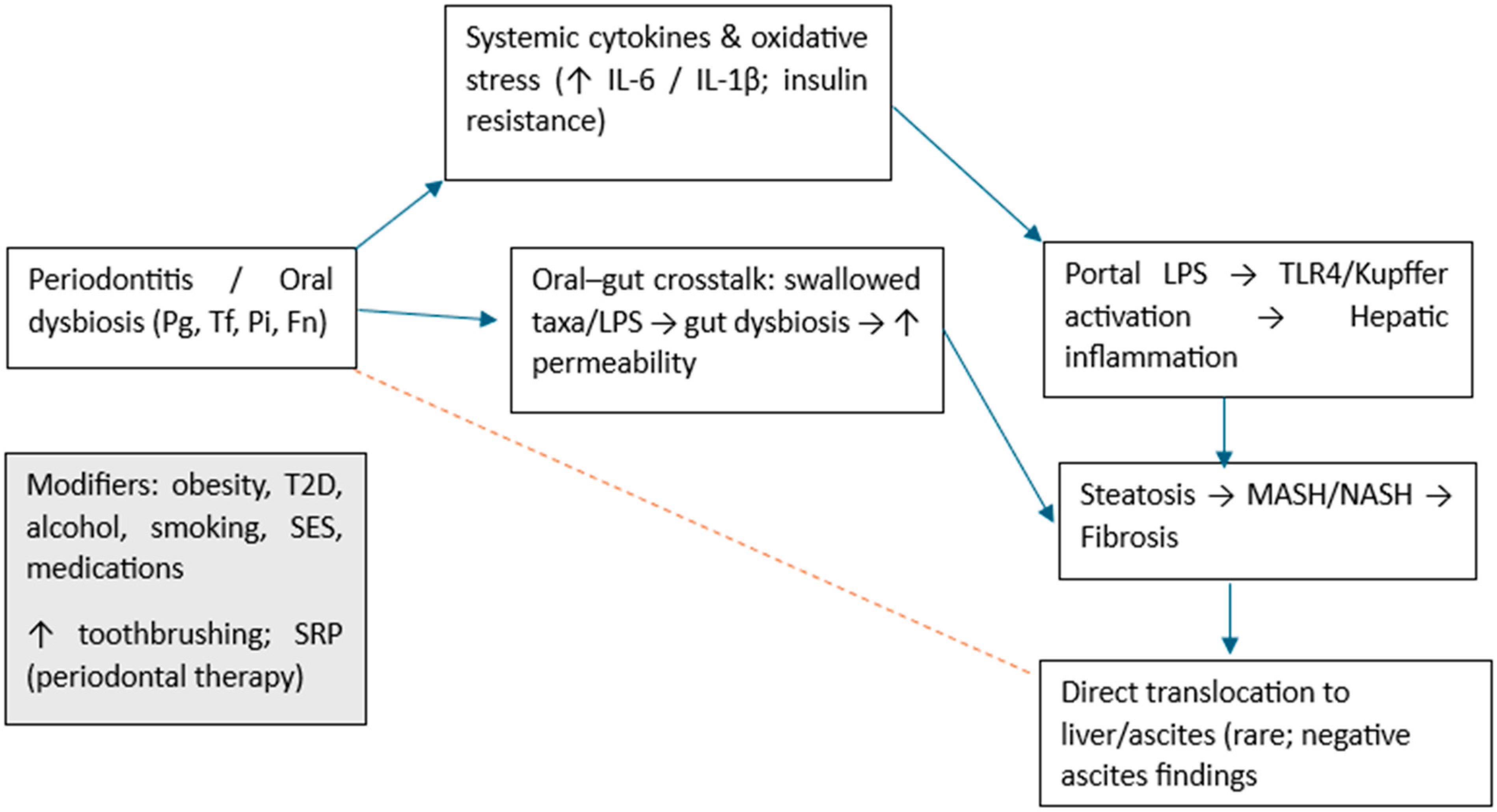
4. Discussion
4.1. Limitations of the Review
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durazzo, M.; Ponzo, E.; Bonetto, S.; Fagoonee, S.; Pellicano, R. Liver diseases in the elderly. Minerva Medica 2019, 110, 35–51. [Google Scholar] [CrossRef]
- Lv, C.; Shi, K.; Guo, Y.; Guo, Z.; Luo, P.; Wang, L.; Wu, Z.; Yu, P. Emerging Roles of Periodontal Pathogen-Derived Outer Membrane Vesicles in NAFLD. Int. Dent. J. 2025, 75, 100825. [Google Scholar] [CrossRef]
- Gan, C.; Yuan, Y.; Shen, H.; Gao, J.; Kong, X.; Che, Z.; Guo, Y.; Wang, H.; Dong, E.; Xiao, J. Liver diseases: Epidemiology, causes, trends and predictions. Signal Transduct. Target. Ther. 2025, 10, 33. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Schmeltzer, P.A.; Kosinski, A.S.; Kleiner, D.E.; Hoofnagle, J.H.; Stolz, A.; Fontana, R.J.; Russo, M.W. Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int. 2016, 36, 603–609. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Mousa, N.; Elmetwalli, A.; Abdel-Razik, A.; Mousa, E.; Abdelsalam, M.; Elbaz, S.; El-Wakeel, N.; Eldars, W.; Gad, E.; Arafa, M.; et al. Periodontitis and Metabolic Dysfunction-Associated Steatotic Liver Disease: Emphasizing the clinical interplay between hepatologists and dentists. Odontology 2025, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cota, J.E.; Dhupar, A.; Spadigam, A.; Carvalho, K. Keyes triad in type 2 diabetes mellitus: A microbiological study. Adv. Biomed. Res. 2021, 10, 36. [Google Scholar] [CrossRef]
- Luo, X.Y.; Huang, W.B.; Lu, C.H.; Gu, W.; Feng, Z.X.; Shen, S.; Chen, M.Z.; Zheng, S.S.; Yang, Z. Application of probiotic therapy in nonalcoholic fatty liver disease: Mediating mechanism and future perspective. Front. Cell. Infect. Microbiol. 2025, 15, 1638372. [Google Scholar] [CrossRef]
- Costa, F.O.; Lages, E.J.P.; Lages, E.M.B.; Cota, L.O.M. Periodontitis in individuals with liver cirrhosis: A case-control study. J. Clin. Periodontol. 2019, 46, 991–998. [Google Scholar] [CrossRef]
- Ballini, A.; Tetè, S.; Scattarella, A.; Cantore, S.; Mastrangelo, F.; Papa, F.; Nardi, G.M.; Perillo, L.; Crincoli, V.; Gherlone, E.; et al. The role of anti-cyclic citrullinated peptide antibody in periodontal disease. Int. J. Immunopathol. Pharmacol. 2010, 23, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef] [PubMed]
- Kuraji, R.; Sekino, S.; Kapila, Y.; Numabe, Y. Periodontal disease-related nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: An emerging concept of oral-liver axis. Periodontol. 2000 2021, 87, 204–240. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Sahingur, S.E. Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis. Periodontol. 2000 2022, 89, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Gopalakrishnan, A.V.; Madhyastha, H. Is periodontitis triggering an inflammatory response in the liver, and does this reaction entail oxidative stress? Odontology 2025, 113, 889–902. [Google Scholar] [CrossRef]
