The Effect of Different White Spot Lesion Treatments on the Enamel Microhardness—An In Vitro Pilot Study
Abstract
1. Introduction
2. Materials and Methods
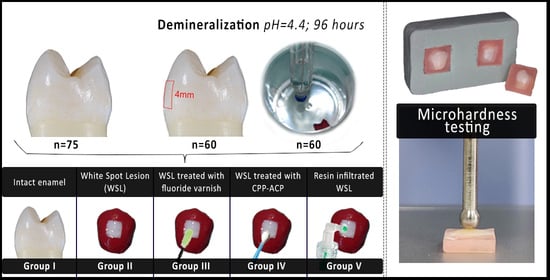
2.1. Sample Collection
2.2. Specimen Preparation
- I—Intact enamel (control group);
- II—Artificial white spot lesion (WSL);
- III—Artificial WSL treated with fluoride varnish;
- IV—Artificial WSL treated with CPP-ACP paste;
- V—Resin-infiltrated artificial WSL.
2.3. Artificial Demineralization
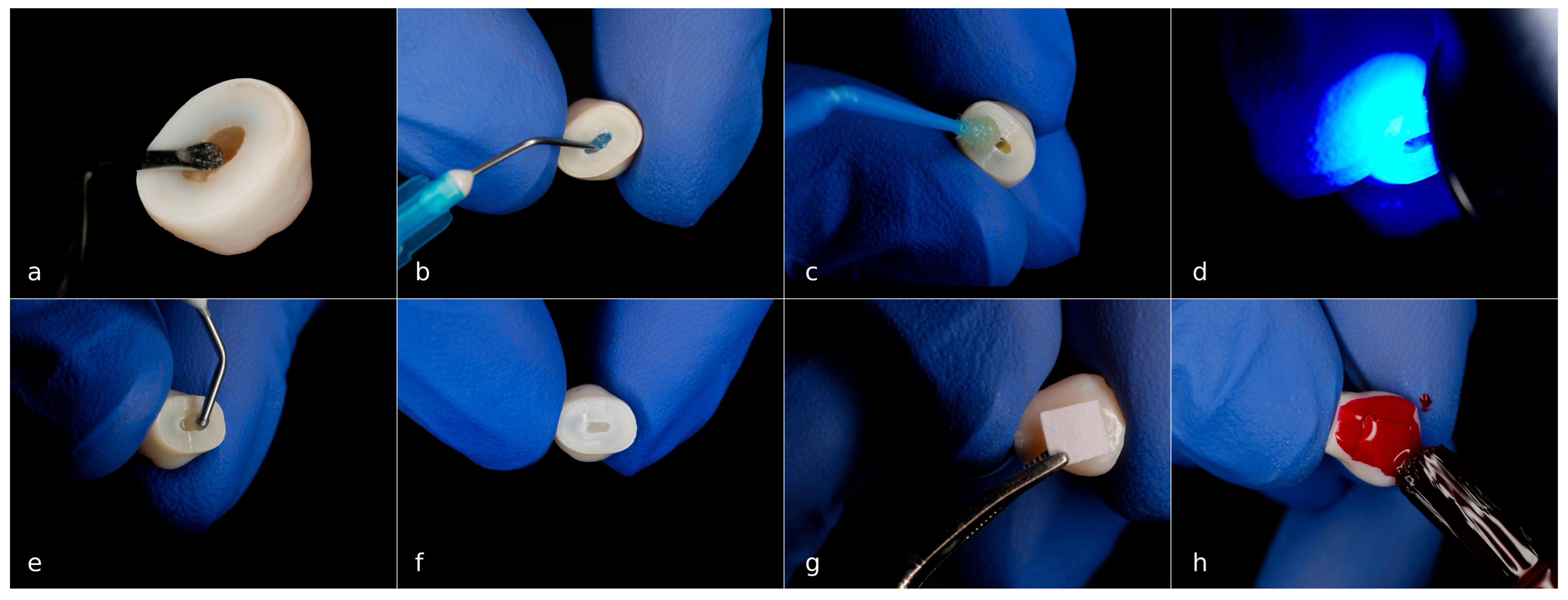
2.4. Material Application
- Group III—Fluoride varnish
- Group IV—CPP-ACP paste
- Group V—Resin infiltration
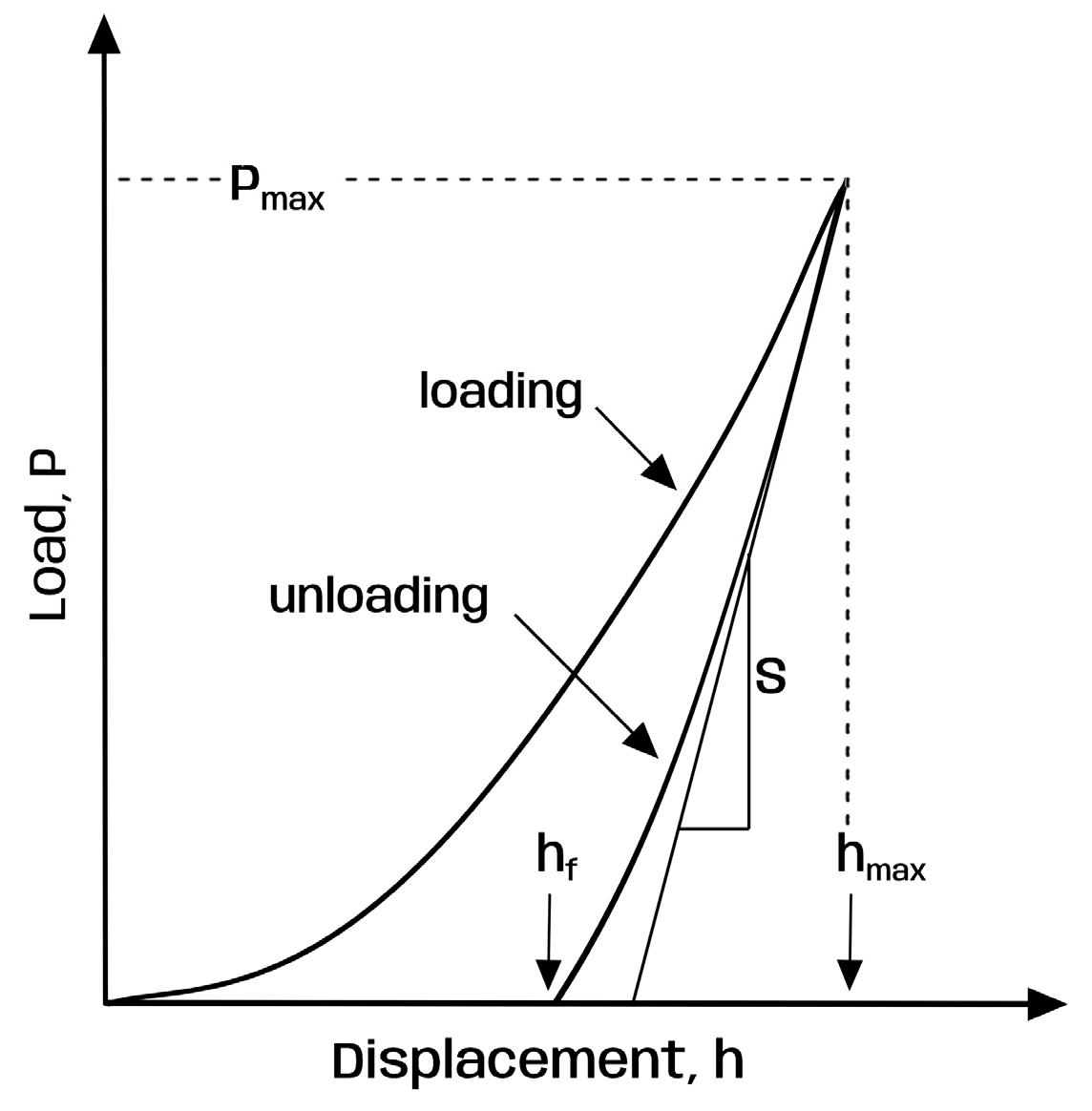
2.5. Surface Microhardness Testing
2.6. Statistical Analysis
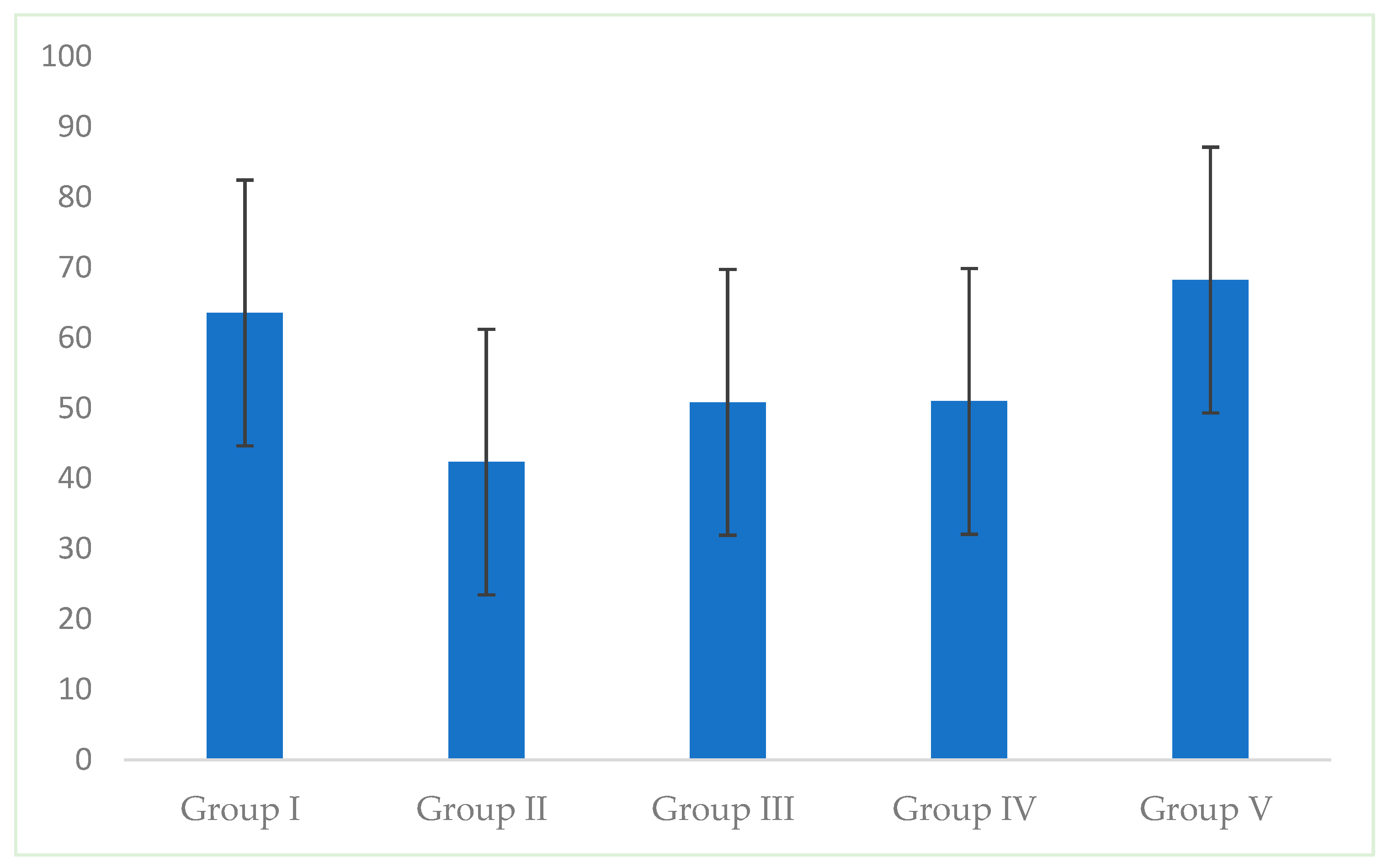
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WSL | White spot lesion |
| CPP-ACP | Casein phosphopeptide–amorphous calcium phosphate |
| RI | Refractive index |
| TEGDMA | Triethlylene glycol dimethacrylate |
| NaF | Sodium fluoride |
Appendix A
Appendix A.1
| Steps | Recommendations |
|---|---|
| Step 1: Icon-etch | Apply Icon-etch with the tip; Let it set for 2 min with periodic smearing; Rinse with water for 30 s; Dry with oil/water-free air spray. |
| Step 2: Icon-dry | Apply Icon-dry with the tip; Let it set for 30 s; Dry with oil/water-free air spray. |
| Step 3: Icon-infiltrant | Apply Icon-infiltrant with the Icon Vestibular Tip; Let it set for 3 min with periodical activation and additional resin coating; Remove excess material with cotton rolls; Light cure for 40 s; Repeat the application of Icon-infiltrant, as previously described, with contact time 1 min; Polishing. |
Appendix A.2

References
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H.M.; Lippert, F. Artificial Caries Lesion Characteristics after Secondary Demineralization with Theobromine-Containing Protocol. Molecules 2021, 26, 300. [Google Scholar] [CrossRef] [PubMed]
- Theodory, T.G.; Kolker, J.L.; Vargas, M.A.; Maia, R.R.; Dawson, D.V. Masking and Penetration Ability of Various Sealants and ICON in Artificial Initial Caries Lesions In Vitro. J. Adhes. Dent. 2019, 21, 265–272. [Google Scholar] [CrossRef]
