Osteoarthritic Bony Alterations of Temporomandibular Joint and Relation to Low Bone Mineral Density in Postmenopausal Edentulous Females
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. TMJ Clinical Examination
2.3. Imaging Assessment of TMJ
2.4. Measurement of BMD
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.C.; Lee, D.G.; Choi, J.H.; Kim, S.T.; Kim, Y.K.; Ahn, H.J. Association between estrogen receptor polymorphism and pain susceptibility in female temporomandibular joint osteoarthritis patients. Int. J. Oral Maxillofac. Surg. 2007, 36, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Yang, X.; Liu, Y.; Huang, Y.; Shi, Z. Evaluation of arthrocentesis with hyaluronic acid injection plus oral glucosamine hydrochloride for temporomandibular joint osteoarthritis in oral-health-related quality of life. J. Craniomaxillofac. Surg. 2014, 42, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lee, S.; Pan, H.C.; Mohammad, A.; Lin, A.; Guo, W.; Chen, E.; Ahn, A.; Li, J.; Ting, K.; et al. Association of Condylar Bone Quality with TMJ Osteoarthritis. J. Dent. Res. 2017, 96, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.W.; Damn, D.D.; Allen, C.M.; Bouquot, J.E. Oral and Maxillofacial Pathology, 2nd ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 2002; p. 755. [Google Scholar]
- Widmalm, S.E.; Westesson, P.L.; Kim, I.K.; Pereira, F.J.; Lundh, H.; Tasaki, M.M. Temporomandibular joint pathosis related to sex, age, and dentition in autopsy material. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Takenoshita, Y.; Ishibashi, K.; Oka, M. Age-related changes in the human mandibular condyle: A morphologic, radiologic, and histologic study. J. Oral Maxillofac. Surg. 1995, 53, 1016–1023. [Google Scholar] [CrossRef]
- Wiberg, B.; Wänman, A. Signs of osteoarthrosis of the temporomandibular joints in young patients: A clinical and radiographic study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 86, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef]
- Barghan, S.; Tetradis, S.; Mallya, S. Application of cone beam computed tomography for assessment of the temporomandibular joints. Aust. Dent. J. 2012, 57 (Suppl. S1), 109–118. [Google Scholar] [CrossRef]
- Axelsson, S. Human and experimental osteoarthrosis of the temporomandibular joint. Morphological and biochemical studies. Swed. Dent. J. Suppl. 1993, 92, 1–45. [Google Scholar]
- Mercuri, L.G. Osteoarthritis, osteoarthrosis, and idiopathic condylar resorption. Oral Maxillofac. Surg. Clin. N. Am. 2008, 20, 169–183. [Google Scholar] [CrossRef]
- Israel, H.A.; Diamond, B.E.; Saed-Nejad, F.; Ratcliffe, A. Correlation between arthroscopic diagnosis of osteoarthritis and synovitis of the human temporomandibular joint and keratan sulfate levels in the synovial fluid. J. Oral Maxillofac. Surg. 1997, 55, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Ioi, H.; Matsumoto, R.; Nishioka, M.; Goto, T.K.; Nakata, S.; Nakasima, A.; Counts, A.L. Relationship of TMJ osteoarthritis / osteoarthrosis to head posture and dentofacial morphology. Orthod. Craniofac. Res. 2008, 11, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, K.; Stamatakis, H.; Tsiklakis, K. Evaluation of the severity of temporomandibular joint osteoarthritic changes related to age using cone beam computed tomography. Dentomaxillofac. Radiol. 2009, 38, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzo, K.L.; Osório, L.B.; Ferrazzo, V.A. CT Images of a Severe TMJ Osteoarthritis and Differential Diagnosis with Other Joint Disorders. Case Rep. Dent. 2013, 2013, 242685. [Google Scholar] [CrossRef]
- Hintze, H.; Wiese, M.; Wenzel, A. Cone beam CT and conventional tomography for the detection of morphological temporomandibular joint changes. Dentomaxillofac. Radiol. 2007, 36, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Honey, O.B.; Scarfe, W.C.; Hilgers, M.J.; Klueber, K.; Silveira, A.M.; Haskell, B.S.; Farman, A.G. Accuracy of cone-beam computed tomography imaging of the temporomandibular joint: Comparisons with panoramic radiology and linear tomography. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Alkhader, M.; Ohbayashi, N.; Tetsumura, A.; Nakamura, S.; Okochi, K.; Momin, M.A.; Kurabayashi, T. Diagnostic performance of magnetic resonance imaging for detecting osseous abnormalities of the temporomandibular joint and its correlation with cone beam computed tomography. Dentomaxillofac. Radiol. 2010, 39, 270–276. [Google Scholar] [CrossRef]
- Walewski, L.Â.; Tolentino, E.S.; Yamashita, F.C.; Iwaki, L.C.V.; da Silva, M.C. Cone beam computed tomography study of osteoarthritic alterations in the osseous components of temporomandibular joints in asymptomatic patients according to skeletal pattern, gender, and age. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 70–77. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Black, D.; Bonjour, J.P.; Dequeker, J.; Ehrlich, G.E.; Kanis, J.; Liberman, U.A.; Masri, B.; Mautalen, C.A.; Meunier, P.J.; et al. Prevention and Management of Osteoporosis; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2003; Volume 921, pp. 1–164.
