Assessment of Common Oral Behaviors in Patients with Temporomandibular Joint Disorders and Their Relationship to Psychosocial Factors
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Setting
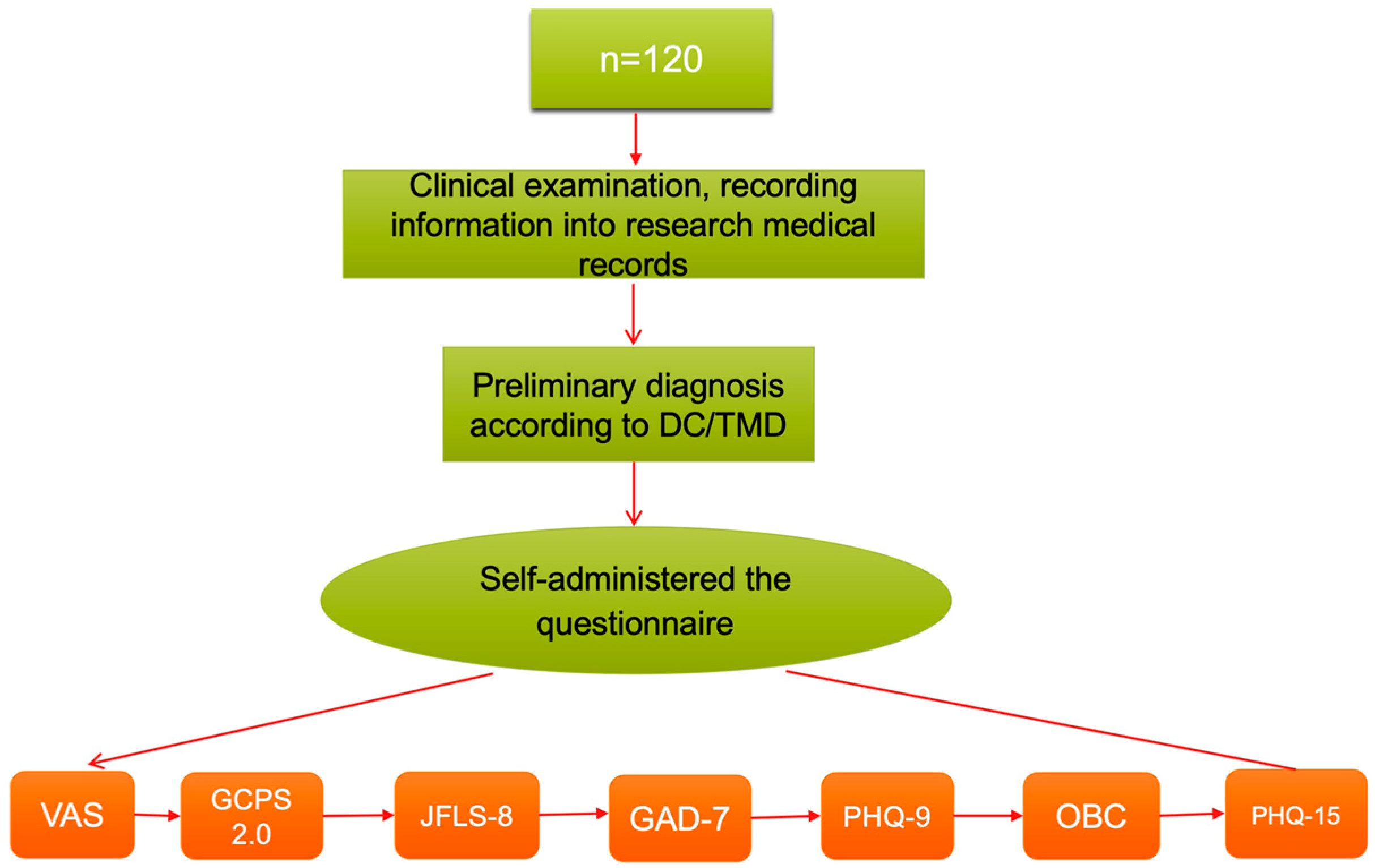
2.3. Participants
2.4. Variables and Data Measurement
2.5. Data Collection and Bias
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Correlation and Univariate Analysis
3.3. Associations with Anxiety and Depression
3.4. Multivariate Analysis
4. Discussion
4.1. Oral Behaviors and TMD Subgroups, Duration of Pain
4.2. Oral Behaviors and Anxiety Disorders, Depression
4.3. Oral Behaviors and Chronic Pain
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Okeson, J.P. Management of Temporomandibular Disorders and Occlusion, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Jung, B.; Yeo, J.; Kim, K.-W.; Cho, J.-H.; Lee, Y.J.; Ha, I.-H. Healthcare utilisation and costs for temporomandibular disorders: A descriptive, cross-sectional study. BMJ Open 2020, 10, e036768. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, R.; Klasser, G.D. Orofacial Pain Guidelines for Assessment, Diagnosis, and Management, 6th ed.; Quintessence Publishing: Batavia, IL, USA, 2018; Volume 1–50, pp. 249–265. [Google Scholar]
- Iodice, G.; Michelotti, A.; D’Antò, V.; Martina, S.; Valletta, R.; Rongo, R. Prevalence of psychosocial findings and their correlation with TMD symptoms in an adult population sample. Prog. Orthod. 2024, 25, 39. [Google Scholar] [CrossRef]
- Huhtela, O.S.; Näpänkangas, R.; Suominen, A.L.; Karppinen, J.; Kunttu, K.; Sipilä, K. Association of psychological distress and widespread pain with sympatoms of temporomandibular disorders and self-reported bruxism in students. Clin. Exp. Dent. Res. 2021, 7, 1154–1166. [Google Scholar] [CrossRef]
- Donnarumma, V.; Cioffi, I.; Michelotti, A.; Cimino, R.; Vollaro, S.; Amato, M. Analysis of the reliability of the Italian Version of the Oral Behaviors Checklist and the relationship between oral behaviors and trait anxiety in healthy individuals. J. Oral. Rehabil. 2018, 45, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, A.; Aghaali, M.; Janatifar, Z.; Saleh, A. Prevalence of Oral Parafunctional Habits in Children and Related Factors: An Observational Cross-sectional Study. Int. J. Clin. Pediatr. Dent. 2023, 16, 308–311. [Google Scholar] [CrossRef]
- Ohlmann, B.; Waldecker, M.; Leckel, M.; Bömicke, W.; Behnisch, R.; Rammelsberg, P.; Schmitter, M. Correlations between Sleep Bruxism and Temporomandibular Disorders. J. Clin. Med. 2020, 9, 611. [Google Scholar] [CrossRef]
- Magalhães, B.G.; Freitas, J.L.d.M.; Barbosa, A.C.d.S.; Gueiros, M.C.S.N.; Gomes, S.G.F.; Rosenblatt, A.; Caldas Júnior, A.d.F. Temporomandibular disorder: Otologic implications and its relationship to sleep bruxism. Braz. J. Otorhinolaryngol. 2017, 84, 614. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, S.; Si, J.; Zhang, L.; Yang, H.; Ye, Z.; Xiong, X. Association Between Oral Behaviors and Painful Temporomandibular Disorders: A Cross-Sectional Study in the General Population. J. Pain Res. 2024, 17, 431. [Google Scholar] [CrossRef]