- Bandekar, S.; Patil, S.; Dudulwar, D.; Moogi, P.P.; Ghosh, S.; Kshirsagar, S. Remineralization potential of fluoride, amorphous calcium phosphate-casein phosphopeptide, and combination of hydroxylapatite and fluoride on enamel lesions: An in vitro comparative evaluation. J. Conserv. Dent. 2019, 22, 305–309. [Google Scholar] [CrossRef]
- Araújo, G.S.; Naufel, F.S.; Alonso, R.C.; Lima, D.A.; Puppin-Rontani, R.M. Influence of Staining Solution and Bleaching on Color Stability of Resin Used for Caries Infiltration. Oper. Dent. 2015, 40, E250–E256. [Google Scholar] [CrossRef]
- Benson, P.E.; Javidi, H.; DiBiase, A.T. What is the value of orthodontic treatment? Br. Dent. J. 2015, 218, 185–190. [Google Scholar] [CrossRef]
- Hasmun, N.; Vettore, M.V.; Lawson, J.A.; Elcock, C.; Zaitoun, H.; Rodd, H.D. Determinants of children’s oral health-related quality of life following aesthetic treatment of enamel opacities. J. Dent. 2020, 98, 103372. [Google Scholar] [CrossRef]
- Rana, N.; Singh, N.; Shaila; Thomas, A.M.; Jairath, R. A comparative evaluation of penetration depth and surface microhardness of Resin Infiltrant, CPP-ACPF and Novamin on enamel demineralization after banding: An in vitro study. Biomater. Investig. Dent. 2021, 8, 64–71. [Google Scholar] [CrossRef]
- Mandava, J.; Reddy, Y.S.; Kantheti, S.; Chalasani, U.; Ravi, R.C.; Borugadda, R.; Konagala, R.K. Microhardness and Penetration of Artificial White Spot Lesions Treated with Resin or Colloidal Silica Infiltration. J. Clin. Diagn. Res. 2017, 11, ZC142–ZC146. [Google Scholar] [CrossRef]
- De Almeida, C.M.; da Rosa, W.L.O.; Meereis, C.T.W.; de Almeida, S.M.; Ribeiro, J.S.; da Silva, A.F.; Lund, R.G. Efficacy of antimicrobial agents incorporated in orthodontic bonding systems: A systematic review and meta-analysis. J. Orthod. 2018, 45, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Aref, N.S.; Alrasheed, M.K. Casein phosphopeptide amorphous calcium phosphate and universal adhesive resin as a complementary approach for management of white spot lesions: An in-vitro study. Progress. Orthod. 