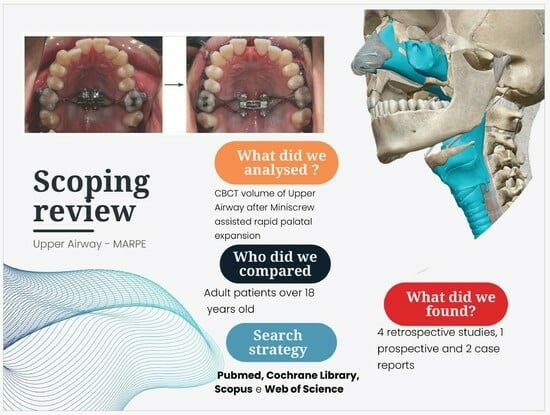
Does Miniscrew-Assisted Rapid Palatal Expansion Influence Upper Airway in Adult Patients? A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Questions
2.2. Eligibility Criteria
- (1)
- Population: Patients with a transverse skeletal discrepancy of maxilla treated with MARPE technique, specifically focusing on individual over 18 years of age. We opted to narrow the scope of the review to studies involving adult patients, aged 18 and above, to minimize the potential influence of physiological growth on outcomes, thereby enhancing the reliability of the results. It is crucial to note that, in growing numbers of patients, variations observed can be attributed to normal development processes, rather than the specific method used.
- (2)
- Intervention: MARPE micro-implant-assisted rapid palatal expansion.
- (3)
- Comparison: Comparing upper airway conditions before treatment (T0) to after treatment (T1) and assessing the sustainability of results over time (T2).
- (4)
- Outcome: Considering volumetric changes or sectional areas on CBCT or CT scans performed before and after the expansion treatment.
- (5)
- Study design: For inclusion, articles needed to have full English text, without restriction on the publication year. Accepted study designs encompassed observational studies, randomized clinical trials, case reports and case series.
2.3. Selection Criteria and Search Strategy
2.4. Study Quality Assessment
2.5. Data Items and Collection
3. Results
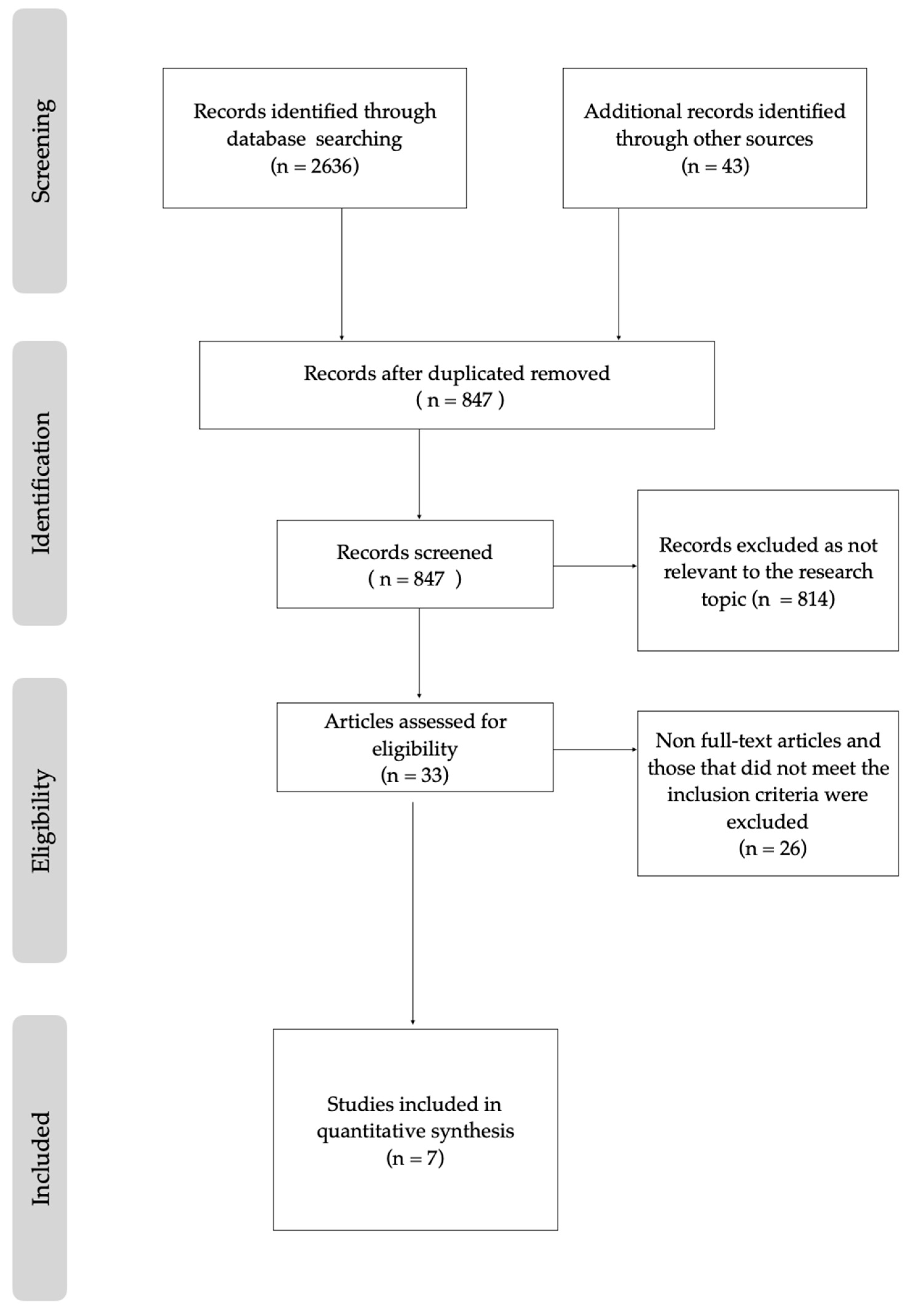
3.1. Study Election
3.2. Study Design
3.3. Type of Appliance and Activation Protocol
3.4. Evaluation Method
3.5. Airway Aerea Localisation Evaluated
3.5.1. Nasal Cavity
3.5.2. Nasopharynx
3.5.3. Oropharynx
3.5.4. Hipopharynx
3.6. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Zhai, M.; Wang, M.; Cui, S.; Cheng, C.; Wang, J.; Wei, F. Three-Dimensional Evaluation Effects of Microimplant-Assisted Rapid Palatal Expansion on the Upper Airway Volume: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1790. [Google Scholar] [CrossRef] [PubMed]
- Kurol, J.; Berglund, L. Longitudinal study and cost-benefit analysis of the effect of early treatment of posterior cross-bites in the primary dentition. Eur. J. Orthod. 1992, 14, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kapetanović, A.; Theodorou, C.I.; Bergé, S.J.; Schols, J.G.J.H.; Xi, T. Efficacy of Miniscrew-Assisted Rapid Palatal Expansion (MARPE) in late adolescents and adults: A systematic review and meta-analysis. Eur. J. Orthod. 2021, 43, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Aloufi, F.; Preston, C.B.; Zawawi, K.H.; Del Fabbro, M.; Vissink, A. Clinical Study Changes in the Upper and Lower Pharyngeal Airway Spaces Associated with Rapid Maxillary Expansion. Int. Sch. Res. Not. 2012, 2012, 290964. [Google Scholar] [CrossRef]
- McDonald, J.P. Airway problems in children—Can the orthodontist help? Ann. Acad. Med. Singap. 1995, 24, 158–162. [Google Scholar]