- Restrepo, C.; Ortiz, A.M.; Henao, A.C.; Manrique, R. Association between psychological factors and temporomandibular disorders in adolescents of rural and urban zones. BMC Oral Health 2021, 21, 140. [Google Scholar] [CrossRef]
- Zhong, Y.; Luo, F.; Li, X.; Zeng, S.; Zhang, S.; Si, J.; Xiong, X.; Fang, S. Associations between oral behaviors, temporomandibular disorder subtypes and psychological distress in adult women: A retrospective case-control study. J. Oral Facial Pain Headache 2024, 38, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Keela, W.; Itthikul, T.; Mitrirattanakul, S.; Pongrojpaw, S. Awake and Sleep Oral Behaviours in Patients With Painful Temporomandibular Disorders. Int. Dent. J. 2023, 74, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Karamat, A.; Smith, J.G.; Melek, L.N.F.; Renton, T. Psychologic Impact of Chronic Orofacial Pain: A Critical Review. J. Oral Facial Pain Headache 2022, 36, 103–140. [Google Scholar] [CrossRef]
- Xu, L.; Cai, B.; Fan, S.; Lu, S.; Dai, K. Association of Oral Behaviors with Anxiety, Depression, and Jaw Function in Patients with Temporomandibular Disorders in China: A Cross-Sectional Study. Med. Sci. Monit. 2021, 27, e929985. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Yang, M.; Lv, X.; Zheng, Y.; Zhang, Q.; Sun, Y.; Wang, J.; Xiong, X. The Association Between Specific Oral Behaviors and the Number of Temporomandibular Disorder Symptoms in the General Population: A Cross-Sectional Study. J Pain Res. 2024, 17, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, M.J.; Lobbezoo, F.; Aartman, I.H.A.; Naeije, M. Validity of the Oral Behaviours Checklist: Correlations between OBC scores and intensity of facial pain. J. Oral Rehabil. 2014, 41, 115–121. [Google Scholar] [CrossRef]
- Emel, D. Prevalence of temporomandibular disorder in Turkish university students: A questionnaire study. Balk. J. Dent. Med. 2019, 23, 80–87. [Google Scholar] [CrossRef]
- Winocur-Arias, O.; Friedman-Rubin, P.; Abu Ras, K.; Lockerman, L.; Emodi-Perlman, A.; Greenbaum, T.; Reiter, S. Local myalgia compared to myofascial pain with referral according to the DC/TMD: Axis I and II results. BMC Oral Health 2022, 22, 27. [Google Scholar] [CrossRef]
- Ohrbach, R. Diagnostic Criteria for Temporomandibular Disorders: Assessment Instruments. 2014. Available online: https://inform-iadr.com/wp-content/uploads/2024/03/DC-TMD-Portuguese-PORT-Assessment-Instruments_2018_09_14.pdf (accessed on 4 August 2025).
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Tanti, I.; Wira, V.V.W.; Pragustine, Y.; Himawan, L.S.; Ariani, N. Validation of the Indonesian version of the graded chronic pain scale 2.0 in pain-related temporomandibular disorders. Med. J. Indones. 2020, 29, 42–46. [Google Scholar] [CrossRef]