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Y.; Cui, T.; Li, J.; Lo, E.C.M.; Hao, G.; Zhi, Q. Comparative evaluation of four treatments for postorthodontic white spot lesions: A randomized controlled trial. Clin. Oral. Investig. 2023, 27, 5957–5968. [Google Scholar] [CrossRef]
- Baafif, H.A.; Alibrahim, I.F.; Alotaibi, S.H.; Alharbi, H.G.; Shubaily, M.N.; Elkwatehy, W.M.A. The Efficacy of Resin Infiltrant and Casein Phosphopeptide-amorphous Calcium Fluoride Phosphate in Treatment of White Spot Lesions (Comparative Study). J. Int. Soc. Prev. Community Dent. 2020, 10, 438–444. [Google Scholar] [CrossRef]
- Dhamija, M.; Tyagi, R.; Kalra, N.; Khatri, A. Efficacy of Resin Infiltration and Fluoride Casein Phosphopeptide Amorphous Calcium Phosphate Varnish on Non-cavitated Active White Spot Lesions in Children: A Randomized Clinical Trial. Pesqui. Bras. Em Odontopediatria E Clínica Integr. 2022, 22, e210094. [Google Scholar] [CrossRef]
- Doğu Kaya, B.; Manav Özen, A.; Yılmaz Atalı, P.; Güngör, A.S.; Dalkılıç, E.; Alkan, E.; Tağtekin, D.; Türkmen, C. Effect of the use of remineralization agents before resin infiltration on the treatment of initial enamel lesions: An in-vitro study. BMC Oral Health 2024, 24, 868. [Google Scholar] [CrossRef]
- Magalhães, A.C.; Moron, B.M.; Comar, L.P.; Wiegand, A.; Buchalla, W.; Buzalaf, M.A. Comparison of cross-sectional hardness and transverse microradiography of artificial carious enamel lesions induced by different demineralising solutions and gels. Caries Res. 2009, 43, 474–483. [Google Scholar] [CrossRef]
- Milanovic, M.; Mandinic, Z.; Juloski, J.; Dimitrijevic, M.; Milicic, B.; Andelski-Radicevic, B.; Pavlović, V.; Beloica, M. Effect of different demineralizing solutions and different exposing times on artificial initial caries lesion formation—An in vitro study. Srpski Arhiv za Celokupno Lekarstvo 2023, 151, 652–657. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Pretty, I.A.; Ellwood, R.P. The caries continuum: Opportunities to detect, treat and monitor the re-mineralization of early caries lesions. J. Dent. 2013, 41 (Suppl. S2), S12–S21. [Google Scholar] [CrossRef] [PubMed]