- Redline, S.; Tishler, P.V.; Tosteson, T.D.; Williamson, J.; Kump, K.; Browner, I.; Ferrette, V.; Krejci, P. The familial aggregation of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 151 Pt 1, 682–687. [Google Scholar] [CrossRef]
- Seto, B.H.; Gotsopoulos, H.; Sims, M.R.; Cistulli, P.A. Maxillary morphology in obstructive sleep apnoea syndrome. Eur. J. Orthod. 2001, 23, 703–714. [Google Scholar] [CrossRef]
- Brunetto, D.P.; Moschik, C.E.; Dominguez-Mompell, R.; Jaria, E.; Sant’Anna, E.F.; Moon, W. Mini-implant assisted rapid palatal expansion (MARPE) effects on adult obstructive sleep apnea (OSA) and quality of life: A multi-center prospective controlled trial. Prog. Orthod. 2022, 23, 3. [Google Scholar] [CrossRef]
- Williams, B.J.D.; Currimbhoy, S.; Silva, A.; O’Ryan, F.S. Complications following surgically assisted rapid palatal expansion: A retrospective cohort study. J. Oral Maxillofac. Surg. 2012, 70, 2394–2402. [Google Scholar] [CrossRef]
- Suri, L.; Taneja, P. Surgically assisted rapid palatal expansion: A literature review. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 290–302. [Google Scholar] [CrossRef]
- Carlson, C.; Sung, J.; McComb, R.W.; MacHado, A.W.; Moon, W. Microimplant-assisted rapid palatal expansion appliance to orthopedically correct transverse maxillary deficiency in an adult. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 716–728. [Google Scholar] [CrossRef]
- Park, J.J.; Park, Y.C.; Lee, K.J.; Cha, J.Y.; Tahk, J.H.; Choi, Y.J. Skeletal and dentoalveolar changes after miniscrew-assisted rapid palatal expansion in young adults: A cone-beam computed tomography study. Korean J. Orthod. 2017, 47, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, Y.; Yang, S. Effect and stability of miniscrew-assisted rapid palatal expansion: A systematic review and meta-analysis. Korean J. Orthod. 2022, 52, 334–344. [Google Scholar] [CrossRef]
- L’ancoraggio Scheletrico Nelle Metodiche di Espansione Palatale Tepedino Michele. Available online: https://www.sido.it/public/media/copertina_fad_2020_sito_pacchetto_last.pdf (accessed on 18 December 2023).
- Cantarella, D.; Dominguez-Mompell, R.; Mallya, S.M.; Moschik, C.; Pan, H.C.; Miller, J.; Moon, W. Changes in the midpalatal and pterygopalatine sutures induced by micro-implant-supported skeletal expander, analyzed with a novel 3D method based on CBCT imaging. Prog. Orthod. 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Elkenawy, I.; Fijany, L.; Colak, O.; Paredes, N.A.; Gargoum, A.; Abedini, S.; Cantarella, D.; Dominguez-Mompell, R.; Sfogliano, L.; Moon, W. An assessment of the magnitude, parallelism, and asymmetry of micro-implant-assisted rapid maxillary expansion in non-growing patients. Prog. Orthod. 2020, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, D.; Dominguez-Mompell, R.; Moschik, C.; Mallya, S.M.; Pan, H.C.; Alkahtani, M.R.; Elkenawy, I.; Moon, W. Midfacial changes in the coronal plane induced by microimplant-supported skeletal expander, studied with cone-beam computed tomography images. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.M.; Dalci, O.; Darendeliler, M.A.; Papageorgiou, S.N.; Papadopoulou, A.K. Volumetric upper airway changes after rapid maxillary expansion: A systematic review and meta-analysis. Eur. J. Orthod. 2017, 39, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Di Carlo, G.; Cornelis, M.A.; Cattaneo, P.M. Three-dimensional analyses of short- and long-term effects of rapid maxillary expansion on nasal cavity and upper airway: A systematic review and meta-analysis. Orthod. Craniofac. Res. 2020, 23, 250–276. [Google Scholar] [CrossRef] [PubMed]
- Alyessary, A.S.; Othman, S.A.; Yap, A.U.J.; Radzi, Z.; Rahman, M.T. Effects of non-surgical rapid maxillary expansion on nasal structures and breathing: A systematic review. Int. Orthod. 2019, 17, 12–19. [Google Scholar] [CrossRef]
- Giudice, A.L.; Spinuzza, P.; Rustico, L.; Messina, G.; Nucera, R. Short-term treatment effects produced by rapid maxillary expansion evaluated with computed tomography: A systematic review with meta-analysis. Korean J. Orthod. 2020, 50, 314–323. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Ratti, S.; Nakra, P.; Shetty, S.; Mohammed, A.; Saidath, K. Evaluation of Soft Tissue and Airway Changes in Individuals Treated with Mini-Implant Assisted Rapid Palatal Expansion (MARPE). J. Long Term Eff. Med. Implants 2022, 32, 7–18. [Google Scholar] [CrossRef]
- Hur, J.-S.; Kim, H.-H.; Choi, J.-Y.; Suh, S.-H.; Baek, S.-H. Investigation of the effects of miniscrew-assisted rapid palatal expansion on airflow in the upper airway of an adult patient with obstructive sleep apnea syndrome using computational fluidstructure interaction analysis. Korean J. Orthod. 2017, 47, 353–364. [Google Scholar] [CrossRef]
- Li, Q.; Tang, H.; Liu, X.; Luo, Q.; Jiang, Z.; Martin, D.; Guo, J. Comparison of dimensions and volume of upper airway before and after mini-implant assisted rapid maxillary expansion. Angle Orthod. 2020, 90, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, P.; Xu, Q.; Hou, Y.; Guo, J. A comparative analysis of aerodynamic and anatomic characteristics of upper airway before and after mini-implant–assisted rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2021, 159, e301–e310. [Google Scholar] [CrossRef]
- Garcez, A.S.; Suzuki, S.S.; Storto, C.J.; Cusmanich, K.G.; Elkenawy, I.; Moon, W. Effects of maxillary skeletal expansion on respiratory function and sport performance in a para-athlete—A case report. Phys. Ther. Sport 2019, 36, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, Y.C.; Lee, K.J.; Lintermann, A.; Han, S.S.; Yu, H.S.; Choi, Y.J. Assessment of changes in the nasal airway after nonsurgical miniscrew-assisted rapid maxillary expansion in young adults. Angle Orthod. 2018, 88, 435–441. [Google Scholar] [CrossRef]