- Rodríguez-Montaño, R.; Martínez-Nieto, M.; González-Alvarez, G.E.; Alarcón-Sánchez, M.A.; Becerra-Ruiz, J.S.; Heboyan, A.; Ruiz-Gaitán, A.; Lomelí-Martínez, S.M. Hepatitis and periodontal health: An emerging oral-liver axis. Ther. Adv. Chronic Dis. 2025, 16, 20406223251368090. [Google Scholar] [CrossRef]
- Liu, L.; Geng, Y.; Xiong, C. Impact of Porphyromonas gingivalis-odontogenic infection on the pathogenesis of non-alcoholic fatty liver disease. Ann. Med. 2023, 55, 2255825. [Google Scholar] [CrossRef]
- Dioguardi, M.; Dello Russo, C.; Scarano, F.; Esperouz, F.; Ballini, A.; Sovereto, D.; Alovisi, M.; Martella, A.; Lo Muzio, L. Analysis of Endodontic Successes and Failures in the Removal of Fractured Endodontic Instruments during Retreatment: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. Healthcare 2024, 12, 1390. [Google Scholar] [CrossRef]
- Longo, A.; Zappatore, M.; Martella, A.; Rucco, C. Enhancing Data Education with Datathons: An Experience with Open Data on Renewable Energy Systems. In Proceedings of the 1st ACM SIGMOD International Workshop on Data Systems Education: Bridging Education Practice with Education Research (DataEd 2022), Philadelphia, PA, USA, 12–17 June 2022; pp. 26–31. [Google Scholar]
- Caione, A.; Guido, A.L.; Martella, A.; Paiano, R.; Pandurino, A. Knowledge base support for dynamic information system management. Inf. Syst. e-Bus. Manag. 2016, 14, 533–576. [Google Scholar] [CrossRef]
- Martella, A.; Longo, A.; Zappatore, M.; Martino, B.D.; Esposito, A. A semantically enabled architecture for interoperable edge-cloud continuum applied to the e-health scenario. Softw. Pract. Exp. 2025, 55, 409–447. [Google Scholar] [CrossRef]
- Kuraji, R.; Ye, C.; Zhao, C.; Gao, L.; Martinez, A.; Miyashita, Y.; Radaic, A.; Kamarajan, P.; Le, C.; Zhan, L.; et al. Nisin lantibiotic prevents NAFLD liver steatosis and mitochondrial oxidative stress following periodontal disease by abrogating oral, gut and liver dysbiosis. npj Biofilms Microbiomes 2024, 10, 3. [Google Scholar] [CrossRef]
- Kuraji, R.; Shiba, T.; Dong, T.S.; Numabe, Y.; Kapila, Y.L. Periodontal treatment and microbiome-targeted therapy in management of periodontitis-related nonalcoholic fatty liver disease with oral and gut dysbiosis. World J. Gastroenterol. 2023, 29, 967–996. [Google Scholar] [CrossRef]
- Nagao, Y.; Tsuji, M. Effects of Hepatitis C Virus Elimination by Direct-Acting Antiviral Agents on the Occurrence of Oral Lichen Planus and Periodontal Pathogen Load: A Preliminary Report. Int. J. Dent. 2021, 2021, 8925879. [Google Scholar] [CrossRef]
