Bone Morphogenetic Protein 7 Promotes the Differentiation of Periodontal Ligament Fibroblasts into F-Spondin-Expressing Cementoblast-like Cells During Root Canal Treatment—An In Vivo Rat Pulpectomy Model and In Vitro Human Fibroblast Study
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Studies
2.1.1. Animals
2.1.2. Preparation of Root Canal Reagents
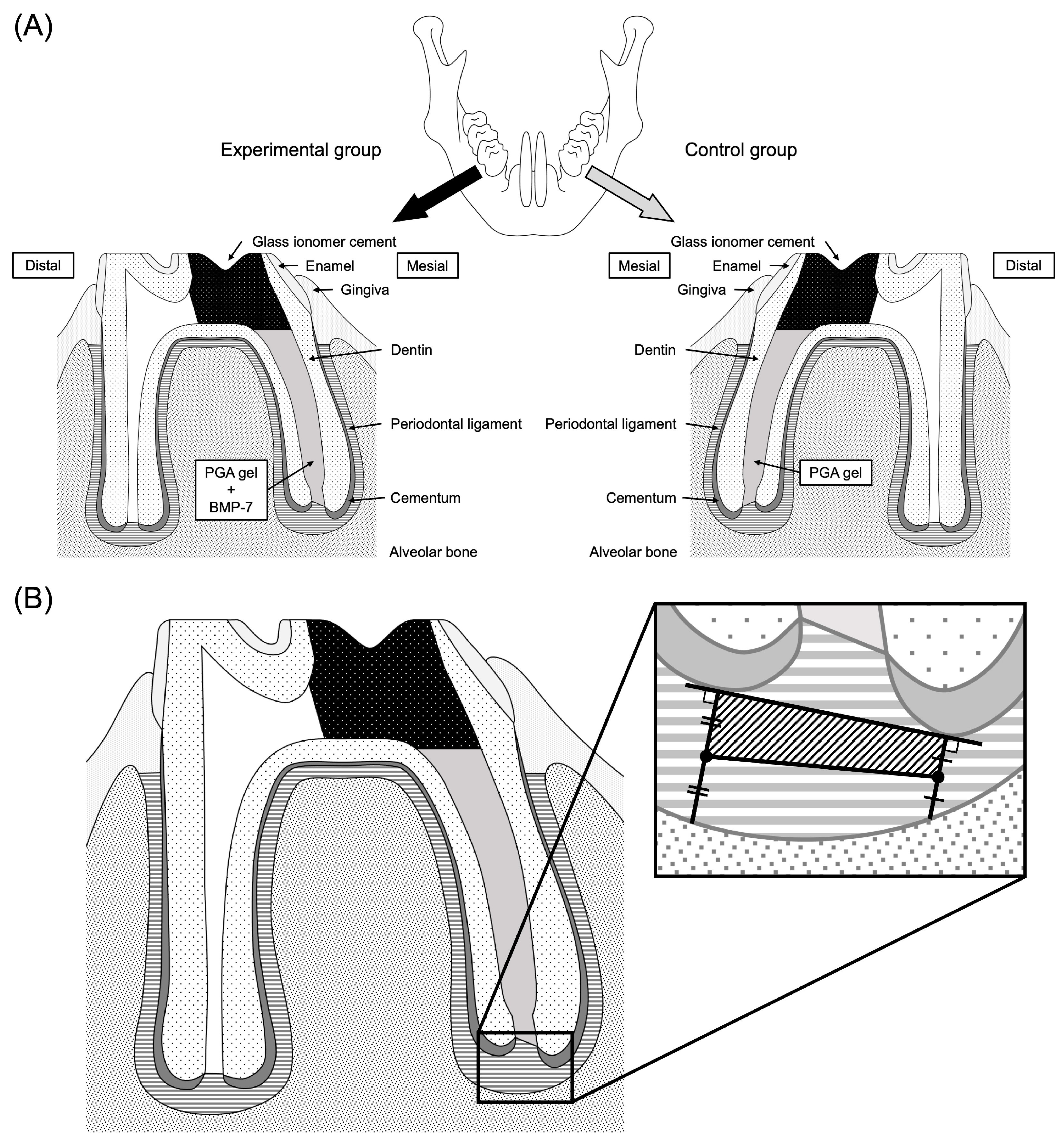
2.1.3. Experimental Procedure
2.1.4. Histological Observations
2.1.5. Immunohistochemical Observations
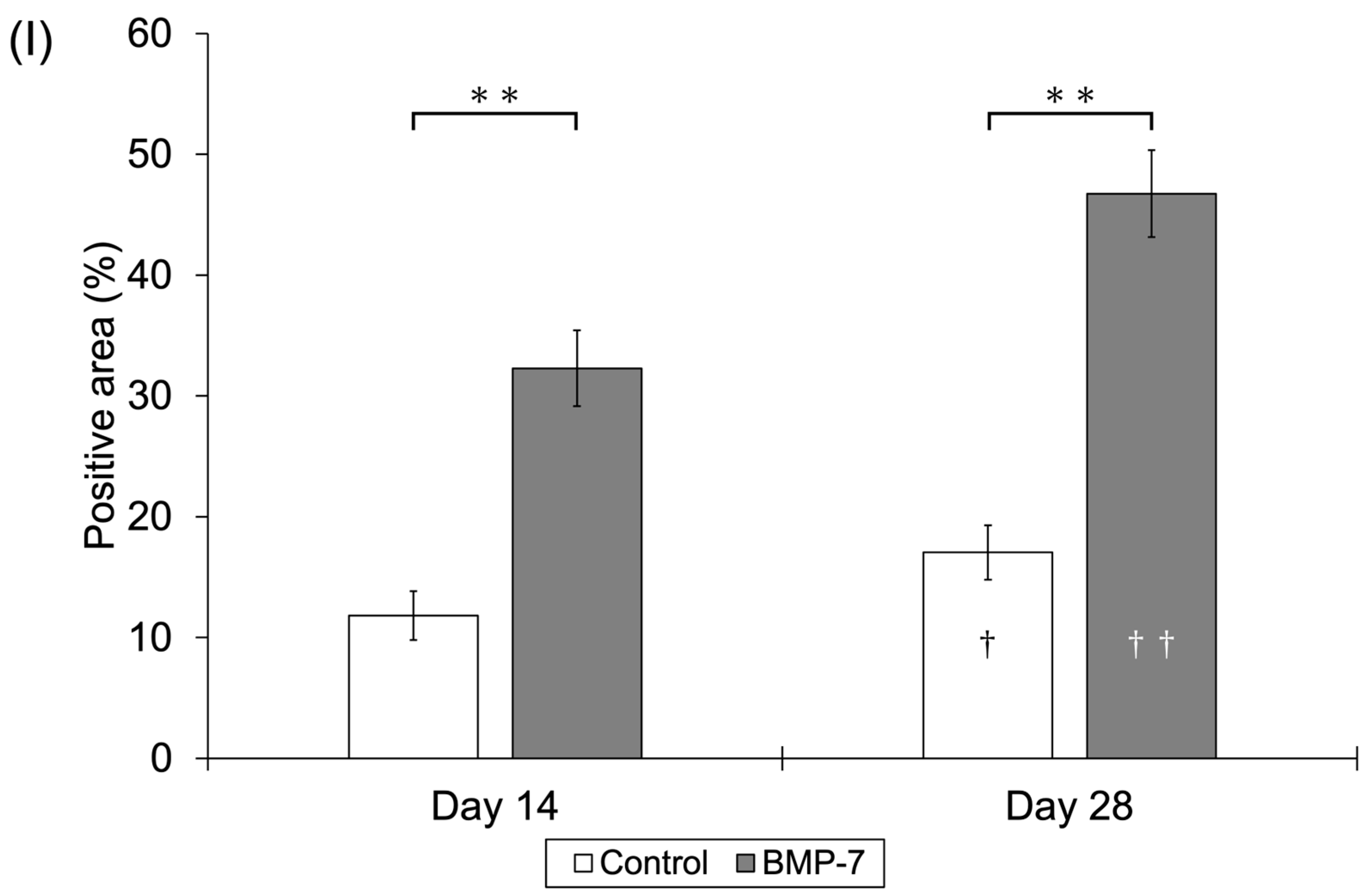
2.1.6. Measurement of Positively Immunostained Structures and Statistical Analysis
2.2. In Vitro Studies
2.2.1. Cells
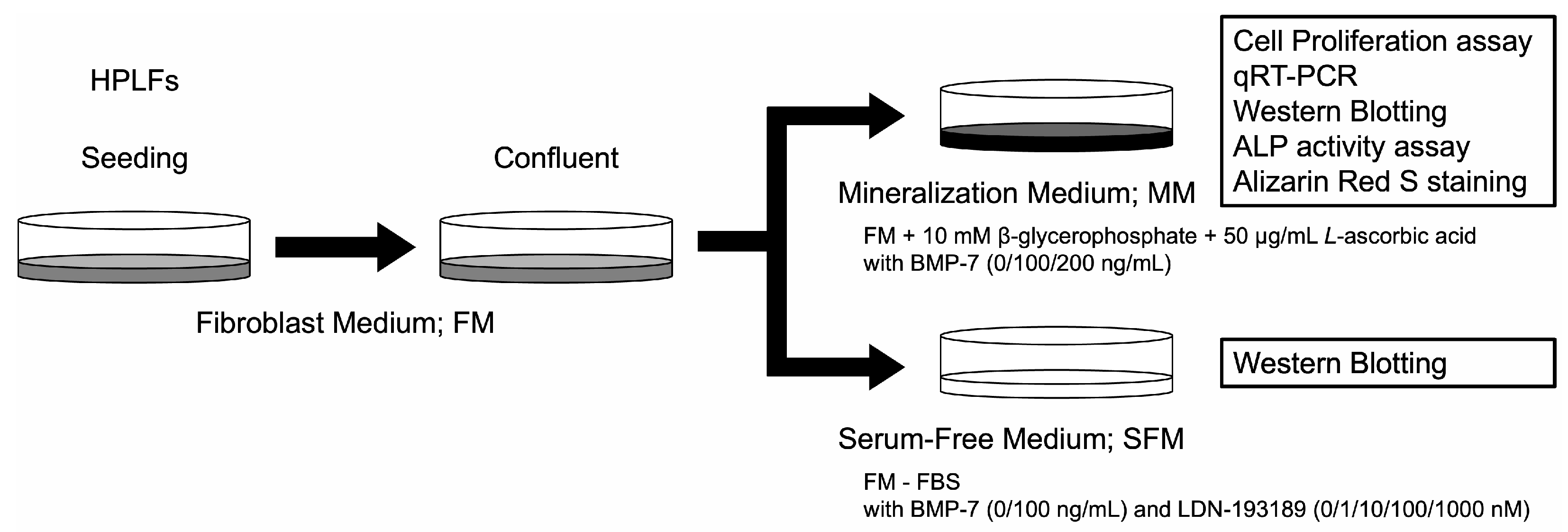
2.2.2. Cell Culture Medium
2.2.3. Cell Proliferation Assay
2.2.4. BMP Signaling Pathway
2.2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Assay
2.2.6. Western Blot Analysis
2.2.7. Alkaline Phosphatase (ALP) Activity Assay
2.2.8. Alizarin Red S (ARS) Staining
2.2.9. Statistical Analysis
3. Results
3.1. In Vivo Studies
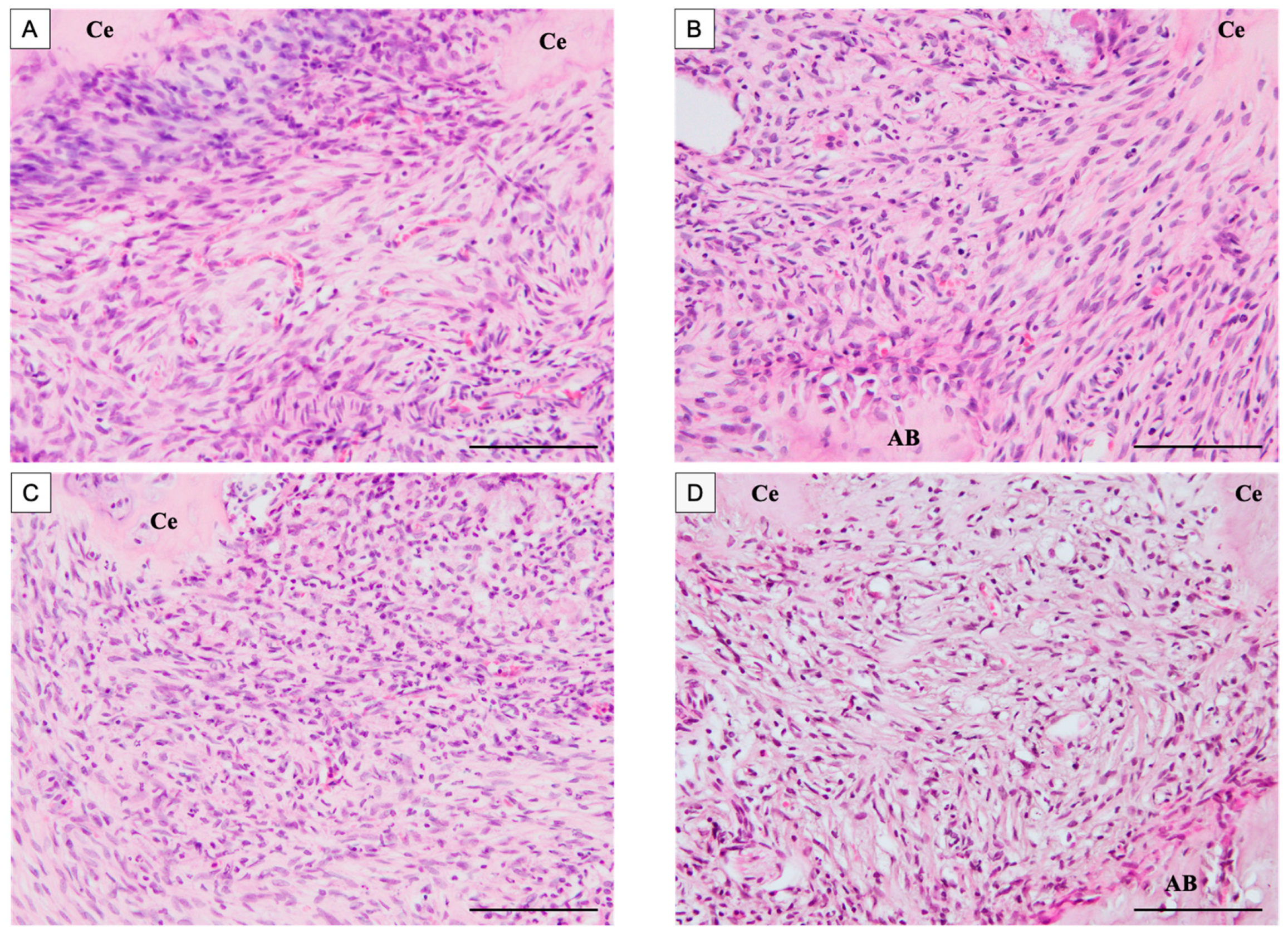
3.1.1. Histological Observations
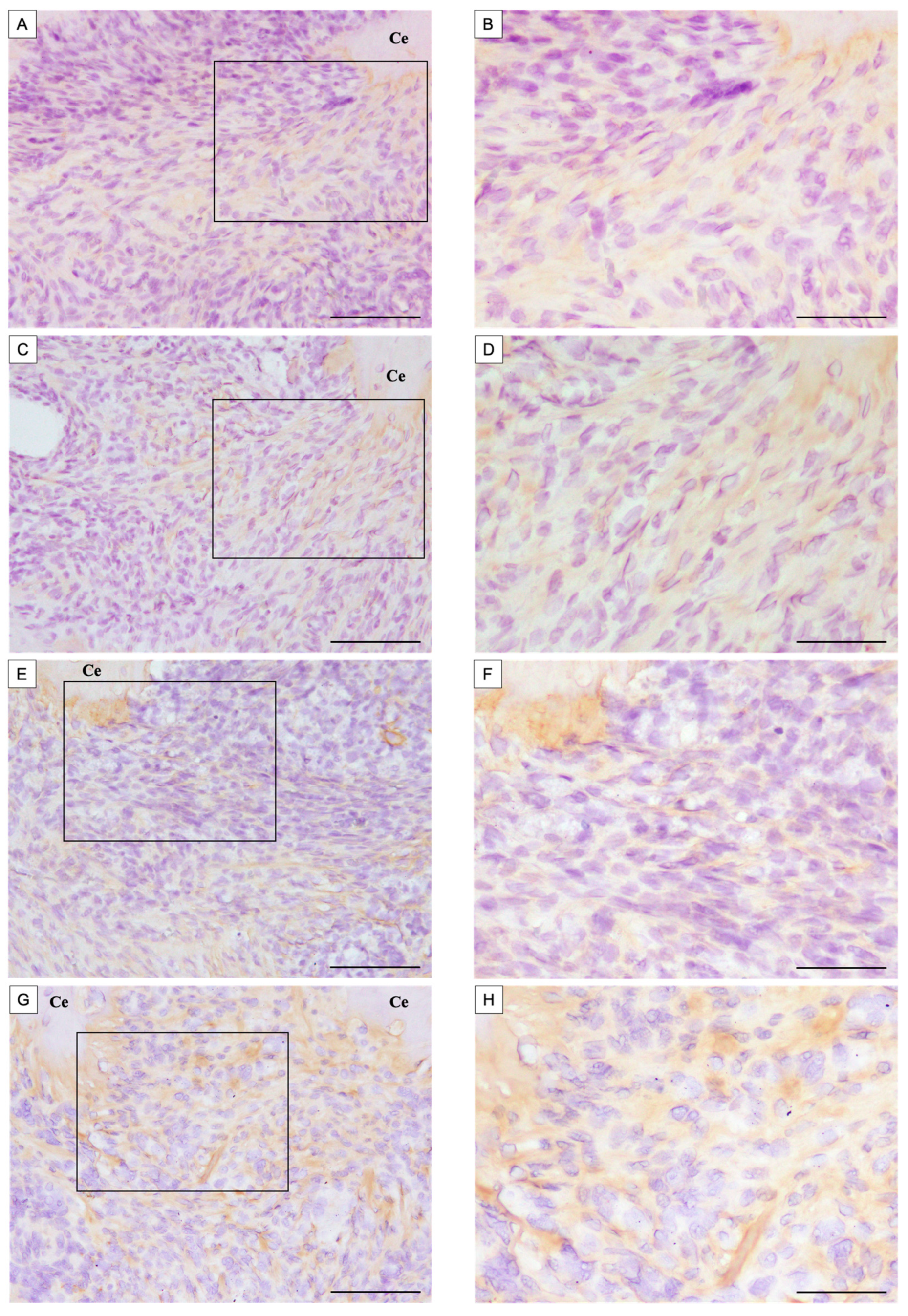
3.1.2. Immunohistochemical Observations
3.2. In Vitro Studies
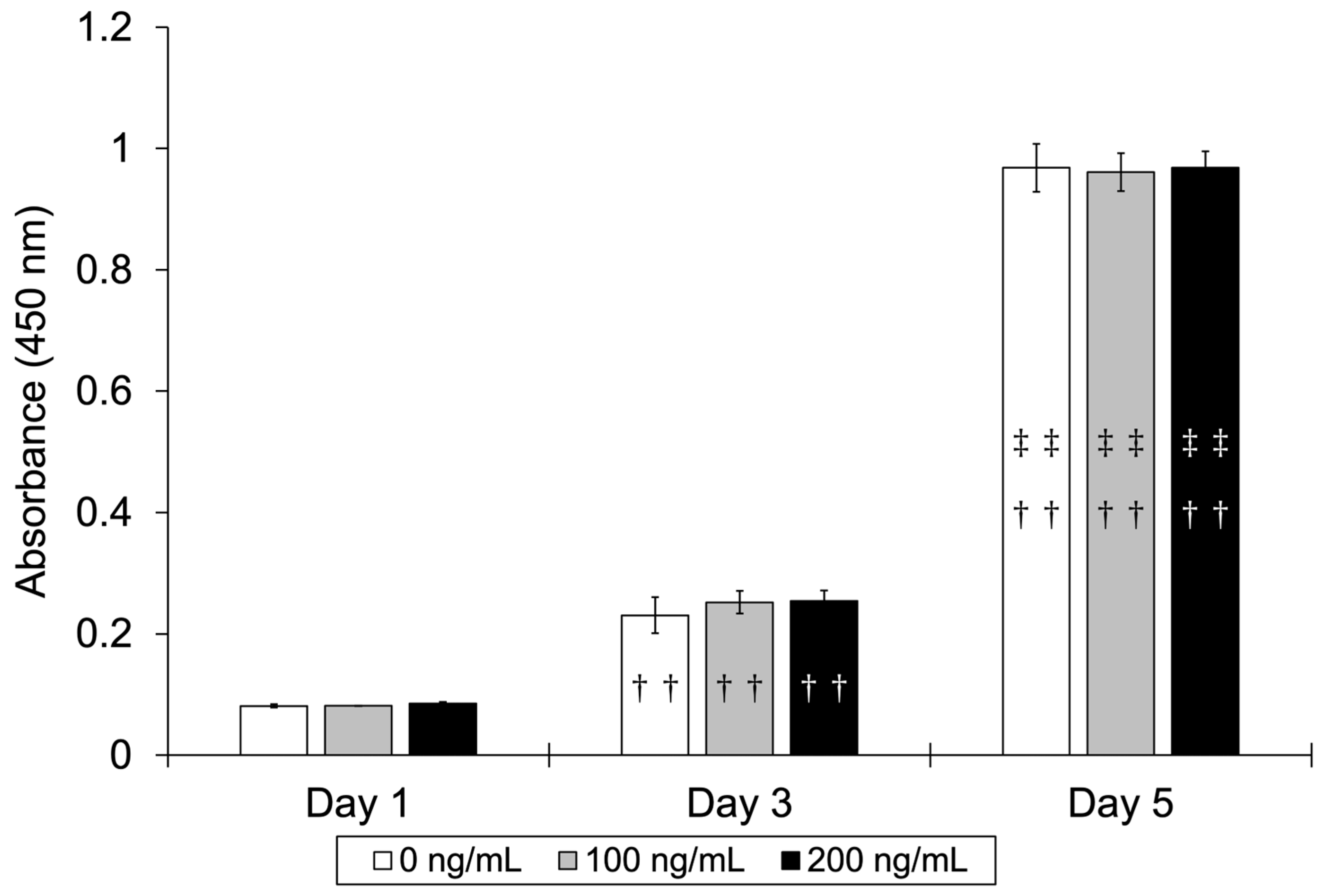
3.2.1. Cell Proliferation Assay
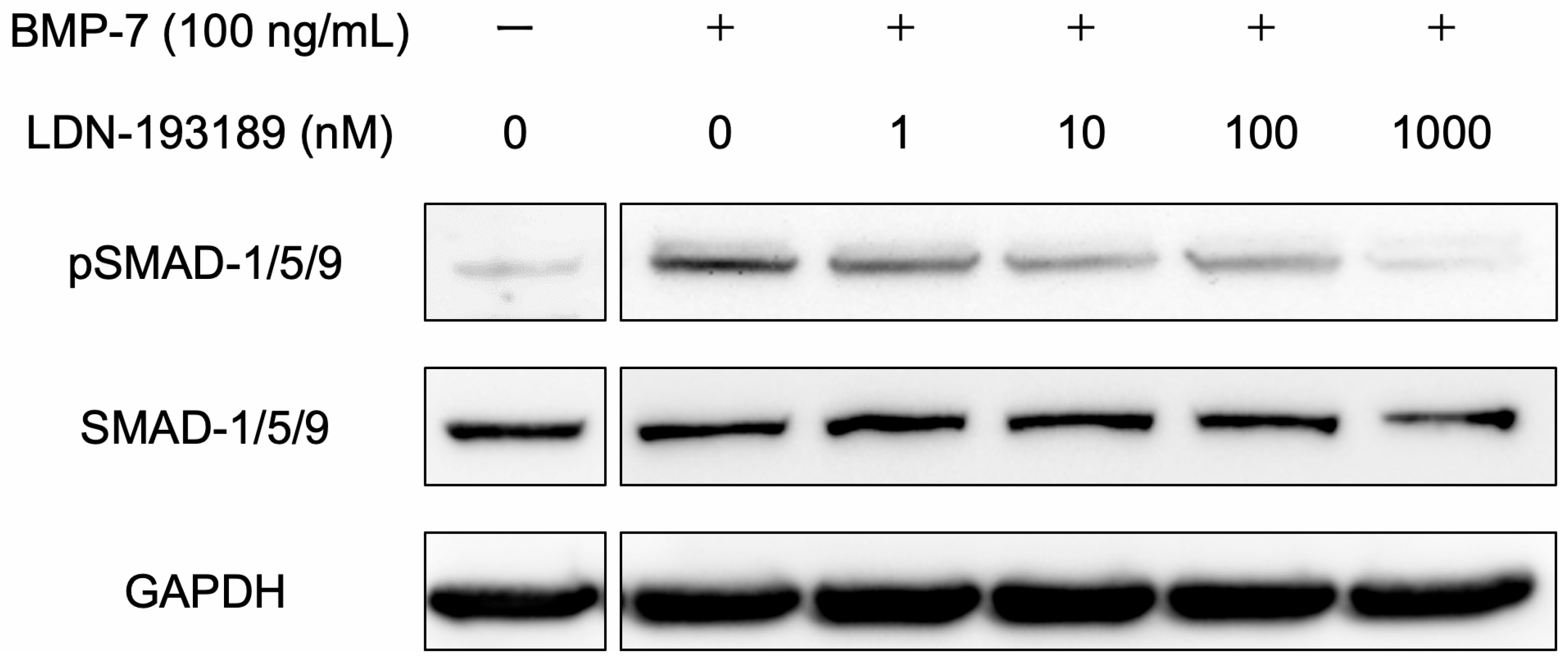
3.2.2. BMP Signaling Pathway