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Kanis, J.A.; Burlet, N.; Cooper, C.; Delmas, P.D.; Reginster, J.Y.; Borgstrom, F.; Rizzoli, R. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2008, 4, 399–428. [Google Scholar] [CrossRef]
- Bultink, I.E.; Lems, W.F. Osteoarthritis and osteoporosis: What is the overlap? Curr. Rheumatol. Rep. 2013, 15, 328. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I.; Kim, M.K. The relationship between osteoarthritis and osteoporosis. J. Bone Miner. Metab. 2014, 32, 101–109. [Google Scholar] [CrossRef]
- Dervis, E. Oral Implications of Osteoporosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2005, 100, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Jagur, O.; Kull, M.; Leibur, E.; Kallikorm, R.; Loorits, D.; Lember, M.; Voog-Oras, U. Relationship between radiographic changes in the temporomandibular joint and bone mineral density: A population based study. Stomatologija 2011, 13, 42–48. [Google Scholar] [PubMed]
- Bäck, K.; Ahlqwist, M.; Hakeberg, M.; Björkelund, C.; Dahlström, L. Relation between osteoporosis and radiographic and clinical signs of osteoarthritis/arthrosis in the temporomandibular joint: A population-based, cross-sectional study in an older Swedish population. Gerodontology 2017, 34, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, S.C.; Durna, D.; Baygutalp, F. Correlations with clinical and radiologic findings and prevalence of osteopenia/osteoporosis in the patients with bilateral temporomandibular joint osteoarthritis. J. Stomatol. Oral Maxillofac. Surg. 2024, 4, 101869. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Kou, X.X.; Meng, Z.; Bi, R.Y.; Liu, Y.; Zhang, J.N.; Zhou, Y.H.; Gan, Y.H. Estrogen aggravates iodoacetate-induced temporomandibular joint osteoarthritis. J. Dent. Res. 2013, 92, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Nekora-Azak, A.; Evlioglu, G.; Ceyhan, A.; Keskin, H.; Berkman, S.; Issever, H. Estrogen replacement therapy among postmenopausal women and its effects on signs and symptoms of temporomandibular disorders. Cranio 2008, 26, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Lora, V.R.; Canales, G.L.; Gonçalves, L.M.; Meloto, C.B.; Barbosa, C.M. Prevalence of temporomandibular disorders in postmenopausal women and relationship with pain and HRT. Braz. Oral Res. 2016, 30, e100. [Google Scholar] [CrossRef]
- Kim, D.; Pirshahid, A.A.; Li, Y.; Varghese, T.; Pope, J.E. Prevalence of osteoporosis in osteoarthritis: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 1687–1693. [Google Scholar] [CrossRef]
