Effect of Laser Irradiation Modes and Photosensitizer Types on Antimicrobial Photodynamic Therapy (aPDT) for Streptococcus sobrinus in the Crown Dentin of Bovine Teeth: An Experimental In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Dentin Plates
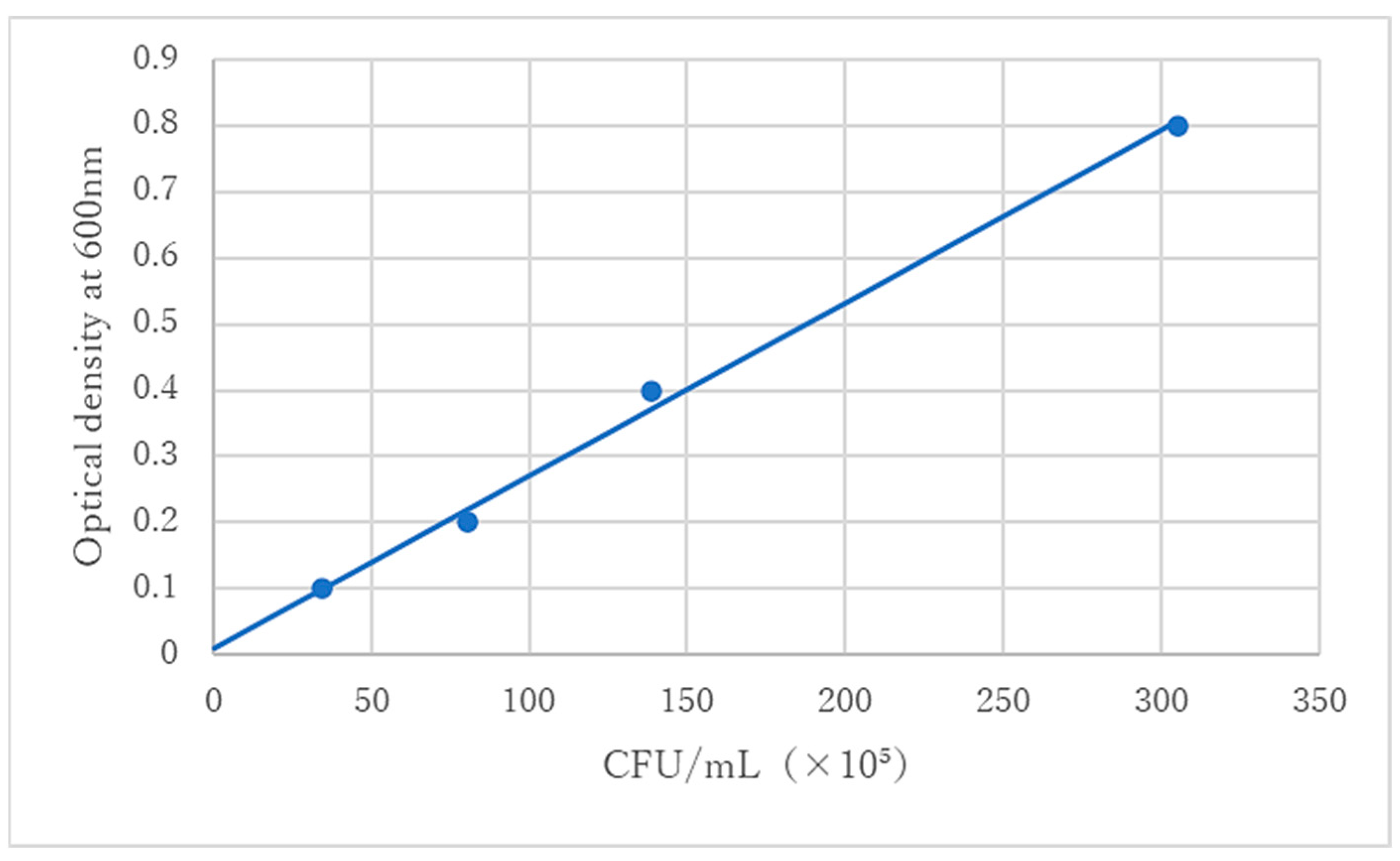
2.2. Preparation of Bacterial Solution
2.3. Preparation of Infected Dentin Plates
2.4. Laser Irradiation Modes
2.5. Adjustment of PS
2.6. Experimental Groups and aPDT Treatment
2.7. Detachment of Viable Bacteria from Infected Dentin Plate
2.8. ATP Assay
2.9. Colony Count Assay
2.10. Scanning Electron Microscopy (SEM) Observation of Infected Dentin Plate after aPDT
2.11. Statistical Analysis
3. Results
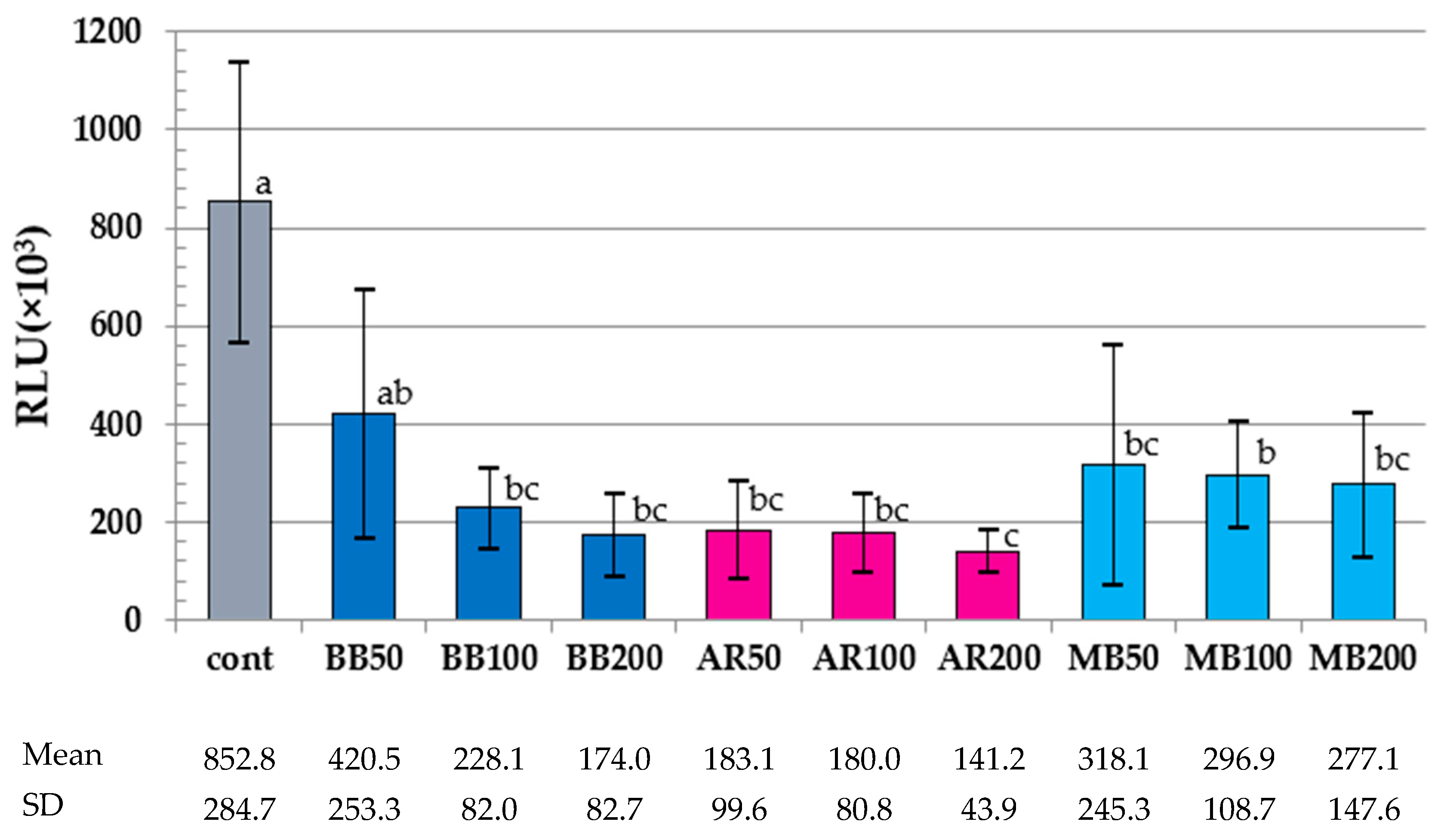
3.1. Results of ATP Assay
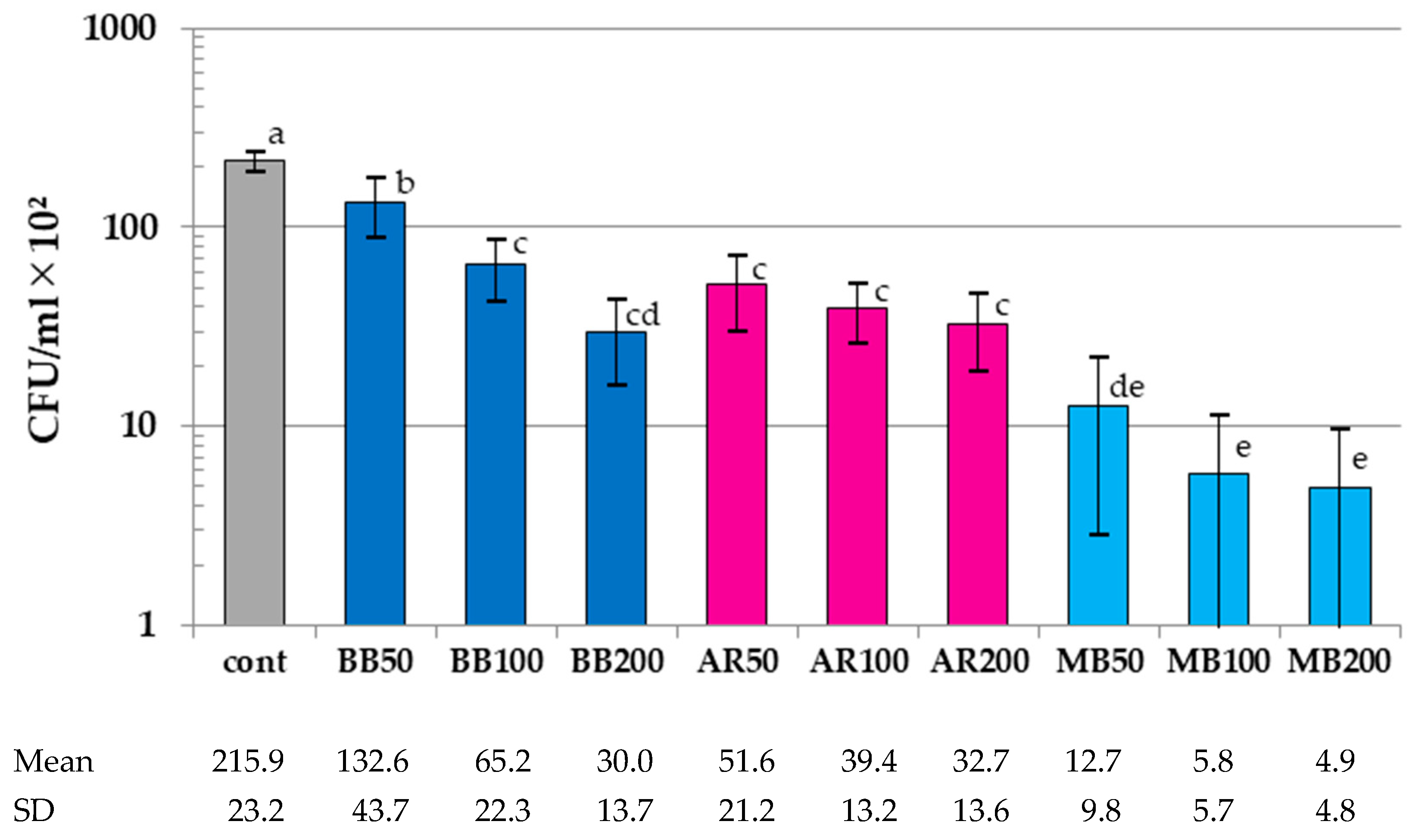
3.2. Results of Colony-Count Assay
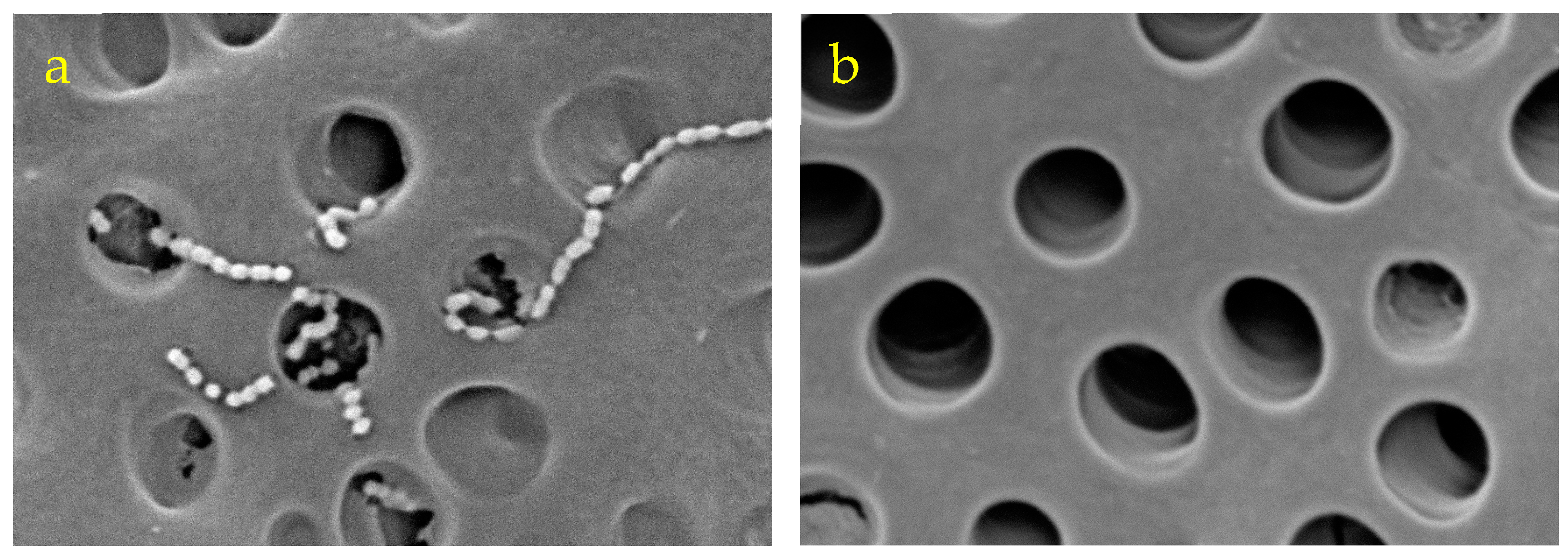
3.3. Scanning Electron Microscope Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitanaka, Y.; Takeuchi, Y.; Hiratsuka, K.; Aung, N.; Sakamaki, Y.; Nemoto, T.; Meinzer, W.; Izumi, Y.; Iwata, T.; Aoki, A. The effect of antimicrobial photodynamic therapy using yellow-green LED and rose bengal on Porphyromonas gingivalis. Photodiagn. Photodyn. Ther. 2020, 32, 102033. [Google Scholar] [CrossRef]
- Cláudio, M.M.; Nuernberg, M.A.A.; Rodrigues, J.V.S.; Belizário, L.C.G.; Batista, J.A.; Duque, C.; Garcia, V.G.; Theodoro, L.H. Effects of multiple sessions of antimicrobial photodynamic therapy (aPDT) in the treatment of periodontitis in patients with uncompensated type 2 diabetes: A randomized controlled clinical study. Photodiagn. Photodyn. Ther. 2021, 35, 102451. [Google Scholar] [CrossRef]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef]