- Murdoch-Kinch, C.A.; McLean, M.E. Minimally invasive dentistry. J. Am. Dent. Assoc. 2003, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Innes, N.P.T.; Chu, C.H.; Fontana, M.; Lo, E.C.M.; Thomson, W.M.; Uribe, S.; Heiland, M.; Jepsen, S.; Schwendicke, F. A Century of Change towards Prevention and Minimal Intervention in Cariology. J. Dent. Res. 2019, 98, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Soveral, M.; Machado, V.; Botelho, J.; Mendes, J.J.; Manso, C. Effect of Resin Infiltration on Enamel: A Systematic Review and Meta-Analysis. J. Funct. Biomater. 2021, 12, 48. [Google Scholar] [CrossRef]
- Prajapati, D.; Nayak, R.; Pai, D.; Upadhya, N.; Bhaskar, V.K.; Kamath, P. Effect of Resin Infiltration on Artificial Caries: An in vitro Evaluation of Resin Penetration and Microhardness. Int. J. Clin. Pediatr. Dent. 2017, 10, 250–256. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Osorio, E.; Prati, C.; Carvalho, R.M. Microhardness of acid-treated and resin infiltrated human dentine. J. Dent. 2005, 33, 349–354. [Google Scholar] [CrossRef]
- Sleem, M.M.; Eid, E.G.; Abdelghany, A.M.; Farahat, D.S. Evaluation of the remineralization potential of different bioactive glass varnishes on white spot lesions: An in vitro study. BMC Oral. Health. 2025, 25, 1284. [Google Scholar] [CrossRef]
- Freitas, M.; Nunes, L.V.; Comar, L.P.; Rios, D.; Magalhaes, A.C.; Honorio, H.M.; Wang, L. In vitro effect of a resin infiltrant on different artificial caries-like enamel lesions. Arch. Oral Biol. 2018, 95, 118–124. [Google Scholar] [CrossRef]
- Torres, C.R.G.; Borges, A.B.; Torres, L.M.S.; Gomes, I.S.; de Oliveira, R.S. Effect of caries infiltration technique and fluoride therapy on the colour masking of white spot lesions. J. Dent. 2011, 39, 202–207. [Google Scholar] [CrossRef]
- Zakizade, M.; Davoudi, A.; Akhavan, A.; Shirban, F. Effect of Resin Infiltration Technique on Improving Surface Hardness of Enamel Lesions: A Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2020, 20, 101405. [Google Scholar] [CrossRef] [PubMed]