- Anéris, F.F.; El Haje, O.; Rosário, H.D.; de Menezes, C.C.; Franzini, C.M.; Custodio, W. The effects of miniscrew-assisted rapid palatal expansion on the upper airway of adults with midpalatal suture in the last two degrees of ossification. J. World Fed. Orthod. 2023, 12, 150–155. [Google Scholar] [CrossRef]
- Marino, A.; Ranieri, R.; Chiarotti, F.; Villa, M.P.; Malagola, C. Rapid maxillary expansion in children with Obstructive Sleep Apnoea Syndrome (OSAS). Eur. J. Paediatr. Dent. 2012, 13, 57–63. [Google Scholar]
- Cistulli, P.A.; Richards, G.N.; Palmisano, R.G.; Unger, G.; Berthon-Jones, M.; Sullivan, C.E. Influence of maxillary constriction on nasal resistance and sleep apnea severity in patients with Marfan’s syndrome. Chest 1996, 110, 1184–1188. [Google Scholar] [CrossRef]
- Persson, M.; Thilander, B. Palatal suture closure in man from 15 to 35 years of age. Am. J. Orthod. 1977, 72, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Magnusson, B.C.; Thilander, B. Sutural closure in rabbit and man: A morphological and histochemical study. J. Anat. 1978, 125 Pt 2, 313–321. [Google Scholar] [PubMed]
- Melsen, B. Palatal growth studied on human autopsy material. A histologic microradiographic study. Am. J. Orthod. 1975, 68, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Korbmacher, H.; Schilling, A.; Püschel, K.; Amling, M.; Kahl-Nieke, B. Age-dependent three-dimensional microcomputed tomography analysis of the human midpalatal suture. J. Orofac. Orthop. 2007, 68, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.; Fricke-Zech, S.; Fialka-Fricke, J.; Dullin, C.; Zapf, A.; Gruber, R.; Sennhenn-kirchner, S.; Kubein-Meesenburg, D.; Sadat-Khonsari, R. Imaging of the midpalatal suture in a porcine model: Flat-panel volume computed tomography compared with multislice computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M. Sutural biology and the correlates of craniosynostosis. Am. J. Med. Genet. 1993, 47, 581–616. [Google Scholar] [CrossRef]
- Angelieri, F.; Cevidanes, L.H.S.; Franchi, L.; Gonçalves, J.R.; Benavides, E.; McNamara, J.A. Midpalatal suture maturation: Classification method for individual assessment before rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 759–769. [Google Scholar] [CrossRef]
- Di Carlo, G.; Polimeni, A.; Melsen, B.; Cattaneo, P.M. The relationship between upper airways and craniofacial morphology studied in 3D. A CBCT study. Orthod. Craniofac. Res. 2015, 18, 1–11. [Google Scholar] [CrossRef]
- Poirrier, A.-L.; Fanielle, J.; Bruwier, A.; Chakar, B.; Poirrier, R. Upper airway imaging in sleep-disordered breathing. Acta Neurol. Belg. 2014, 114, 87–93. [Google Scholar] [CrossRef]
- Di Carlo, G.; Gurani, S.F.; Pinholt, E.M.; Cattaneo, P.M. A new simple three-dimensional method to characterize upper airway in orthognathic surgery patient. Dentomaxillofac. Radiol. 2017, 46, 20170042. [Google Scholar] [CrossRef]
- Luzzi, V.; Ierardo, G.; Di Carlo, G.; Saccucci, M.; Polimeni, A. Obstructive sleep apnea syndrome in the pediatric age: The role of the dentsit. Eur. Rev. Pharmacol. Sci. 2019, 23, 9–14. [Google Scholar] [CrossRef]