- Garcez, A.S.; Hamblin, M.R. Methylene blue and hydrogen peroxide for photodynamic inactivation in root canal—A new protocol for use in endodontics. Eur. Endod. J. 2017, 2, 29. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Leelanarathiwat, K.; Katsuta, Y.; Katsuragi, H.; Watanabe, F. Antibacterial activity of blue high-power light-emitting diode-activated flavin mononucleotide against Staphylococcus aureus biofilm on a sandblasted and etched surface. Photodiagn. Photodyn. Ther. 2020, 31, 101855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Song, J.; Ping, Y.; Li, M. The application of antimicrobial photodynamic therapy (aPDT) in the treatment of peri-implantitis. Comput. Math. Methods. Med. 2022, 2022, 3547398. [Google Scholar] [CrossRef]
- Nemezio, M.A.; de Souza Farias, S.S.; Borsatto, M.C.; Aires, C.P.; Corona, S.A.M. Effect of methylene blue-induced photodynamic therapy on a Streptococcus mutans biofilm model. Photodiagn. Photodyn. Ther. 2017, 20, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Besegato, J.F.; de Melo, P.B.G.; Abreu Bernardi, A.C.; Souza, M.T.; Zanotto, E.D.; Bagnato, V.S.; de Souza Rastelli, A.N. Using antimicrobial photodynamic therapy with ultrasound devices and bioactive glasses as a combined approach for treating dentin caries lesions. Pathogens 2023, 17, 1052. [Google Scholar] [CrossRef] [PubMed]
- Comeau, P.; Manso, A.A. Systematic evaluation of curcumin concentrations and blue light parameters towards antimicrobial photodynamic therapy against cariogenic microorganisms. Pharmaceutics 2023, 15, 2707. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.A.; Tonon, C.C.; Spolidório, D.M.P.; Bagnato, V.S.; Giusti, J.S.M.; Santos-Pinto, L. Photodynamic potential of curcumin and blue LED against Streptococcus mutans in a planktonic culture. Photodiagnosis. Photodyn. Ther. 2013, 10, 313–319. [Google Scholar] [CrossRef]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers. Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef]