- Kamata, Y.; Kessoku, T.; Shimizu, T.; Kobayashi, T.; Kurihashi, T.; Sato, S.; Kuraji, S.; Aoyama, N.; Iwasaki, T.; Takashiba, S.; et al. Efficacy and safety of PERIOdontal treatment versus usual care for Nonalcoholic liver disease: Protocol of the PERION multicenter, two-arm, open-label, randomized trial. Trials 2020, 21, 291. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, L.; Wang, J.; Liu, C.; Li, Y.; Wu, Y. pckA-deficient Porphyromonas gingivalis W83 shows reduction in hemagglutination activity and alteration in the distribution of gingipain activity. Eur. J. Oral Sci. 2018, 126, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, D.V.; Emelyanova, N.Y. Risk factors for chronic periodontitis in patients with non-alcoholic fatty liver disease. Mod. Gastroenterol. 2022, 5–6, 12–16. [Google Scholar] [CrossRef]
- Lê, S.; Minty, M.; Boyer, É.; Blasco-Baque, V.; Bonnaure-Mallet, M.; Meuric, V. Oral microbiota and liver. Med. Sci. 2024, 40, 42–48. [Google Scholar] [CrossRef]
- Tan, L.; Xu, S.Q. Association between serum antibodies to oral microorganisms and nonalcoholic fatty liver disease in adults. BMC Oral Health 2024, 24, 1352. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Iino, C.; Furusawa, K.; Yoshida, K.; Chinda, D.; Sawada, K.; Mikami, T.; Nakaji, S.; Fukuda, S.; Sakuraba, H. Effect of Oral Microbiota Composition on Metabolic Dysfunction-Associated Steatotic Liver Disease in the General Population. J. Clin. Med. 2025, 14, 2013. [Google Scholar] [CrossRef]
- Matsui, T.; Morozumi, T.; Yamamoto, Y.; Kobayashi, T.; Takuma, R.; Yoneda, M.; Nogami, A.; Kessoku, T.; Tamura, M.; Nomura, Y.; et al. Relationship of Metabolic Dysfunction-Associated Steatohepatitis-Related Hepatocellular Carcinoma with Oral and Intestinal Microbiota: A Cross-Sectional Pilot Study. Medicina 2024, 60, 1150. [Google Scholar] [CrossRef]
- Pischke, S.; Ashouri, M.M.; Peters, U.; Shiprov, A.; Schulze Zur Wiesch, J.; Sterneck, M.; Fischer, F.; Huebener, P.; Mader, M.; Fischer, L.; et al. High incidence of periodontitis in patients with ascitic decompensated cirrhosis. World J. Hepatol. 2023, 15, 1325–1332. [Google Scholar] [CrossRef]
- Pischke, S.; Shiprov, A.; Peters, U.; Schulze Zur Wiesch, J.; Kluwe, J.; Westphal, T.; Fischer, F.; Mader, M.; Fründt, T.; Horvatits, K.; et al. High prevalence of periodontal disease in patients with NASH- possible association of poor dental health with NASH severity. Ann. Hepatol. 2023, 28, 100887. [Google Scholar] [CrossRef]
- Kamata, Y.; Kessoku, T.; Shimizu, T.; Sato, S.; Kobayashi, T.; Kurihashi, T.; Morozumi, T.; Iwasaki, T.; Takashiba, S.; Hatanaka, K.; et al. Periodontal Treatment and Usual Care for Nonalcoholic Fatty Liver Disease: A Multicenter, Randomized Controlled Trial. Clin. Transl. Gastroenterol. 2022, 13, e00520. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kamata, Y.; Kessoku, T.; Shimizu, T.; Kobayashi, T.; Kurihashi, T.; Takashiba, S.; Hatanaka, K.; Hamada, N.; Kodama, T.; et al. A cross-sectional study assessing the relationship between non-alcoholic fatty liver disease and periodontal disease. Sci. Rep. 2022, 12, 13621. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ikeya, T.; Okuyama, S.; Fukuda, K.; Kobayashi, D. Association between the Frequency of Daily Toothbrushing and Development of Nonalcoholic Fatty Liver Disease. Dig. Dis. 2021, 39, 646–652. [Google Scholar] [CrossRef]