- Neres, E.Y.; Moda, M.D.; Chiba, E.K.; Briso, A.L.F.; Pessan, J.P.; Fagundes, T.C. Microhardness and Roughness of Infiltrated White Spot Lesions Submitted to Different Challenges. Oper. Dent. 2017, 42, 428–435. [Google Scholar] [CrossRef]
- Paris, S.; Schwendicke, F.; Seddig, S.; Muller, W.D.; Dorfer, C.; Meyer-Lueckel, H. Micro-hardness and mineral loss of enamel lesions after infiltration with various resins: Influence of infiltrant composition and application frequency in vitro. J. Dent. 2013, 41, 543–548. [Google Scholar] [CrossRef]
- Gajewski, V.E.; Pfeifer, C.S.; Fróes-Salgado, N.R.; Boaro, L.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- Seppä, L. Fluoride varnishes in caries prevention. Med. Princ. Pract. 2004, 13, 307–311. [Google Scholar] [CrossRef]
- Piesiak-Pańczyszyn, D.; Zakrzewski, W.; Piszko, A.; Piszko, P.J.; Dobrzyński, M. Review on fluoride varnishes currently recommended in dental prophylaxis. Polim. Med. 2023, 53, 141–151. [Google Scholar] [CrossRef]
- Sh, P.; Raghu, R.; Shetty, A.; Gautham, P.; Reddy, S.; Srinivasan, R. Effect of organic versus inorganic fluoride on enamel microhardness: An in vitro study. J. Conserv. Dent. 2013, 16, 203–207. [Google Scholar] [CrossRef]
- Vyavhare, S.; Sharma, D.S.; Kulkarni, V.K. Effect of three different pastes on remineralization of initial enamel lesion: An in vitro study. J. Clin. Pediatr. Dent. 2015, 39, 149–160. [Google Scholar] [CrossRef]
- Li, J.; Xie, X.; Wang, Y.; Yin, W.; Antoun, J.S.; Farella, M.; Mei, L. Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in vivo: A systematic review. J. Dent. 2014, 42, 769–777. [Google Scholar] [CrossRef] [PubMed]
- El-zankalouny, S.M.; Abd El Fattah, W.M.; El-Shabrawy, S. Penetration depth and enamel microhardness of resin infiltrant and traditional techniques for treatment of artificial enamel lesions. Alex. Dent. J. 2016, 41, 20–25. [Google Scholar] [CrossRef]