- Andersen, R.; Loebel, N.; Hammond, D.; Wilson, M. Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J. Clin. Dent. 2007, 18, 34–38. [Google Scholar]
- Kunert, M.; Lukomska-Szymanska, M. Bio-inductive materials in direct and indirect pulp capping-a review article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef]
- Nanda, R.; Koul, M.; Srivastava, S.; Upadhyay, V.; Dwivedi, R. Clinical evaluation of 3 mix and other mix in non-instrumental endodontic treatment of necrosed primary teeth. J. Oral Biol. Craniofac. Res. 2014, 4, 114–119. [Google Scholar] [CrossRef][Green Version]
- Reis, A.C.M.; Regis, W.F.M.; Rodrigues, L.K.A. Scientific evidence in antimicrobial photodynamic therapy: An alternative approach for reducing cariogenic bacteria. Photodiagnosis Photodyn. Ther. 2019, 26, 179–189. [Google Scholar] [CrossRef]
- Cieplika, F.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Hiller, K.; Maisch, T.; Karygianni, L. Antimicrobial photodynamic therapy as an adjunct for treatment of deep carious lesions—A systematic review. Photodiagnosis Photodyn. Ther. 2017, 18, 54–62. [Google Scholar] [CrossRef]
- Faria, L.V.; Fernandes, T.O.; Guimarães, L.S.; Cajazeira, M.R.R.; Antunes, L.S.; Antunes, L.A.A. Does selective caries removal in combination with antimicrobial photodynamic therapy affect the clinical performance of adhesive restorations of primary or permanent teeth? A systematic review with meta-analysis. J. Clin. Pediatr. Dent. 2022, 46, 1–14. [Google Scholar] [CrossRef]
- Nagai, Y.; Suzuki, A.; Katsuragi, H.; Shinkai, K. Effect of antimicrobial photodynamic therapy (aPDT) on the sterilization of infected dentin in vitro. Odontology 2018, 106, 154–161. [Google Scholar] [CrossRef]
- Yoshii, D.; Katsuragi, H.; Shinkai, K. Bactericidal effect of antimicrobial photodynamic therapy (aPDT) on dentin plate infected with Lactobacillus acidophilus. Odontology 2021, 109, 67–75. [Google Scholar] [CrossRef]