- Takamisawa, K.; Sugita, N.; Komatsu, S.; Wakasugi, M.; Yokoseki, A.; Yoshihara, A.; Kobayashi, T.; Nakamura, K.; Onodera, O.; Momotsu, T.; et al. Association between serum IgG antibody titers against Porphyromonas gingivalis and liver enzyme levels: A cross-sectional study in Sado Island. Heliyon 2020, 6, e05531. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Tanigawa, T. Red complex periodontal pathogens are risk factors for liver cirrhosis. Biomed. Rep. 2019, 11, 199–206. [Google Scholar] [CrossRef]
- Zhou, Y.; Vatsalya, V.; Gobejishvili, L.; Lamont, R.J.; McClain, C.J.; Feng, W. Porphyromonas gingivalis as a Possible Risk Factor in the Development/Severity of Acute Alcoholic Hepatitis. Hepatol. Commun. 2019, 3, 293–304. [Google Scholar] [CrossRef]
- Jensen, A.; Ladegaard Grønkjær, L.; Holmstrup, P.; Vilstrup, H.; Kilian, M. Unique subgingival microbiota associated with periodontitis in cirrhosis patients. Sci. Rep. 2018, 8, 10718. [Google Scholar] [CrossRef]
- Nakahara, T.; Hyogo, H.; Ono, A.; Nagaoki, Y.; Kawaoka, T.; Miki, D.; Tsuge, M.; Hiraga, N.; Hayes, C.N.; Hiramatsu, A.; et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J. Gastroenterol. 2018, 53, 269–280. [Google Scholar] [CrossRef]
- Nagao, Y.; Kawahigashi, Y.; Sata, M. Association of Periodontal Diseases and Liver Fibrosis in Patients With HCV and/or HBV infection. Hepat. Mon. 2014, 14, e23264. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Naka, S.; Nakano, K.; Wada, K.; Endo, H.; Mawatari, H.; Imajo, K.; Nomura, R.; Hokamura, K.; Ono, M.; et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012, 12, 16. [Google Scholar] [CrossRef]
- Emelyanov, D.V.; Cherelyuk, N.I. Nonalcoholic fatty liver disease as an endogenous factor of dysbiosis in the structure of oral microbiome. Ukr. Ther. J. 2022, 2022, 42–47. [Google Scholar] [CrossRef]
- Emelyanov, D.V.; Emelyanova, N.Y. The results of dysbiosis in the structure of periodontopathogens of the II order in patients with non-alcoholic fatty liver disease. Mod. Gastroenterol. 2023, 3, 29–35. [Google Scholar] [CrossRef]
- Li, D.; Xi, W.; Zhang, Z.; Ren, L.; Deng, C.; Chen, J.; Sun, C.; Zhang, N.; Xu, J. Oral microbial community analysis of the patients in the progression of liver cancer. Microb. Pathog. 2020, 149, 104479. [Google Scholar] [CrossRef]
- Takuma, R.; Morozumi, T.; Yamamoto, Y.; Kobayashi, T.; Matsui, T.; Yoneda, M.; Kessoku, T.; Nogami, A.; Tamura, M.; Kamata, Y.; et al. Association between Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma and Periodontopathic Bacteria: A Cross-Sectional Pilot Study. Appl. Sci. 2023, 13, 3893. [Google Scholar] [CrossRef]