- Hietaharju, M.; Näpänkangas, R.; Sipilä, K.; Teerijoki-Oksa, T.; Tanner, J.; Kemppainen, P.; Tolvanen, M.; Suvinen, T. Importance of the Graded Chronic Pain Scale as a Biopsychosocial Screening Instrument in TMD Pain Patient Subtyping. J. Oral Facial Pain Headache 2021, 35, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Inform International Network for Orofacial Pain and Related Disorders Methodology, A Consortium Focused on Clinical Translation Research. Available online: https://inform-iadr.com/ (accessed on 5 January 2023).
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- University of Virginia Library. Using and Interpreting Cronbach’s Alpha. 2015. Available online: https://data.library.virginia.edu/using-and-interpreting-cronbachs-alpha/ (accessed on 5 January 2023).
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Balk, G.A.; Stewart, R.E. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain 2014, 155, 2545–2550. [Google Scholar] [CrossRef]
- Winocur, E.; Littner, D.; Adams, I.; Gavish, A. Oral habits and their association with signs and symptoms of temporomandibular disorders in adolescents: A gender comparison. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 482–487. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk, A.; Wójcicki, M. Global Prevalence of Sleep Bruxism and Awake Bruxism in Pediatric and Adult Populations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4259. [Google Scholar] [CrossRef]
- Michelotti, A.; Iodice, G.; Vollaro, S.; Steenks, M.H.; Farella, M. Evaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw muscles. J. Am. Dent. Assoc. 2012, 143, 47–53. [Google Scholar] [CrossRef]
- van der Meulen, M.J.; Lobbezoo, F.; Aartman, I.H.A.; Naeije, M. Self-reported oral parafunctions and pain intensity in temporomandibular disorder patients. J. Orofac. Pain 2006, 20, 31–35. [Google Scholar]
- Yap, A.U.; Kim, S.; Lee, B.-M.; Jo, J.H.; Park, J.W. Sleeping and waking-state oral behaviors in TMD patients: Their correlates with jaw functional limitation and psychological distress. Clin. Oral Investig. 2024, 28, 332. [Google Scholar] [CrossRef]
- Pavlou, I.A.; Spandidos, D.A.; Zoumpourlis, V.; Papakosta, V.K. Neurobiology of bruxism: The impact of stress (Review). Biomed. Rep. 2024, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Alrashdan, M.S.; Al-Omiri, M.K. Psychosocial profiles and their correlation with physical diagnosis in temporomandibular disorders, a preliminary report. Cranio J. Craniomandib. Pract. 2022, 43, 91–99. [Google Scholar] [CrossRef]
- Khawaja, S.; Nickel, J.; Iwasaki, L.; Crow, H.; Gonzalez-Stucker, Y. Association between waking-state oral parafunctional behaviors and bio-psychosocial characteristics. J. Oral Rehabil. 2015, 42, 651–656. [Google Scholar] [CrossRef]
- Thakur, P. Association Between Temporomandibular Disorders Pain, Oral Behaviors, Anxiety and Stress. 2019. Available online: https://hdl.handle.net/11299/202896 (accessed on 28 November 2023).
