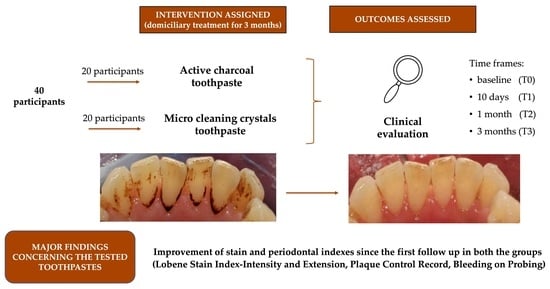
Evaluation of the Efficacy of Low-Particle-Size Toothpastes against Extrinsic Pigmentations: A Randomized Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
- -
- Adult patients
- -
- Presence of extrinsic stains
- -
- Underage patients
- -
- Patients suffering from neurological or psychiatric disorders
- -
- Pregnant women
- -
- Patients undergoing anticancer chemotherapy
2.3. Interventions and Outcomes
- -
- PCR—plaque control record by O’Leary [19] allows to assess the compliance of the patient and their level of oral hygiene at home by identifying the areas of biofilm accumulation. Each tooth was divided into four surfaces: mesial, distal, vestibular, palatal/lingual. The presence/absence of plaques for all surfaces was recorded. PCR is therefore calculated as a percentage value, dividing the number of plaque-containing surfaces by the total number of available surfaces, multiplied by 100.
- -
- BoP—bleeding on probing [20] allows to assess the level of gingival inflammation, calculated after gentle probing with a periodontal probe. All teeth underwent periodontal probing on the three buccal surfaces (mesiobuccal, medial and distobuccal). BoP was considered positive when bleeding occurred 20 s after probing. As for the PCR, a percentage value was obtained dividing the total positive sites by the total sites, multiplied by 100.
- -
- -
- Control group: patients had to use Colgate® Sensation White (Colgate-Palmolive, New York, NY, USA) for home oral care twice a day
- -
- Trial group: patients had to use Blanx Black® (Coswell S.p.A., Funo di Argelato, BO, Italy) for home oral care twice a day
2.4. Sample Size
2.5. Randomization and Blinding
2.6. Statistical Analysis
3. Results
3.1. Participant Flow and Baseline Data
3.2. Plaque Control Record
3.3. Bleeding on Probing
3.4. Lobene Stain Index-Intensity
3.5. Lobene Stain Index-Extension
3.6. Harms and Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casado, B.G.S.; Moraes, S.L.D.; Souza, G.F.M.; Guerra, C.M.F.; Souto-Maior, J.R.; Lemos, C.A.A.; Vasconcelos, B.C.E.; Pellizzer, E.P. Efficacy of Dental Bleaching with Whitening Dentifrices: A Systematic Review. Int. J. Dent. 2018, 2018, 7868531. [Google Scholar] [CrossRef]
- Mahmood, I.; Pettinato, M. Impact of Intrinsic and Extrinsic Factors on the Pharmacokinetics of Peptides: When Is the Assessment of Certain Factors Warranted? Antibodies 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Duque, C.C.; Soares, D.G.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Bleaching effectiveness, hydrogen peroxide diffusion, and cytotoxicity of a chemically activated bleaching gel. Clin. Oral Investig. 2014, 18, 1631–1637. [Google Scholar] [PubMed]
- Soares, D.G.; Marcomini, N.; Duque, C.C.O.; Bordini, E.A.F.; Zuta, U.O.; Basso, F.G.; Hebling, J.; Costa, C.A.S. Increased whitening efficacy and reduced cytotoxicity are achieved by the chemical activation of a highly concentrated hydrogen peroxide bleaching gel. J. Appl. Oral Sci. 2019, 27, e20180453. [Google Scholar] [CrossRef]
- Joiner, A.; Luo, W. Tooth colour and whiteness: A review. J. Dent. 2017, 67, S3–S10. [Google Scholar] [CrossRef]
- Young, N.; Fairley, P.; Mohan, V.; Jumeaux, C. A study of hydrogen peroxide chemistry and photochemistry in tea stain solution with relevance to clinical tooth whitening. J. Dent. 2012, 2, e11–e16. [Google Scholar] [CrossRef]
- Sulieman, M. An overview of tooth discoloration: Extrinsic, intrinsic and internalized stains. Dent. Updat. 2005, 32, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.; Addy, M. Tooth discolouration and staining: A review of the literature. Br. Dent. J. 2001, 190, 309–316. [Google Scholar] [CrossRef]
- Nakonieczna-Rudnicka, M.; Bachanek, T.; Madejczyki, M.; Grajewskai, I.; Kobyłecka, E. Teeth whitening versus the influence of extrinsic factors on teeth stains. Przegl. Lek. 2015, 72, 126–130. [Google Scholar]