- Komazaki, R.; Katagiri, S.; Takahashi, H.; Maekawa, S.; Shiba, T.; Takeuchi, Y.; Kitajima, Y.; Ohtsu, A.; Udagawa, S.; Sasaki, N.; et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci. Rep. 2017, 7, 13950. [Google Scholar] [CrossRef]
- Jirillo, E.; Caccavo, D.; Magrone, T.; Piccigallo, E.; Amati, L.; Lembo, A.; Kalis, C.; Gumenscheimer, M. The role of the liver in the response to LPS: Experimental and clinical findings. J. Endotoxin Res. 2002, 8, 319–327. [Google Scholar] [CrossRef]
- Shrestha, D.; Choi, Y.H.; Zhang, J.; Hazlett, L.J.; Merchant, A.T. Relationship between serologic markers of periodontal bacteria and metabolic syndrome and its components. J. Periodontol. 2015, 86, 418–430. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, Y.-M.; Lee, G.-N.; Song, H.C.; Ahn, Y.-B.; Han, K.; Ko, S.-H. Association between toothbrushing and non-alcoholic fatty liver disease. PLoS ONE 2021, 16, e0243686. [Google Scholar] [CrossRef] [PubMed]
- De Munck, T.J.I.; Xu, P.; Verwijs, H.J.A.; Masclee, A.A.M.; Jonkers, D.; Verbeek, J.; Koek, G.H. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020, 40, 2906–2916. [Google Scholar] [CrossRef]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef]
- Akiba, Y.; Maruta, K.; Takajo, T.; Narimatsu, K.; Said, H.; Kato, I.; Kuwahara, A.; Kaunitz, J.D. Lipopolysaccharides transport during fat absorption in rodent small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G1070–G1087. [Google Scholar] [CrossRef]
- Sookoian, S.; Salatino, A.; Castaño, G.O.; Landa, M.S.; Fijalkowky, C.; Garaycoechea, M.; Pirola, C.J. Intrahepatic bacterial metataxonomic signature in non-alcoholic fatty liver disease. Gut 2020, 69, 1483. [Google Scholar] [CrossRef]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Villard, A.; Boursier, J.; Andriantsitohaina, R. Bacterial and eukaryotic extracellular vesicles and nonalcoholic fatty liver disease: New players in the gut-liver axis? Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G485–G495. [Google Scholar] [CrossRef]
- Ballini, A.; Scacco, S.; Boccellino, M.; Santacroce, L.; Arrigoni, R. Microbiota and Obesity: Where Are We Now? Biology 2020, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Genco, R. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S106–S112. [Google Scholar] [CrossRef] [PubMed]
- Chancharoenthana, W.; Kamolratanakul, S.; Udompornpitak, K.; Wannigama, D.L.; Schultz, M.J.; Leelahavanichkul, A. Alcohol-induced gut permeability defect through dysbiosis and enterocytic mitochondrial interference causing pro-inflammatory macrophages in a dose dependent manner. Sci. Rep. 2025, 15, 14710. [Google Scholar] [CrossRef]
- Mullany, D.V.; Pilcher, D.V.; Dobson, A.J. Associations Between Socioeconomic Status, Patient Risk, and Short-Term Intensive Care Outcomes. Crit. Care Med. 2021, 49, e849–e859. [Google Scholar] [CrossRef]