- Love, R.M.; Jenkinson, H.F. Invasion of dentinal tubules by oral bacteria. Crit. Rev. Oral Biol. Med. 2002, 13, 171–183. [Google Scholar] [CrossRef]
- Brittan, J.L.; Sprague, S.V.; Macdonald, E.L.; Love, R.M.; Jenkinson, H.F.; West, N.X. In Vivo Model for Microbial Invasion of Tooth Root Dentinal Tubules. J. Appl. Oral Sci. 2016, 24, 126–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conrads, G.; de Soet, J.J.; Song, L.; Henne, K.; Sztajer, H.; Wagner-Döbler, I.; Zeng, A.P. Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level. J. Oral Microbiol. 2014, 6, 26189. [Google Scholar] [CrossRef] [PubMed]
- Farva, K.; Sattar, H.; Ullah, H.; Raziq, A.; Mehmood, M.D.; Tareen, A.K.; Sultan, I.N.; Zohra, Q.; Khan, M.W. Phenotypic analysis, molecular characterization, and antibiogram of caries-causing bacteria isolated from dental patients. Microorganisms 2023, 11, 1952. [Google Scholar] [CrossRef]
- Kishi, M.; Abe, A.; Kishi, K.; Ohara-Nemoto, Y.; Kimura, S.; Yonemitsu, M. Relationship of quantitative salivary levels of Streptococcus mutans and S. sobrinus in mothers to caries status and colonization of mutans streptococci in plaque in their 2.5-year-old children. Community Dent. Oral Epidemiol. 2009, 37, 241–249. [Google Scholar] [CrossRef]
- de Soet, J.J.; Toors, F.A.; de Graaff, J. Acidogenesis by oral streptococci at different pH values. Caries Res. 1989, 23, 14–17. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Lemos, J.A.C.; Abranches, J.; Gonçalves, R.B.; Burne, R.A. Adaptive acid tolerance response of Streptococcus sobrinus. J. Bacteriol. 2004, 186, 6383–6390. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Malta, I.; Duarte, M.; Dinis, M.; Tavares, D.; Videira, A.; Ferreira, P. Enolase from Streptococcus sobrinus is an immunosuppressive protein. Cell Microbiol. 2004, 6, 79–88. [Google Scholar] [CrossRef]