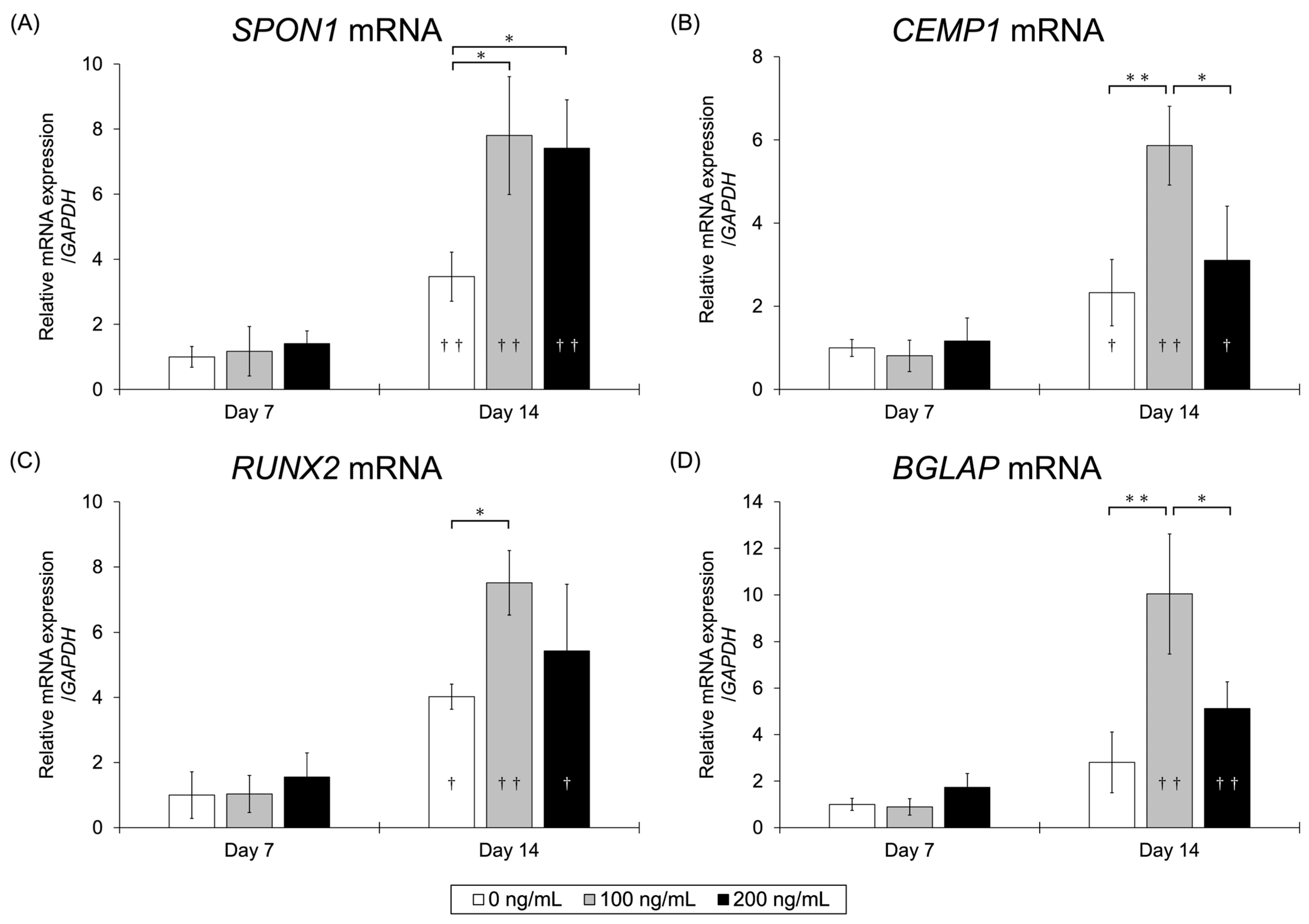
3.2.3. qRT-PCR Assay
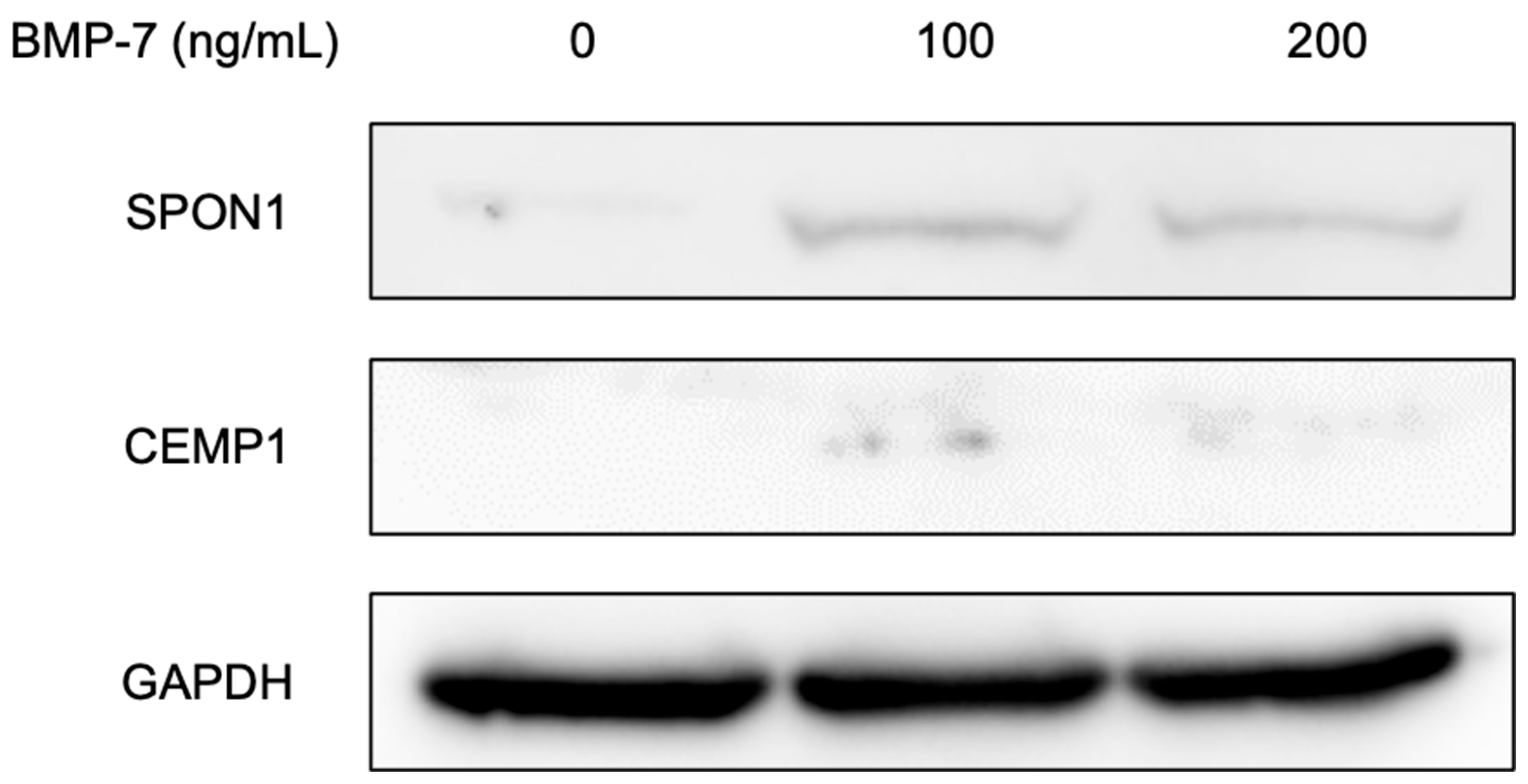
3.2.4. Western Blot Analysis
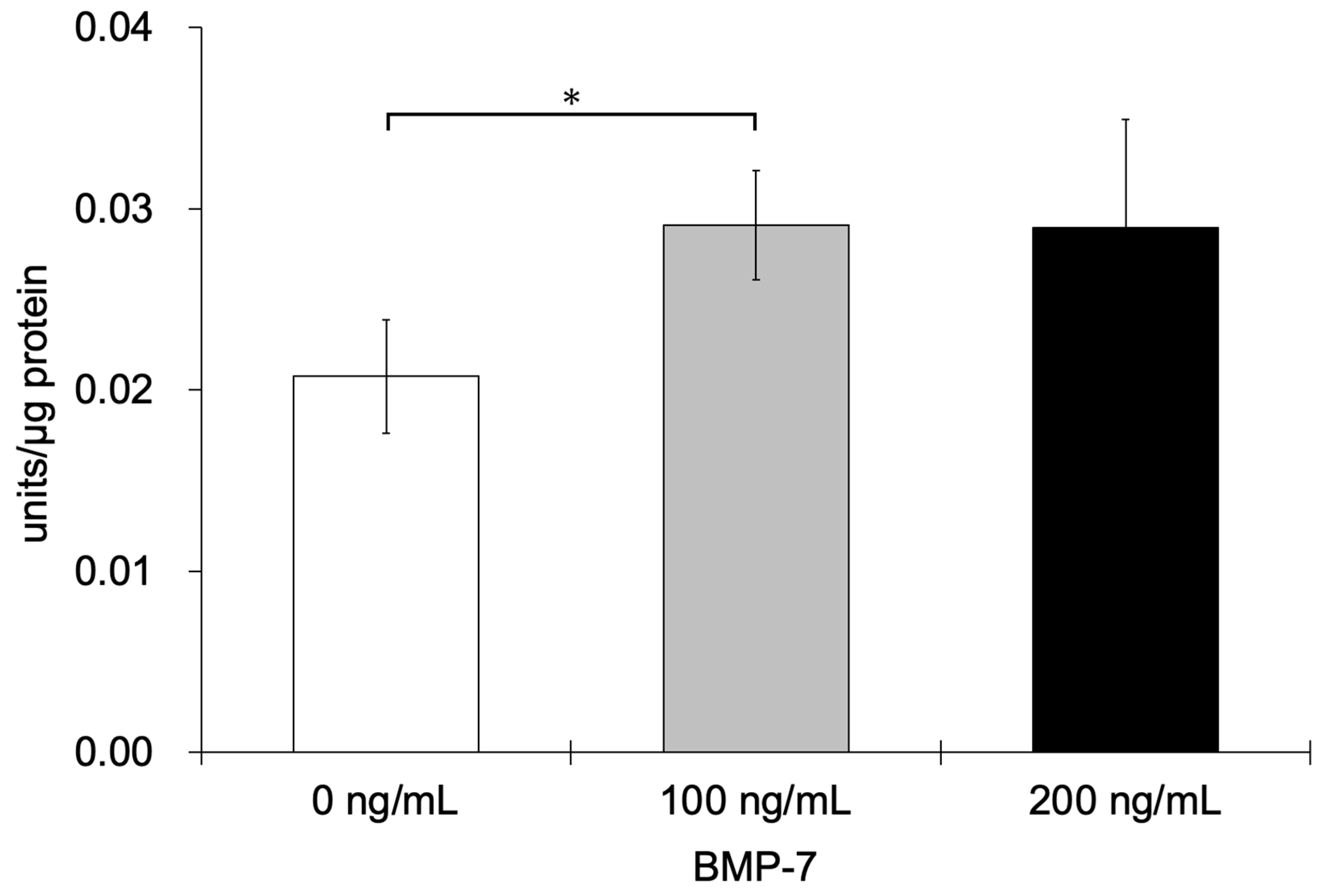
3.2.5. ALP Activity Assay
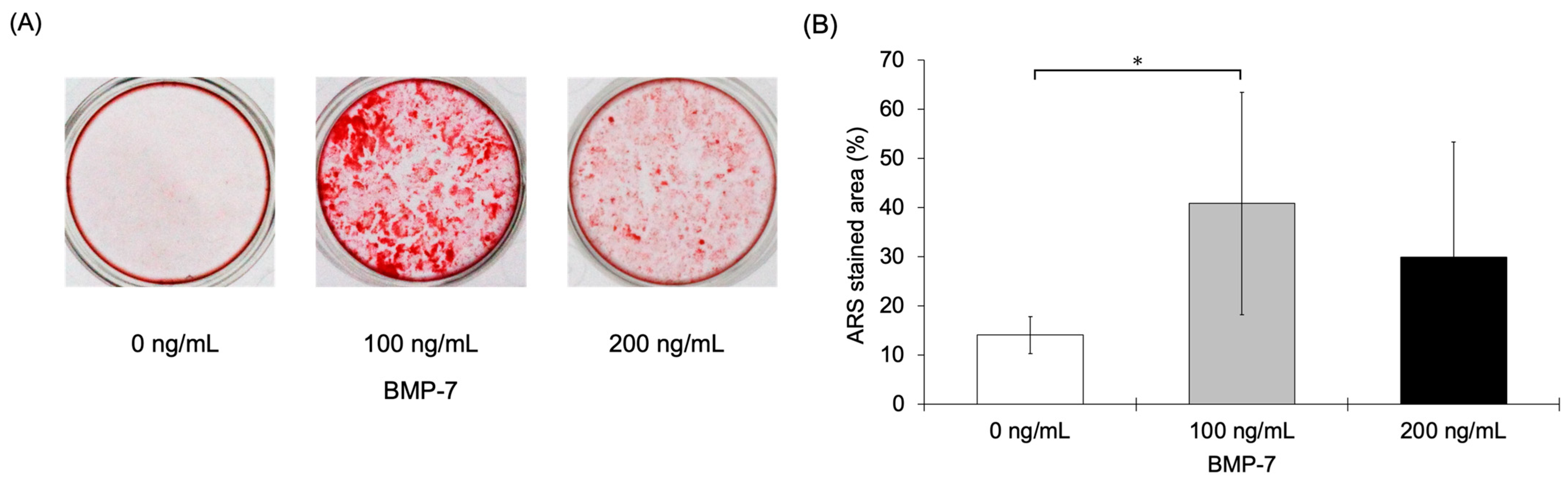
3.2.6. ARS Staining
4. Discussion
Limitation of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricucci, D.; Lin, L.M.; Spångberg, L.S.W. Wound healing of apical tissues after root canal therapy: A long-term clinical, radiographic, and histopathologic observation study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2009, 108, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, M.R.; Hernandez, M.E.F.T.; Silva, L.A.B.; Tanomaru-Filho, M. Effect of a calcium hydroxide-based root canal dressing on periapical repair in dogs: A histological study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 102, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mente, J.; Hage, N.; Pfefferle, T.; Koch, M.J.; Dreyhaupt, J.; Staehle, H.J.; Friedman, S. Mineral trioxide aggregate apical plugs in teeth with open apical foramina: A retrospective analysis of treatment outcome. J. Endod. 2009, 35, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Aluganti Narasimhulu, C.; Singla, D.K. The role of bone morphogenetic protein 7 (BMP-7) in inflammation in heart diseases. Cells 2020, 9, 280. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; Shi, C.; Sun, H. BMP signaling in the development and regeneration of tooth roots: From mechanisms to applications. Front. Cell Dev. Biol. 2023, 11, 1272201. [Google Scholar] [CrossRef]
- Badr, A.M.; Shalaby, H.K.; Awad, M.A.; Hashem, M.A. Assessment of bone morphogenetic protein-7 loaded chitosan/β-Glycerophosphate hydrogel on periodontium tissues regeneration of class III furcation defects. Saudi Dent. J. 2023, 35, 760–767. [Google Scholar] [CrossRef]
- Jung, Y.H.; Park, J.Y.; Kim, H.J.; Lee, S.M.; Kim, S.H.; Yun, J.H. Regenerative potential of bone morphogenetic protein 7-engineered mesenchymal stem cells in ligature-induced periodontitis. Tissue Eng. Part A 2023, 29, 200–210. [Google Scholar] [CrossRef]
- Fraser, D.; Caton, J.; Benoit, D.S.W. Periodontal wound healing and regeneration: Insights for engineering new therapeutic approaches. Front. Dent. Med. 2022, 3, 815810. [Google Scholar] [CrossRef]
- Aryal, A.C.S.; Islam, M.S. Potential role of BMP7 in regenerative dentistry. Int. Dent. J. 2024, 74, 901–909. [Google Scholar] [CrossRef]
- Kitagawa, M.; Ao, M.; Miyauchi, M.; Abiko, Y.; Takata, T. F-spondin regulates the differentiation of human cementoblast-like (HCEM) cells via BMP7 expression. Biochem. Biophys. Res. Commun. 2012, 418, 229–233. [Google Scholar] [CrossRef]