- Sun, B.; Liu, X.; Jiang, Y.; Qi, S.; Guan, Z.; Li, H. Proteomics reveals periodontitis-driven oxidative stress and lipid metabolism disruption in NAFLD. Front. Endocrinol. 2025, 16, 1600015. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.; Ayares, G.; Taboun, Z.; Malhi, G.; Idalsoaga, F.; Mortuza, R.; Souyet, M.; Ramirez-Cadiz, C.; Díaz, L.A.; Arrese, M. Periodontal disease and cirrhosis: Current concepts and future prospects. eGastroenterology 2025, 3, e100140. [Google Scholar] [CrossRef] [PubMed]
| Databases | Search | Records | Date |
|---|---|---|---|
| Ebsco | liver disease AND periodontitis | 528 | 1 October 2025 |
| LILACS | liver disease AND periodontitis | 22 | 1 October 2025 |
| DOAJ | liver disease AND periodontitis | 108 | 1 October 2025 |
| Scilit (MDPI) | liver disease AND periodontitis | 652 | 1 October 2025 |
| Web of science | liver disease AND periodontitis | 461 | 1 October 2025 |
| First Author, Data, Reference | Country | Study Design | Source Database | Reason for Exclusion |
|---|---|---|---|---|
| Kuraji et al., 2024 [22] | Japan, USA, China | In vitro | PubMed, Web of Science, Scopus | In vitro |
| Kuraji et al., 2023 [23] | Japan, USA | Narrative review | PubMed, Web of Science, Scopus | Review |
| Nagao and Tsuji, 2021 [24] | Japan | Prospective case series, pilot study | PubMed, Web of Science, Scopus | Only four cases |
| Kamata et al., 2020 [25] | Japan | Protocol RCT | PubMed, Web of Science, Scopus, DOJA | Protocol |
| Wu et al., 2018 [26] | China | In vitro | PubMed, Web of Science, Scopus | In vitro |
| Emelyanov and Emelyanova, 2022 [27] | Ukraine | Cross-sectional | Scopus | Data already reported |
| Lê et al., 2024 [28] | France | Narrative review | Scopus | Review |
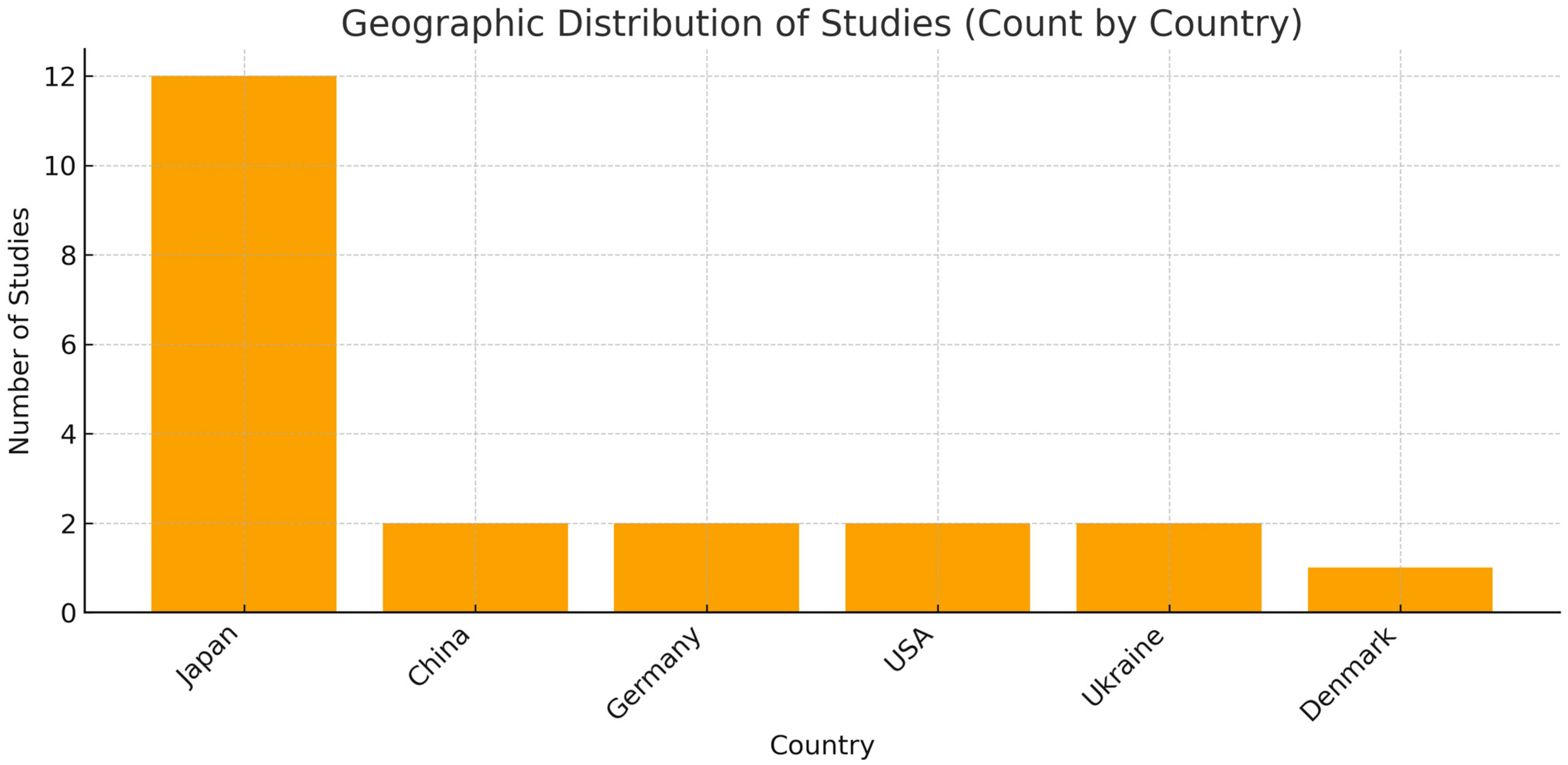
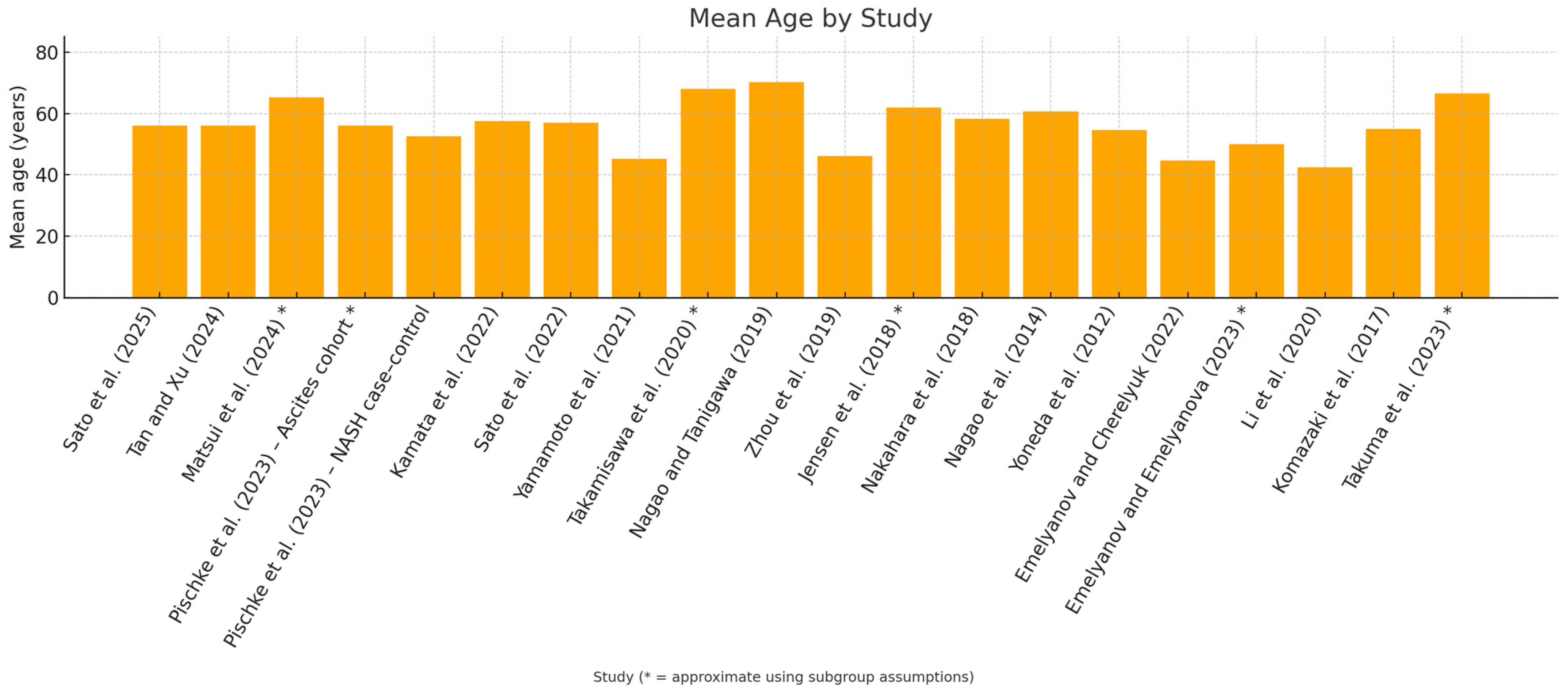
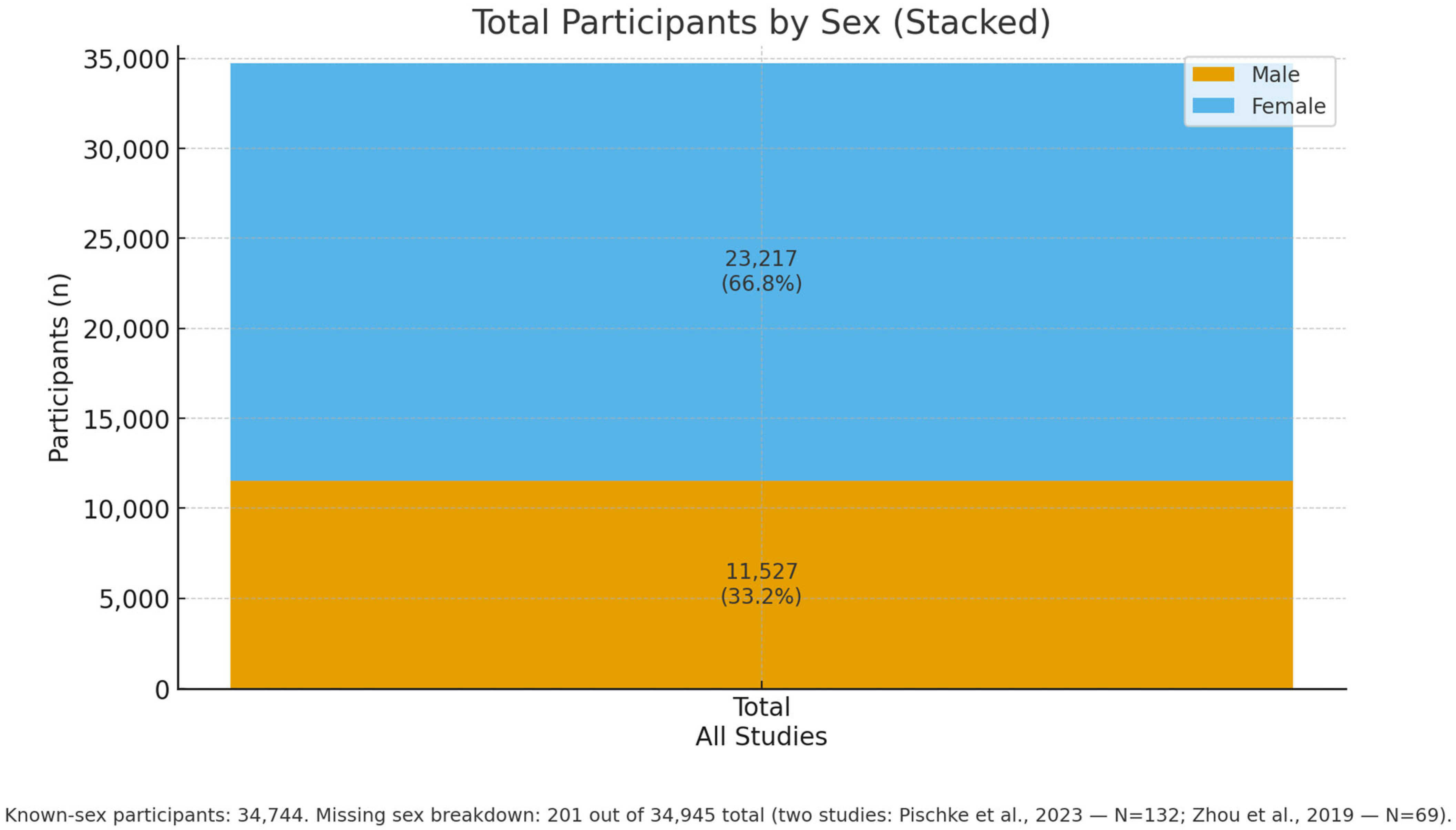
| First Author, Data, Reference | Country | Study Design | N of Patients Included (M/F) | Mean Age, Range, SD | Type of Liver Disease, s | Patient Liver Disease |
|---|---|---|---|---|---|---|
| Sato et al., 2025 [30] | Japan | Cross-sectional | 712 (312/400) | 56.0 ± 14 | MASLD | 277 |
| Tan and Xu, 2024 [29] | China (population USA) | Cross-sectional | 6330 (3383/2947) | 56.02 ± 10.56 | NAFLD | 1804 (890\914) |
| Matsui et al., 2024 [31] | Japan, China | Cross-sectional | 41 (25/16) MASH, 19 (13/6) MASH-HCC | 59 (55–70) MASH, 79 (64–82) | MASH, MASH-HCC | 60 |
| Pischke et al., 2023 [32] | Germany | Prospective cohort study | 27 (15/12) | 56 (37–76) periodontitis; 59 (56–68) no periodontitis | Ascitic decompensated cirrhosis | 27 |
| Pischke et al., 2023 [33] | Germany | Prospective cohort study | 32 (16/16) NASH, 100 C | 52 ± 13 periodontitis; 56 ± 15 no periodontitis | NASH | 32 |