- Peck, C.C.; Goulet, J.P.; Lobbezoo, F.; Schiffman, E.L.; Alstergren, P.; Anderson, G.C.; de Leeuw, R.; Jensen, R.; Michelotti, A.; Ohrbach, R.; et al. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J. Oral Rehabil. 2014, 41, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hollender, L.; Anderson, Q.; Kartha, K.; Ohrbach, R.; Truelove, E.L.; John, M.T.; Schiffman, E.L. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): Development of image analysis criteria and examiner reliability for image analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Nikitina, E.; Soboleva, U.; Slaidina, A. Evaluation of temporomandibular joint condyle changes and bone mineral density to postmenopausal females with complete dentures using cone beam computed tomography. In Proceedings of the 40th European Prosthodontic Association (EPA), 65th German Society for Prosthetic Dentistry and Biomaterials (DGPro), Halle, Germany, 15–17 September 2016. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Rando, C.; Waldron, T. TMJ osteoarthritis: A new approach to diagnosis. Am. J. Phys. Anthropol. 2012, 148, 45–53. [Google Scholar] [CrossRef]
- Osteoarthritis. PrimeView. Nat. Rev. Dis. Primers. 2016, 2, 16073. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Whyte, A.; Boeddinghaus, R.; Bartley, A.; Vijeyaendra, R. Imaging of the temporomandibular joint. Clin. Radiol. 2021, 76, 76.E21–76.E35. [Google Scholar] [CrossRef] [PubMed]
- Görürgöz, C.; İçen, M.; Kurt, M.H.; Aksoy, S.; Bakırarar, B.; Rozylo-Kalinowska, I.; Orhan, K. Degenerative changes of the mandibular condyle in relation to the temporomandibular joint space, gender and age: A multicenter CBCT study. Dent. Med. Probl. 2023, 60, 127–135. [Google Scholar] [CrossRef]
- Cevidanes, L.H.; Hajati, A.K.; Paniagua, B.; Lim, P.F.; Walker, D.G.; Palconet, G.; Nackley, A.G.; Styner, M.; Ludlow, J.B.; Zhu, H.; et al. Quantification of condylar resorption in temporomandibular joint osteoarthritis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 110–117. [Google Scholar] [CrossRef]
- Kijowski, R.; Blankenbaker, D.; Stanton, P.; Fine, J.; De Smet, A. Correlation between radiographic findings of osteoarthritis and arthroscopic findings of articular cartilage degeneration within the patellofemoral joint. Skelet. Radiol. 2006, 35, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Nishiyama, H.; Hayashi, T. Follow-up study of condylar bony changes using helical computed tomography in patients with temporomandibular disorder. Dentomaxillofac. Radiol. 2007, 36, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Rudisch, A.; Emshoff, R.; Maurer, H.; Kovacs, P.; Bodner, G. Pathologic-sonographic correlation in temporomandibular joint pathology. Eur. Radiol. 