- Shaik, Z.A.; Rambabu, T.; Sajjan, G.; Varma, M.; Satish, K.; Raju, V.B.; Ganguru, S.; Ventrapati, N. Quantitative Analysis of Remineralization of Artificial Carious Lesions with Commercially Available Newer Remineralizing Agents Using SEM-EDX- In Vitro Study. J. Clin. Diagn. Res. 2017, 11, ZC20–ZC23. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Martins, I.; Pessanha, S.; Coelho, A.; Arantes-Oliveira, S.; Marques, P.F. Evaluation of the Efficacy of CPP-ACP Remineralizing Mousse in Molar-Incisor Hypomineralized Teeth Using Polarized Raman and Scanning Electron Microscopy-An In Vitro Study. Biomedicines 2022, 10, 3086. [Google Scholar] [CrossRef] [PubMed]
- Tosco, V.; Vitiello, F.; Monterubbianesi, R.; Gatto, M.L.; Orilisi, G.; Mengucci, P.; Putignano, A.; Orsini, G. Assessment of the Remineralizing Potential of Biomimetic Materials on Early Artificial Caries Lesions after 28 Days: An In Vitro Study. Bioengineering 2023, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C.; Cai, F.; Cochrane, N.J.; Shen, P.; Walker, G.D.; Morgan, M.V.; Reynolds, C. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J. Dent. Res. 2008, 87, 344–348. [Google Scholar] [CrossRef]
- Subramaniam, P.; Telegeti, S. Effect of different concentrations of fluoride varnish on enamel surface microhardness: An in vitro randomized controlled study. J. Indian Assoc. Public Health Dent. 2016, 14, 344–347. [Google Scholar] [CrossRef]
- Babu, K.L.; Subramaniam, P.; Teleti, S. Effect of varnish containing casein phosphopeptides-amorphous calcium phosphate and fluoride on surface microhardness of enamel—An in vitro study. Saudi J. Oral. Sci. 2020, 7, 29–34. [Google Scholar] [CrossRef]
- Singh, V.; Naik, S.; Vashisth, P.; Sharma, S.; Chandak, A.; Murry, J.N. Comparative Evaluation of Longevity of Fluoride Release from Three Different Fluoride Varnishes: An Observational Study. Int. J. Clin. Pediatr. Dent. 2024, 17, 341–345. [Google Scholar] [CrossRef]

| Material | Composition | Manufacturer |
|---|---|---|
| Fluor Protector S | 1.5% ammonium fluoride (7700 ppmF in solution); Ethanol; Water; Polymer; Additive; Saccharin, mint aroma | Ivoclar Vivadent, Schaan, Liechtenstein |