- Beertsen, W.; McCulloch, C.A.; Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontol. 2000 1997, 13, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Lekic, P.; McCulloch, C.A.G. Periodontal ligament cell populations: The central role of fibroblasts in creating a unique tissue. Anat. Rec. 1996, 245, 327–341. [Google Scholar] [CrossRef]
- Klar, A.; Baldassare, M.; Jessell, T.M. F-spondin: A gene expressed at high levels in the floor plate encodes a secreted protein that promotes neural cell adhesion and neurite extension. Cell 1992, 69, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Inoue, K.; Nakajima, K.; Tachikawa, T.; Yamazaki, H.; Isobe, T.; Sugawara, A.; Ogawa, M.; Tanaka, C.; Saito, M.; et al. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci. Rep. 2014, 4, 6044. [Google Scholar] [CrossRef]
- Yu, P.B.; Deng, D.Y.; Lai, C.S.; Hong, C.C.; Cuny, G.D.; Bouxsein, M.L.; Hong, D.W.; McManus, P.M.; Katagiri, T.; Sachidanandan, C.; et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat. Med. 2008, 14, 1363–1369. [Google Scholar] [CrossRef]
- Klatte-Schulz, F.; Giese, G.; Differ, C.; Minkwitz, S.; Ruschke, K.; Puts, R.; Knaus, P.; Wildemann, B. An investigation of BMP-7 mediated alterations to BMP signalling components in human tenocyte-like cells. Sci. Rep. 2016, 6, 29703. [Google Scholar] [CrossRef]