- Zimmerman, J.N.; Vora, S.R.; Pliska, B.T. Reliability of upper airway assessment using CBCT. Eur. J. Orthod. 2019, 41, 101–108. [Google Scholar] [CrossRef]
- Li, S.; Shi, H.; Bao, J.; Dong, W.; Wang, G.; Yu, J. The effects of body posture on upper airway in OSAHS patients. Lin Chuang Er Bi Yan Hou Ke Za Zhi 2004, 18, 737–739. [Google Scholar]
- Yildirim, N.; Fitzpatrick, M.F.; Whyte, K.F.; Jalleh, R.; Wightman, A.J.; Douglas, N.J. The effect of posture on upper airway dimensions in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am. Rev. Respir. Dis. 1991, 144, 845–847. [Google Scholar] [CrossRef]
- Van Holsbeke, C.S.; Verhulst, S.L.; Vos, W.G.; De Backer, J.W.; Vinchurkar, S.C.; Verdonck, P.R.; van Doorn, J.W.; Nadjmi, N.; De Backer, W.A. Change in upper airway geometry between upright and supine position during tidal nasal breathing. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hellsing, E. Changes in the pharyngeal airway in relation to extension of the head. Eur. J. Orthod. 1989, 11, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Takeda, S.; Kanazawa, M.; Yamazaki, A.; Fujiwara, Y.; Mizoguchi, I. The effect of head posture on the pharyngeal airway space (PAS). Int. J. Oral Maxillofac. Surg. 2002, 31, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, D.P.; Sant’Anna, E.F.; Machado, A.W.; Moon, W. Non-surgical treatment of transverse deficiency in adults using Microimplant-assisted Rapid Palatal Expansion (MARPE). Dent. Press. J. Orthod. 2017, 22, 110–125. [Google Scholar] [CrossRef] [PubMed]

| Authors and Year | Study Design | Participants | Expansion Device | Expansion Protocol | Measurement Method | Software | Airway Regions | Outcomes | Change Percentage % | Follow Up Point |
|---|---|---|---|---|---|---|---|---|---|---|
| Garcez et al., 2019 [27] | Case report | 1 pt male Age 18 | MSE | Twice a day (0.25 mm) until necessary expansion was achieved | CBCT volume Respiratory test | ITK-SNAP | Nasal cavity Pharyngeal airway | Increases in both nasal cavity and oropharyngeal volume | 31% | T0: before expansion T1: immediately after expansion |
| Kim et al., 2018 [28] | Retrospective clinical study | 14 pts (10 f, 4 m) mean age: 22.7 years range: 18.3–26.5 years | Four banded Hyrax MRE supported by four miniscrew | Once a day (0.2 mm/turn) until the required expansion was achieved | CBCT volume | On Demand 3d | Nasal cavity Nasopharynx | Volume and cross-sectional area of nasal cavity increased after MARME and were maintained after one year | NC-V 9.9% (T0–T1) 5.5% (T1–T2)—15.4% (T0–T2) NF 6.4% (T0–T1)—4.1% (T1–T2) 10.5% (T0–T2) | T0: before expansion T1: immediately after expansion T2: after one year exapnsion |