2006, 16, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Dumbuya, A.; Gomes, A.F.; Marchini, L.; Zeng, E.; Comnick, C.L.; Melo, S.L.S. Bone changes in the temporomandibular joints of older adults: A cone-beam computed tomography study. Spec. Care Dentist. 2020, 40, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Larheim, T.A.; Abrahamsson, A.K.; Kristensen, M.; Arvidsson, L.Z. Temporomandibular joint diagnostics using CBCT. Dentomaxillofac. Radiol. 2015, 44, 20140235. [Google Scholar] [CrossRef] [PubMed]
- Cömert Kiliç, S.; Kiliç, N.; Sümbüllü, M.A. Temporomandibular joint osteoarthritis: Cone beam computed tomography findings, clinical features, and correlations. Int. J. Oral Maxillofac. Surg. 2015, 44, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, T.; Nakashima, M.; Okuda, T.; Tatematsu, N. Effect of estrogen replacement on temporomandibular joint remodeling in ovariectomized rats. J. Oral Maxillofac. Surg. 2000, 58, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Richette, P. Impact of treatments for osteoporosis on osteoarthritis progression. Osteoporos. Int. 2012, 8, S881–S883. [Google Scholar] [CrossRef] [PubMed]
- Giesen, E.B.; Ding, M.; Dalstra, M.; van Eijden, T.M. Changed morphology and mechanical properties of cancellous bone in the mandibular condyles of edentate people. J. Dent. Res. 2004, 83, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Shi, L.; Lu, H.; Liu, Z.; Yu, M.; Wang, Y.; Wang, H. Influence of edentulism on the structure and function of temporomandibular joint. Heliyon 2023, 9, e20307. [Google Scholar] [CrossRef]
- Zarb, G.A.; Carlsson, G.E. Temporomandibular disorders: Osteoarthritis. J. Orofac. Pain 1999, 13, 295–306. [Google Scholar] [PubMed]
- Mejersjö, C.; Wenneberg, B. Diclofenac sodium and occlusal splint therapy in TMJ osteoarthritis: A randomized controlled trial. J. Oral Rehabil. 2008, 35, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, B.N.; Rancic, N.K.; Bojanovic, M.R.; Stojanovic, S.K.; Zivkovic, V.G.; Djordjevic, D.B.; Stankovic, A.M. Is Osteoarthritis Always Associated with Low Bone Mineral Density in Elderly Patients? Medicina 2022, 58, 1207. [Google Scholar] [CrossRef] [PubMed]
| Overall N (%) | OA N (%) | NonOA N (%) | |
|---|---|---|---|
| Indeterminant signs of OA | |||
| Flattening | 38 (47.5%) | 6 (7.5%) | 32 (40%) |
| Subcortical sclerosis | 33 (41.3%) | 6 (7.5%) | 17 (21.3%) |
| Signs of OA | |||
| Subcortical cyst | 8 (10%) | 8 (10%) | 0 |
| Erosion | 4 (5%) | 4 (5%) | 0 |
| Osteophytes | 0 | 0 | 0 |
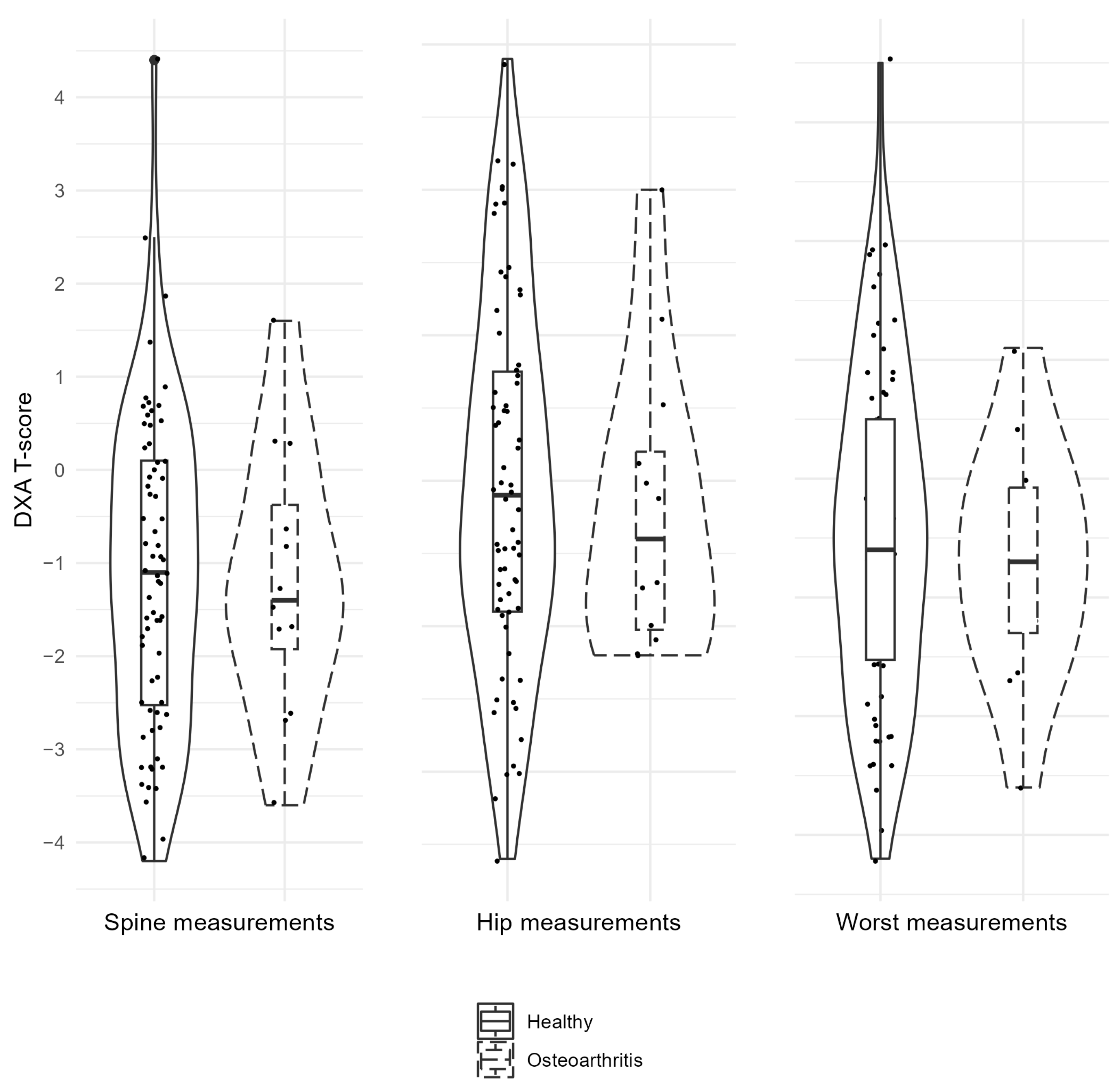
| Factor (N) | Descriptives | DXA Spine | DXA Hip | DXA Worst | Age |
|---|---|---|---|---|---|
| OA (12) | Mean (SD) | −1.2 (1.5) | −1.2 (1.0) | −1.7 (1.0) | 72.3 (10.1) |