- Martin, E.I.; Ressler, K.J.; Binder, E.; Nemeroff, C.B. The Neurobiology of Anxiety Disorders: Brain Imaging, Genetics, and Psychoneuroendocrinology. Psychiatr. Clin. N. Am. 2009, 32, 549–575. [Google Scholar] [CrossRef]
- Maletic, V.; Robinson, M.; Oakes, T.; Iyengar, S.; Ball, S.G.; Russell, J. Neurobiology of depression: An integrated view of key findings. Int. J. Clin. Pract. 2007, 61, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Ohrbach, R.; Dworkin, S.F. AAPT Diagnostic Criteria for Chronic Painful Temporomandibular Disorders. J. Pain 2019, 20, 1276–1292. [Google Scholar] [CrossRef]
- Ohrbach, R.; Fillingim, R.B.; Mulkey, F.; Gonzalez, Y.; Gordon, S.; Gremillion, H.; Lim, P.-F.; Ribeiro-Dasilva, M.; Greenspan, J.D.; Knott, C.; et al. Clinical Findings and Pain Symptoms as Potential Risk Factors for Chronic TMD: Descriptive Data and Empirically Identified Domains from the OPPERA Case-Control Study. J. Pain 2011, 12, T27–T45. [Google Scholar] [CrossRef]
- Medin Ceylan, C.; Cigdem Karacay, B. The relationship between the Oral Behavioral Checklist and the Jaw Functional Limitation Scale in temporomandibular joint pain. J. Oral Health Oral Epidemiol. 2024, 13, 112–117. [Google Scholar] [CrossRef]
- Koca, C.; Yıldırım, B.; Bilgir, E. Effects of bruxism on temporomandibular joint internal derangement in patients with unilateral temporomandibular joint pain: The role of magnetic resonance imaging diagnostics. Cranio. 2021, 42, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, M.L.; Parenti, S.I.; Bortolotti, F.; Della Godenza, V.; Vandi, S.; Pizza, F.; Plazzi, G.; Alessandri-Bonetti, G. Sleep Bruxism and Orofacial Pain in Patients with Sleep Disorders: A Controlled Cohort Study. J. Clin. Med. 2023, 12, 2997. [Google Scholar] [CrossRef]
- Adams, L.M.; Turk, D.C. Psychosocial factors and central sensitivity syndromes. Curr. Rheumatol. Rev. 2015, 11, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Cortines Laxe, L.; Lacerda-Santos, R. Distribution of anxiety and depression among different subtypes of temporomandibular disorder: A systematic review and meta-analysis. J. Oral Rehabil. 2022, 49, 754–767. [Google Scholar] [CrossRef] [PubMed]
| GCPS (n, %) | OBC 0–16 | OBC 17–24 | OBC ≥ 25 | p-Value |
|---|---|---|---|---|
| Grade 0 | 2 (100) | 0 (0) | 0 (0) | 0.032 ** |
| Grade I | 13 (40.6) | 12 (37.5) | 7 (21.9) | |
| Grade II | 35 (48.6) | 27 (37.5) | 10 (13.9) | |
| Grade III | 2 (18.2) | 3 (27.3) | 6 (54.5) | |
| Grade IV | 0 (0) | 1 (33.3) | 2 (66.7) | |
| 120 (100.0) | 52 (43.3) | 43 (35.8) | 25 (20.8) |
| Oral Behavior | PAIN DISORDER SUBGROUPS | |||||
|---|---|---|---|---|---|---|
| No Pain (n, %) | Myalgia (n, %) | Arthralgia (n, %) | Combined Pain (n, %) | Total (n, %) | p-Value | |
| Sleep in a position that puts pressure on the jaw | 2 (2.0) | 15 (15.2) | 8 (8.1) | 74 (74.7) | 99 (82.5) | 0.044 ** |