| Kamata et al., 2022 [34] | Japan | Randomized controlled trial | 40 (18/22), 20 SRP, 20 | 61 ± 10 SRP, 54 ± 15 | NAFLD | 40 |
| Sato et al., 2022 [35] | Japan | Cross-sectional | 164 (92/72) | 57 ± 15 | NAFLD | 164 |
| Yamamoto et al., 2021 [36] | Japan | Retrospective longitudinal study | 25804 (6.901/18903) | ≅ 45.2 ± 13.9 | NAFLD | 6901 |
| Takamisawa et al., 2020 [37] | Japan | Cross-sectional | 388 (192/196) | ≅ 68.1 | Subclinical liver dysfunction | 388 |
| Nagao and Tanigawa, 2019 [38] | Japan | Cross-sectional | 47 (30/17) | 70.2 ± 8.8, (49–89) | Chronic hepatitis C; liver cirrhosis, | 47 |
| Zhou et al., 2019 [39] | Usa | Cross-sectional | 69 | AAH: 46,1 ± 11.7; 47.0 ± 13.2 (health) | AAH, Acute Alcoholic Hepatitis | 47 |
| Jensen et al., 2018 [40] | Denmark | Cross-sectional | 21 (16/5) | 62 (59–74) | Cirrhosis | 21 |
| Nakahara et al., 2018 [41] | Japan | Retrospective | 200 (94/106) | 58.2 ± 13.2 | NAFLD | 200 |
| Nagao et al., 2014 [42] | Japan | Retrospective | 351 (147/204) | 60.62 ± 11.2, (17–87) | HCV, HBV | 351 |
| Yoneda et al., 2012 [43] | Japan | Retrospective | 210 (103/107) | 52.9 ± 2.4 (health); 54.6 ± 1.2 (NAFLD) | NAFLD 48, NASH 102 | 150 |
| Emelyanov and Cherelyuk, 2022 [44] | Ukraine | Cross-sectional | 146 (68/78) | 44.6 ± 10.8 (NAFLD); 44.2 ± 7.2 (health) | NAFLD 126 | 126 |
| Emelyanov and Emelyanova, 2023 [45] | Ukraine | Cross-sectional | 108 (43/65) | 50.0 (42– 58) NAFLD) | NAFLD 44 | 44 |
| Li et al., 2020, [46] | China | Cross-sectional | 24 (12/12) | ≅42.5 ± 3.6 | Hepatitis B, hepatitis B cirrhosis; liver cancer | 18 |
| Takuma et al., 2023 [47] | Japan | Cross-sectional pilot study | 60 (36/24)—NASH 40 (22/18), NASH-HCC 20 (14/6) (M/F) | NASH 60.5 (55–70), NASH-HCC 78.5 (65–81.8) | NASH 40, NASH-HCC 20 | 60 |
| Komazaki et al., 2017 [48] | Japan | Cross-sectional | 52 (27/25) | 55 ± 13.8 | NAFLD | 52 |
| Study (Year) | Outcome (Definition) | Exposure (Definition) | OR 95% CI | p Value | Notes |
|---|---|---|---|---|---|
| Yoneda et al., 2012 [43] | NAFLD (biopsy-proven) vs. controls | Salivary P. gingivalis (PCR/fimA) | 2.615 (1.001–6.832) | 0.049 | Adjusted for age, diabetes, and BMI. |
| Nagao and Tanigawa, 2019 [38] | High oral ‘red complex’ burden (microbiological endpoint) | P. gingivalis fimA II genotype | 4.08 (1.12–15.96) | 0.0336 | Endpoint is oral microbiological load; cirrhosis is an independent covariate in the model. |
| Nakahara et al., 2018 [41] | Advanced fibrosis in NAFLD | Serum anti-P. gingivalis fimA type 4 positivity | 2.081 (1.098–3.943) | 0.025 | Adjusted association reported. |
| Sato et al., 2022 [35] | Advanced fibrosis in NAFLD (MRE ≥ 3.4 kPa) | Salivary P. gingivalis ≥ 0.01% (PCR) | 4.05 (1.056–15.52) | 0.04 | Adjusted association present. |
| Tan and Xu, 2024 [29] | NAFLD (ultrasound, NHANES III) | Mixed anti-P. gingivalis antibodies (serum) | 0.996 (0.963–1.030) | 0.81 | No association after survey-weighted adjustment. |