| NonOA (68) | Mean (SD) | −1.1 (1.7) | −1.0 (1.2) | −1.5 (1.4) | 72.0 (8.7) |
| CI, 95% | −1.0; 1.1 | −0.6; 0.9 | −0.7; 1.0 | −5.9; 5.2 | |
| p-Value | 0.458 | 0.277 | 0.170 | 0.856 |
| Factor | Positive/Negative Finding (N) | Descriptives | DXA Spine | DXA Hip | DXA Worst | Age |
|---|---|---|---|---|---|---|
| Flattening | Positive (38) | Mean (SD) | −1.1 (1.5) | −1.0 (1.3) | −1.5 (1.2) | 73.1 (8.1) |
| Negative (42) | Mean (SD) | −1.1 (1.7) | −1.1 (1.1) | −1.6 (1.3) | 71.1 (9.5) | |
| CI, 95% | −0.8; 0.7 | −0.7; 0.4 | −0.7; 0.5 | −6.0; 1.9 | ||
| p-Value | 0.721 | 0.302 | 0.819 | 0.447 | ||
| Subcortical sclerosis | Positive (33) | Mean (SD) | −1.4 (1.5) | −1.1 (1.1) | −1.7 (1.4) | 70.8 (9.3) |
| Negative (47) | Mean (SD) | −0.9 (1.7) | −1.1 (1.3) | −1.5 (1.3) | 72.9 (8.5) | |
| CI, 95% | −0.2; 1.3 | −0.5; 0.5 | −0.4; 0.8 | −1.8; 6.2 | ||
| p-Value | 0.685 | 0.347 | 0.937 | 0.555 | ||
| Subcortical cyst | Positive (8) | Mean (SD) | −1.4 (1.0) | −0.8 (1.1) | −1.5 (0.9) | 71.8 (11.2) |
| Negative (72) | Mean (SD) | −1.1 (1.7) | −1.1 (1.2) | −1.6 (1.4) | 72.1 (8.6) | |
| CI, 95% | −0.9; 1.5 | −1.1; 0.7 | −1.1; 0.9 | −6.3; 6.9 | ||
| p-Value | 0.104 | 0.428 | 0.199 | 0.582 | ||
| Erosion | Positive (4) | Mean (SD) | −0.8 (2.3) | −1.8 (0.6) | −2.2 (1.1) | 73.5 (8.9) |
| Negative (76) | Mean (SD) | −1.1 (1.6) | −1.0 (1.2) | −1.5 (1.3) | 72.0 (8.9) | |
| CI, 95% | −2.0; 1.3 | −0.4; 2.0 | −0.6; 2.1 | −10.6; 7.5 | ||
| p-Value | 0.364 | 0.095 | 0.342 | 0.681 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumpane, L.; Nikitina, E.; Neimane, L.; Abeltins, A.; Soboleva, U.; Slaidina, A. Osteoarthritic Bony Alterations of Temporomandibular Joint and Relation to Low Bone Mineral Density in Postmenopausal Edentulous Females. Dent. J. 2024, 12, 238. https://doi.org/10.3390/dj12080238
Krumpane L, Nikitina E, Neimane L, Abeltins A, Soboleva U, Slaidina A. Osteoarthritic Bony Alterations of Temporomandibular Joint and Relation to Low Bone Mineral Density in Postmenopausal Edentulous Females. Dentistry Journal. 2024; 12(8):238. https://doi.org/10.3390/dj12080238
Chicago/Turabian StyleKrumpane, Laura, Evija Nikitina, Laura Neimane, Andris Abeltins, Una Soboleva, and Anda Slaidina. 2024. "Osteoarthritic Bony Alterations of Temporomandibular Joint and Relation to Low Bone Mineral Density in Postmenopausal Edentulous Females" Dentistry Journal 12, no. 8: 238. https://doi.org/10.3390/dj12080238
APA StyleKrumpane, L., Nikitina, E., Neimane, L., Abeltins, A., Soboleva, U., & Slaidina, A. (2024). Osteoarthritic Bony Alterations of Temporomandibular Joint and Relation to Low Bone Mineral Density in Postmenopausal Edentulous Females. Dentistry Journal, 12(8), 238. https://doi.org/10.3390/dj12080238