| Tooth Mousse | CPP-ACP (RECALDENT), Water, Glycerol, D-sorbitol, CMC-Na, Propylene glycol, Silicon dioxide, Titanium dioxide, Xylitol, Phosphoric acid, Flavoring, Zinc oxide, Sodium-saccharin, Ethyl p-hydroxybenzoate, Magnesium oxide, Guar gum, Propyl p-hydroxybenzoate, Butyl p-hydroxybenzoate | GC, Tokyo, Japan |
| ICON | Icon-Etch: Hydrochloric acid (15% HCl), Pyrogenic silicic acid; Surface-active substances; Icon-Dry: 99% ethanol; Icon-Infiltrant: Methacrylate-based resin matrix; Initiators; Additives | DMG, Hamburg, Germany |
| Group I Intact Enamel | Group II WSL | Group III Fluoride Varnish | Group IV CPP-ACP Paste | Group V Resin Infiltration | |
|---|---|---|---|---|---|
| Mean | 63.57 A | 42.35 B | 50.84 A,B | 50.99 A,B | 68.23 A |
| S.D. | 18.89 | 17.98 | 14.35 | 15.31 | 21.45 |
| p | 0.001 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanović, M.; Beloica, M.; Mandinić, Z.; Juloski, J.; Petrović, M.; Kosanović, D.; Todorović, M.; Dimitrijević, M.; Jakovljević, A.; Vorkapić, M.; et al. The Effect of Different White Spot Lesion Treatments on the Enamel Microhardness—An In Vitro Pilot Study. Dent. J. 2025, 13, 496. https://doi.org/10.3390/dj13110496
Milanović M, Beloica M, Mandinić Z, Juloski J, Petrović M, Kosanović D, Todorović M, Dimitrijević M, Jakovljević A, Vorkapić M, et al. The Effect of Different White Spot Lesion Treatments on the Enamel Microhardness—An In Vitro Pilot Study. Dentistry Journal. 2025; 13(11):496. https://doi.org/10.3390/dj13110496
Chicago/Turabian StyleMilanović, Milena, Miloš Beloica, Zoran Mandinić, Jelena Juloski, Miloš Petrović, Dušan Kosanović, Miloš Todorović, Maja Dimitrijević, Aleksandar Jakovljević, Miloš Vorkapić, and et al. 2025. "The Effect of Different White Spot Lesion Treatments on the Enamel Microhardness—An In Vitro Pilot Study" Dentistry Journal 13, no. 11: 496. https://doi.org/10.3390/dj13110496
APA StyleMilanović, M., Beloica, M., Mandinić, Z., Juloski, J., Petrović, M., Kosanović, D., Todorović, M., Dimitrijević, M., Jakovljević, A., Vorkapić, M., & Stanimirović, D. (2025). The Effect of Different White Spot Lesion Treatments on the Enamel Microhardness—An In Vitro Pilot Study. Dentistry Journal, 13(11), 496. https://doi.org/10.3390/dj13110496