| Takamisawa et al. (2020) [37] | Elevated ALT (females), T3 vs. T1 anti-P. gingivalis IgG | ELISA anti-P. gingivalis IgG (tertiles) | 2.80 (1.22–6.44) | <0.05 | Outcome-specific; study reports enzyme-specific, sex-stratified models (no single composite OR). |
| Domain (RoB 2) | Judgment | Rationale (Concise) |
|---|---|---|
| 1. Bias arising from the randomization process | Low risk | Computer-generated sequence with concealed allocation via a central system; baseline characteristics balanced; no evidence of compromised randomization. |
| 2. Bias due to deviations from intended interventions | Some concerns | Open-label behavioral intervention (SRP vs. toothbrushing) could influence adherence or co-interventions; primary analysis by ITT, but lack of blinding may introduce performance bias. |
| 3. Bias due to missing outcome data | Low risk | No substantial differential attrition for the 12-week primary endpoint; outcome data largely complete in both arms. |
| 4. Bias in measurement of the outcome | Low risk | Outcomes largely objective (biochemistry, elastography/imaging) with standardized assays; lack of blinding unlikely to affect measurement. |
| 5. Bias in selection of the reported result | Low risk | Registered protocol with prespecified primary endpoint and analysis plan; reported estimates and CIs consistent with protocol—no indication of selective reporting. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dioguardi, M.; Lo Muzio, E.; Guerra, C.; Sovereto, D.; Laneve, E.; Martella, A.; Aiuto, R.; Garcovich, D.; Caloro, G.A.; Cantore, S.; et al. Liver Disease and Periodontal Pathogens: A Bidirectional Relationship Between Liver and Oral Microbiota. Dent. J. 2025, 13, 503. https://doi.org/10.3390/dj13110503
Dioguardi M, Lo Muzio E, Guerra C, Sovereto D, Laneve E, Martella A, Aiuto R, Garcovich D, Caloro GA, Cantore S, et al. Liver Disease and Periodontal Pathogens: A Bidirectional Relationship Between Liver and Oral Microbiota. Dentistry Journal. 2025; 13(11):503. https://doi.org/10.3390/dj13110503
Chicago/Turabian StyleDioguardi, Mario, Eleonora Lo Muzio, Ciro Guerra, Diego Sovereto, Enrica Laneve, Angelo Martella, Riccardo Aiuto, Daniele Garcovich, Giorgia Apollonia Caloro, Stefania Cantore, and et al. 2025. "Liver Disease and Periodontal Pathogens: A Bidirectional Relationship Between Liver and Oral Microbiota" Dentistry Journal 13, no. 11: 503. https://doi.org/10.3390/dj13110503
APA StyleDioguardi, M., Lo Muzio, E., Guerra, C., Sovereto, D., Laneve, E., Martella, A., Aiuto, R., Garcovich, D., Caloro, G. A., Cantore, S., Lo Muzio, L., & Ballini, A. (2025). Liver Disease and Periodontal Pathogens: A Bidirectional Relationship Between Liver and Oral Microbiota. Dentistry Journal, 13(11), 503. https://doi.org/10.3390/dj13110503