| Tang et al., 2021 [26] | Retrospective clinical study | 30 pts (21 f, 9 m) mean age: 23.8 ± 3.90 years; range: 18–33 years | MSE type II | Once a day (0.13 mm/turn) until the required expansion was achieved | CBCT volume Computational fluid dynamics | Dolphin Images | Total Pharynx Nasopharynx Oropharynx Hypopharynx | Enlargements of the volume of total pharynx, nasopharynx and oropharynx were found | Pharynx 9.9% Nasopharynx 20.7% Oropharynx 8.84% | T0: before expansion T1: after 3 months |
| Li et al., 2020 [25] | Retrospective clinical study | 22 pts (18 f, 4 m) mean age: 22.6 ± 4.5 years; range: 18–35 years | MSE | Twice a day (0.25 mm) until necessary expansion was achieved | CBCT volume | Dolphin Images | Nasal cavity Nasopharynx Retropalatal Retroglossal Hypopharynx | Volume of Nasal cavity and Nasopharynx increased significantly | V-NC 16.2% V-NPA 14.1% V-RPA 5.7% V-RGA 11.26 V-HPA −11.6% | T0: before expansion T1: immediately after expansion |
| Hur et al., 2017 [24] | Case report | 1 pt male Age 18.7 | Not reported | Not reported | CBCT volume and areas Computational fluid dynamics | ICEM-CFD | Nasal cavity Pharynx | The cross-sectional areas at most planes in nasal cavity and the upper half of the pharynx were significantly increased | N/A | T0: before expansion T1: after 6 months |
| Aneris et al., 2023 [29] | Controlled clinical trail | 20 pts (man-to-woman ratio of 1:5,) mean age: 24.5 ± 6.2 years; range: 18–30 years | Hybrid with four miniscrew—Pec Lab, Bio Horizonte | Not reported | CBCT volume | Osirix MD | Total upper Retropalatal Retroglossal | Increases of all volumetric parameters and minimal transverse airway constriction (p < 0.05) | 14% | T0: before expansion T1: after 120 days |
| Shetty et al., 2022 [23] | Retrospective clinical study | 10 pts Range: 18–30 years | Not reported | Not reported | CBCT volume and linear measurements | Planmeca Romexis | Retropalatal Retroglossal Total airway | Slight decreases of retropalatal and retroglossal airway. All variations was found to be statististically insignificant | N/A | T0: before expansion T1: immediately after expansion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benetti, M.; Montresor, L.; Cantarella, D.; Zerman, N.; Spinas, E. Does Miniscrew-Assisted Rapid Palatal Expansion Influence Upper Airway in Adult Patients? A Scoping Review. Dent. J. 2024, 12, 60. https://doi.org/10.3390/dj12030060
Benetti M, Montresor L, Cantarella D, Zerman N, Spinas E. Does Miniscrew-Assisted Rapid Palatal Expansion Influence Upper Airway in Adult Patients? A Scoping Review. Dentistry Journal. 2024; 12(3):60. https://doi.org/10.3390/dj12030060
Chicago/Turabian StyleBenetti, Mariachiara, Luca Montresor, Daniele Cantarella, Nicoletta Zerman, and Enrico Spinas. 2024. "Does Miniscrew-Assisted Rapid Palatal Expansion Influence Upper Airway in Adult Patients? A Scoping Review" Dentistry Journal 12, no. 3: 60. https://doi.org/10.3390/dj12030060
APA StyleBenetti, M., Montresor, L., Cantarella, D., Zerman, N., & Spinas, E. (2024). Does Miniscrew-Assisted Rapid Palatal Expansion Influence Upper Airway in Adult Patients? A Scoping Review. Dentistry Journal, 12(3), 60. https://doi.org/10.3390/dj12030060