- Okada, M.; Kawamura, M.; Oda, Y.; Yasuda, R.; Kojima, T.; Kurihara, H. Caries prevalence associated with Streptococcus mutans and Streptococcus sobrinus in Japanese schoolchildren. Int. J. Paediatr. Dent. 2012, 22, 342–348. [Google Scholar] [CrossRef]
- Korona-Glowniak, I.; Skawinska-Bednarczyk, A.; Wrobel, R.; Pietrak, J.; Tkacz-Ciebiera, I.; Maslanko-Switala, M.; Krawczyk, D.; Bakiera, A.; Borek, A.; Malm, A.; et al. Streptococcus sobrinus as a predominant oral bacteria related to the occurrence of dental caries in polish children at 12 years old. Int. J. Environ. Res. Public Health 2022, 19, 15005. [Google Scholar] [CrossRef] [PubMed]
- Mylona, V.; Anagnostaki, E.; Parker, S.; Cronshaw, M.; Lynch, E.; Grootveld, M. Laser-assisted aPDT protocols in randomized controlled clinical trials in dentistry: A systematic review. Dent. J. 2020, 8, 107. [Google Scholar] [CrossRef]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the outcome of various laser therapy applications in root canal disinfection: A systematic review. Photodiagn. Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef]
- Cabral, F.V.; Souza, T.H.; Sellera, F.H.; Fontes, A.; Ribeiro, M.S. Strengthening collaborations at the biology-physics interface: Trends in antimicrobial photodynamic therapy. Biophys Rev. 2023, 15, 685–697. [Google Scholar] [CrossRef]
- Nammour, S.; Zeinoun, T.; Bogaerts, I.; Lamy, M.; Geerts, S.O.; Bou Saba, S.; Lamard, L.; Peremans, A.; Limme, M. Evaluation of dental pulp temperature rise during photo-activated decontamination (PAD) of caries: An in vitro study. Lasers. Med. Sci. 2010, 25, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Granados-Tavera, K.; Zambrano-Angulo, M.; Hidalgo-Rosa, Y.; Zarate, X.; Cárdenas-Jirón, G. Tuning the visible-NIR absorption of azulenocyanine-based photosensitizers. J. Mol. Model. 2022, 28, 344. [Google Scholar] [CrossRef]