- Matsumoto, N.; Minakami, M.; Hatakeyama, J.; Haruna, C.; Morotomi, T.; Izumi, T.; Anan, H. Histologic evaluation of the effects of Emdogain gel on injured root apex in rats. J. Endod. 2014, 40, 1989–1994. [Google Scholar] [CrossRef]
- Williams, J.C.; Maitra, S.; Anderson, M.J.; Christiansen, B.A.; Reddi, A.H.; Lee, M.A. BMP-7 and bone regeneration: Evaluation of dose-response in a rodent segmental defect model. J. Orthop. Trauma 2015, 29, e336–e341. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Torii, D.; Tsutsui, T.W.; Watanabe, N.; Konishi, K. Bone morphogenetic protein 7 induces cementogenic differentiation of human periodontal ligament-derived mesenchymal stem cells. Odontology 2016, 104, 1–9. [Google Scholar] [CrossRef]
- Hakki, S.S.; Foster, B.L.; Nagatomo, K.J.; Bozkurt, S.B.; Hakki, E.E.; Somerman, M.J.; Nohutcu, R.M. Bone morphogenetic protein-7 enhances cementoblast function in vitro. J. Periodontol. 2010, 81, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.A.G.; McKenna, E.; Robey, P.G.; Shajib, M.S.; Crawford, R.W.; Doran, M.R.; Futrega, K. Inhibition of BMP signaling with LDN 193189 can influence bone marrow stromal cell fate but does not prevent hypertrophy during chondrogenesis. Stem Cell Rep. 2022, 17, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Ezawa, N.; Akashi, Y.; Nakajima, K.; Kokubun, K.; Furusawa, M.; Matsuzaka, K. The effects of tricalcium-silicate-nanoparticle-containing cement: In vitro and in vivo studies. Materials 2023, 16, 4451. [Google Scholar] [CrossRef]
- Komaki, M.; Iwasaki, K.; Arzate, H.; Narayanan, A.S.; Izumi, Y.; Morita, I. Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J. Cell. Physiol. 2012, 227, 649–657. [Google Scholar] [CrossRef]
- Lim, J.C.; Bae, S.H.; Lee, G.; Ryu, C.J.; Jang, Y.J. Activation of β-catenin by TGF-β1 promotes ligament-fibroblastic differentiation and inhibits cementoblastic differentiation of human periodontal ligament cells. Stem Cells 2020. Advance online publication. [Google Scholar] [CrossRef]
- Nagata, M.; Chu, A.K.Y.; Ono, N.; Welch, J.D.; Ono, W. Single-cell transcriptomic analysis reveals developmental relationships and specific markers of mouse periodontium cellular subsets. Front. Dent. Med. 2021, 2, 679937. [Google Scholar] [CrossRef]
- Bae, W.J.; Auh, Q.S.; Lim, H.C.; Kim, G.T.; Kim, H.S.; Kim, E.C. Sonic hedgehog promotes cementoblastic differentiation via activating the BMP pathways. Calcif. Tissue Int. 2016, 99, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Aida, Y.; Kurihara, H.; Kato, K. Wnt3a promotes differentiation of human bone marrow-derived mesenchymal stem cells into cementoblast-like cells. Vitr. Cell. Dev. Biol.-Anim. 2018, 54, 468–476. [Google Scholar] [CrossRef]