| GAD-7 | ||||||
| None (n, %) | Mild (n, %) | Moderate (n, %) | Severe (n, %) | Total (n, %) | p-value | |
| Clench or grind teeth when asleep | 25 (44.7) | 13 (23.2) | 13 (23.2) | 5 (8.9) | 56 (46.7) | 0.007 * |
| Sleep in a position that puts pressure on the jaw | 51 (51.5) | 29 (29.3) | 14 (14.1) | 5 (5.1) | 99 (82.5) | 0.041 * |
| Grind teeth together during walking hours | 16 (37.2) | 14 (32.6) | 9 (20.9) | 4 (9.3) | 43 (35.8) | 0.011 ** |
| Use chewing gum | 26 (54.2) | 13 (27.1) | 4 (8.3) | 5 (10.4) | 48 (40) | 0.014 ** |
| Oral Behaviors | GAD-7 | PHQ-9 | ||||
|---|---|---|---|---|---|---|
| Coef | OR (95% CI) | p-Value | Coef | OR (95% CI) | p-Value | |
| Clench or grind teeth when asleep | 1.081 | 2.95 (1.30–6.67) | 0.01 | −0.106 | 0.90 (0.37–2.19) | 0.815 |
| Sleep in a position that puts pressure on the jaw | 1.032 | 2.81 (0.81–9.72) | 0.104 | 0.229 | 1.26 (0.35–4.50) | 0.724 |
| Grind teeth together during walking hours | 0.75 | 2.12 (0.91–4.94) | 0.083 | 0.111 | 1.12 (0.45–2.77) | 0.81 |
| Use chewing gum | 0.259 | 1.30 (0.55–3.06) | 0.555 | 0.658 | 1.93 (0.75–4.96) | 0.171 |
| Chew food on one side only | 0.184 | 1.20 (0.30–4.82) | 0.795 | −0.293 | 0.75 (0.18–3.11) | 0.687 |
| Sustained talking | 0.041 | 1.04 (0.44–2.48) | 0.926 | 0.407 | 1.50 (0.60–3.78) | 0.387 |
| Yawning | −0.173 | 0.84 (0.28–2.52) | 0.758 | −0.13 | 0.88 (0.27–2.85) | 0.828 |
| Variable | Coef (95% CI) | p-Value |
|---|---|---|
| GAD-7 | 0.454 (−0.03–0.94) | 0.067 |
| PHQ-9 | 0.243 (−0.24–0.72) | 0.317 |
| JFLS-8 | −0.044 (−0.20–0.11) | 0.57 |
| PHQ-15 | 0.011 (−0.49–0.51) | 0.965 |
| VAS | 1.829 (0.51–3.15) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoa, N.N.; Viet Hai, H.; Binh, T.T.; Dong, T.T.; Minh Quyen, T.T.; Do, T. Assessment of Common Oral Behaviors in Patients with Temporomandibular Joint Disorders and Their Relationship to Psychosocial Factors. Dent. J. 2025, 13, 480. https://doi.org/10.3390/dj13100480
Hoa NN, Viet Hai H, Binh TT, Dong TT, Minh Quyen TT, Do T. Assessment of Common Oral Behaviors in Patients with Temporomandibular Joint Disorders and Their Relationship to Psychosocial Factors. Dentistry Journal. 2025; 13(10):480. https://doi.org/10.3390/dj13100480
Chicago/Turabian StyleHoa, Nguyen Ngoc, Hoang Viet Hai, Tran Thai Binh, To Thanh Dong, Tran Thi Minh Quyen, and Toan Do. 2025. "Assessment of Common Oral Behaviors in Patients with Temporomandibular Joint Disorders and Their Relationship to Psychosocial Factors" Dentistry Journal 13, no. 10: 480. https://doi.org/10.3390/dj13100480
APA StyleHoa, N. N., Viet Hai, H., Binh, T. T., Dong, T. T., Minh Quyen, T. T., & Do, T. (2025). Assessment of Common Oral Behaviors in Patients with Temporomandibular Joint Disorders and Their Relationship to Psychosocial Factors. Dentistry Journal, 13(10), 480. https://doi.org/10.3390/dj13100480