- Hosoya, Y.; Taguchi, T.; Arita, S.; Tay, F.R. Clinical evaluation of polypropylene glycol-based caries detecting dyes for primary and permanent carious dentin. J. Dent. 2008, 36, 1041–1047. [Google Scholar] [CrossRef]
- Jori, G.; Camerin, M.M.; Guidorin, L.S.; Coppellotti, O. Antimicrobial photodynamic therapy: Basic principles. In Photodynamic Inactivation of Microbial Pathogens—Medical and Environmental Applications; Hamblin, M.R., Jori, G., Eds.; RSC: Cambridge, UK, 2011; pp. 1–18. [Google Scholar]
- Maliszewska, I.; Wanarska, E.; Thompson, A.C.; Samuel, I.D.W.; Matczyszyn, K. Biogenic gold nanoparticles decrease methylene blue photobleaching and enhance antimicrobial photodynamic therapy. Molecules. 2021, 26, 623. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, K.; Nakamura, K.; Ikai, H.; Kanno, T.; Kohno, M.; Sasaki, K.; Niwano, Y. Bactericidal action of photogenerated singlet oxygen from photosensitizers used in plaque disclosing agents. PLoS ONE 2012, 7, e37871. [Google Scholar] [CrossRef] [PubMed]
- Niedre, M.J.; Secord, A.J.; Patterson, M.S.; Wilson, B.C. In vitro tests of the validity of singlet oxygen luminescence measurements as a dose metric in photodynamic therapy. Cancer Res. 2003, 63, 7986–7994. [Google Scholar] [PubMed]
- Yamamoto, J.; Yamamoto, S.; Hirano, T.; Li, S.; Koide, M.; Kohno, E.; Okada, M.; Inenaga, C.; Tokuyama, T.; Yokota, N.; et al. Monitoring of singlet oxygen is useful for predicting the photodynamic effects in the treatment for experimental glioma. Clin. Cancer Res. 2006, 12, 7132–7139. [Google Scholar] [CrossRef]
- Yang, R.; Guo, S.; Xiao, S.; Ding, Y. Enhanced wound healing and osteogenic potential of photodynamic therapy on human gingival fibroblasts. Photodiagn. Photodyn. Ther. 2020, 32, 101967. [Google Scholar] [CrossRef]