- Sawada, K.; Shimomura, J.; Takedachi, M.; Murata, M.; Morimoto, C.; Kawasaki, K.; Kawakami, K.; Iwayama, T.; Murakami, S. Activation of periodontal ligament cell cytodifferentiation by juxtacrine signaling from cementoblasts. J. Periodontol. 2024, 95, 256–267. [Google Scholar] [CrossRef]
- Hidaka, T.; Nagasawa, T.; Shirai, K.; Kado, T.; Furuichi, Y. FGF-2 induces proliferation of human periodontal ligament cells and maintains differentiation potentials of STRO-1+/CD146+ periodontal ligament cells. Arch. Oral. Biol. 2012, 57, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J. Dent. Res. 2005, 84, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; You, T.M.; Jang, Y.J. Galectin-3 plays an important role in BMP7-induced cementoblastic differentiation of human periodontal ligament cells by interacting with extracellular components. Stem Cells Int. 2023, 2023, 5924286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasawa, H.; Akashi, Y.; Nakajima, K.; Kokubun, K.; Furusawa, M.; Matsuzaka, K. Bone Morphogenetic Protein 7 Promotes the Differentiation of Periodontal Ligament Fibroblasts into F-Spondin-Expressing Cementoblast-like Cells During Root Canal Treatment—An In Vivo Rat Pulpectomy Model and In Vitro Human Fibroblast Study. Dent. J. 2025, 13, 494. https://doi.org/10.3390/dj13110494
Iwasawa H, Akashi Y, Nakajima K, Kokubun K, Furusawa M, Matsuzaka K. Bone Morphogenetic Protein 7 Promotes the Differentiation of Periodontal Ligament Fibroblasts into F-Spondin-Expressing Cementoblast-like Cells During Root Canal Treatment—An In Vivo Rat Pulpectomy Model and In Vitro Human Fibroblast Study. Dentistry Journal. 2025; 13(11):494. https://doi.org/10.3390/dj13110494
Chicago/Turabian StyleIwasawa, Hiroki, Yoshihiko Akashi, Kei Nakajima, Katsutoshi Kokubun, Masahiro Furusawa, and Kenichi Matsuzaka. 2025. "Bone Morphogenetic Protein 7 Promotes the Differentiation of Periodontal Ligament Fibroblasts into F-Spondin-Expressing Cementoblast-like Cells During Root Canal Treatment—An In Vivo Rat Pulpectomy Model and In Vitro Human Fibroblast Study" Dentistry Journal 13, no. 11: 494. https://doi.org/10.3390/dj13110494
APA StyleIwasawa, H., Akashi, Y., Nakajima, K., Kokubun, K., Furusawa, M., & Matsuzaka, K. (2025). Bone Morphogenetic Protein 7 Promotes the Differentiation of Periodontal Ligament Fibroblasts into F-Spondin-Expressing Cementoblast-like Cells During Root Canal Treatment—An In Vivo Rat Pulpectomy Model and In Vitro Human Fibroblast Study. Dentistry Journal, 13(11), 494. https://doi.org/10.3390/dj13110494