- Miyata, S.; Miyaji, H.; Kawasaki, H.; Yamamoto, M.; Nishida, E.; Takita, H.; Akasaka, T.; Ushijima, N.; Iwanaga, T.; Sugaya, T. Antimicrobial photodynamic activity and cytocompatibility of Au25(Capt)18 clusters photoexcited by blue LED light irradiation. Int. J. Nanomed. 2017, 12, 2703–2716. [Google Scholar] [CrossRef]

| Laser Irradiation Protocol | Photo Sensitizer | ||
|---|---|---|---|
| BB | AR | MB | |
| 50 mW × 120 s | BB50 | AR50 | MB50 |
| 100 mW × 60 s | BB100 | AR100 | MB100 |
| 200 mW × 30 s | BB200 | AR200 | MB200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, Y.; Yoshii, D.; Katsuragi, H.; Shinkai, K. Effect of Laser Irradiation Modes and Photosensitizer Types on Antimicrobial Photodynamic Therapy (aPDT) for Streptococcus sobrinus in the Crown Dentin of Bovine Teeth: An Experimental In Vitro Study. Dent. J. 2024, 12, 59. https://doi.org/10.3390/dj12030059
Yamaguchi Y, Yoshii D, Katsuragi H, Shinkai K. Effect of Laser Irradiation Modes and Photosensitizer Types on Antimicrobial Photodynamic Therapy (aPDT) for Streptococcus sobrinus in the Crown Dentin of Bovine Teeth: An Experimental In Vitro Study. Dentistry Journal. 2024; 12(3):59. https://doi.org/10.3390/dj12030059
Chicago/Turabian StyleYamaguchi, Yohei, Daiki Yoshii, Hiroaki Katsuragi, and Koichi Shinkai. 2024. "Effect of Laser Irradiation Modes and Photosensitizer Types on Antimicrobial Photodynamic Therapy (aPDT) for Streptococcus sobrinus in the Crown Dentin of Bovine Teeth: An Experimental In Vitro Study" Dentistry Journal 12, no. 3: 59. https://doi.org/10.3390/dj12030059
APA StyleYamaguchi, Y., Yoshii, D., Katsuragi, H., & Shinkai, K. (2024). Effect of Laser Irradiation Modes and Photosensitizer Types on Antimicrobial Photodynamic Therapy (aPDT) for Streptococcus sobrinus in the Crown Dentin of Bovine Teeth: An Experimental In Vitro Study. Dentistry Journal, 12(3), 59. https://doi.org/10.3390/dj12030059