- Koc Vural, U.; Bagdatli, Z.; Yilmaz, A.E.; Yalçın Çakır, F.; Altundaşar, E.; Gurgan, S. Effects of charcoal-based whitening toothpastes on human enamel in terms of color, surface roughness, and microhardness: An in vitro study. Clin. Oral Investig. 2021, 25, 5977–5985. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, G.; Wang, D.; Yang, J.; Zhang, Y.; Garnett, J.A.; Chen, Y.; Wang, Y.; Xia, B. Comparative Microbial Profiles of Caries and Black Extrinsic Tooth Stain in Primary Dentition. Caries Res. 2021, 55, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Bhardwaj, A.; Kansil, S.; Kaur, R.; Kaur, S.; Gambhir, R.S. Efficacy evaluation of rubber cup and air polishing techniques using glycine in plaque and stain removal-A clinical trial. J. Family Med. Prim. Care 2021, 10, 636–641. [Google Scholar] [PubMed]
- Joiner, A. Whitening toothpastes: A review of the literature. J. Dent. 2010, 38 (Suppl. 2), e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Devila, A.; Lasta, R.; Zanella, L.; Agnol, M.D.; Rodrigues-Junior, S.A. Efficacy and Adverse Effects of Whitening Dentifrices Compared with Other Products: A Systematic Review and Meta-analysis. Oper. Dent. 2020, 45, E77–E90. [Google Scholar] [CrossRef] [PubMed]
- Ghajari, M.F.; Shamsaei, M.; Basandeh, K.; Galouyak, M.S. Abrasiveness and whitening effect of charcoal-containing whitening toothpastes in permanent teeth. Dent. Res. J. 2021, 18, 51. [Google Scholar]
- Dionysopoulos, D.; Papageorgiou, S.; Malletzidou, L.; Gerasimidou, O.; Tolidis, K. Effect of novel charcoal-containing whitening toothpaste and mouthwash on color change and surface morphology of enamel. J. Conserv. Dent. 2020, 23, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.K.; Bashirelahi, N.; Reynolds, M.A. Charcoal and charcoal-based dentifrices: A literature review. J. Am. Dent. Assoc. 2017, 148, 661–670. [Google Scholar] [CrossRef]
- Sowinski, J.; Petrone, D.M.; Battista, G.; Petrone, M.E.; DeVizio, W.; Volpe, A.R. Clinical comparison of two tartar control dentifrices: A twelve-week study. J. Clin. Dent. 1998, 9, 101–104. [Google Scholar]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Almiñana-Pastor, P.J.; Segarra-Vidal, M.; López-Roldán, A.; Alpiste-Illueca, F.M. A controlled clinical study of periodontal health in anticoagulated patients: Assessment of bleeding on probing. J. Clin. Exp. Dent. 2017, 9, e1431–e1438. [Google Scholar] [CrossRef]
- Lobene, R.R. Effect of dentifrices on tooth stains with controlled brushing. J. Am. Dent. Assoc. 1968, 77, 849–855. [Google Scholar] [CrossRef]
- Yin, W.; Li, X.; He, S.; Ma, H.; Hu, D.; Zhang, Y.P.; Delgado, E.; DeVizio, W.; Mateo, L.R. Extrinsic stain removal efficacy of a new desensi-tizing dentifrice containing 8.0% arginine, calcium carbonate and 1450 ppm fluoride. Am. J. Dent. 2010, 23, 36A–40A. [Google Scholar]
- Gudipaneni, R.K.; Kumar, V.; Jesudass, G.; Peddengatagari, S.; Duddu, Y. Short term comparative evaluation of antimicrobial efficacy of tooth paste containing lactoferrin, lysozyme, lactoperoxidase in children with severe early childhood caries: A clinical study. J. Clin. Diagn. Res. 2014, 8, ZC18. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Van Loveren, C.; Duckworth, R.M. Anti-calculus and whitening toothpastes. Monogr. Oral Sci. 2013, 23, 61–74. [Google Scholar] [PubMed]
- Nathoo, S.; Petrone, M.E.; DeVizio, W.; Chaknis, P.; Volpe, A.R. A six-week clinical study to compare the stain removal efficacy of three dentifrices. J. Clin. Dent. 2002, 13, 91–94. [Google Scholar] [PubMed]
- Heidari, A.; Shahrabi, M.; Shahrabi, M.S. Efficacy of Three Toothpastes in Iron Stain Removal from Primary Teeth. Int. J. Clin. Pediatr. Dent. 2019, 12, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Alofi, R.S.; Alsuayri, H.A.; Mohey, L.S.; Alofi, A.S. Efficiency of activated charcoal powder in stain removal and effect on surface roughness compared to whitening toothpaste in resin composite: In vitro study. Saudi Dent. J. 2021, 33, 1105–1110. [Google Scholar] [CrossRef]
- Greenwall, L.H.; Greenwall-Cohen, J.; Wilson, N.H.F. Charcoal-containing dentifrices. Br. Dent. J. 2019, 226, 697–700. [Google Scholar] [CrossRef]
- Sanchez, N.; Fayne, R.; Burroway, B. Charcoal: An ancient material with a new face. Clin. Dermatol. 2020, 38, 262–264. [Google Scholar] [CrossRef]
- Jowett, A.K.; Marlow, I.; Rawlinson, A. A double blind randomised controlled clinical trial comparing a novel anti-stain and calculus reducing dentifrice with a standard fluoride dentifrice. J. Dent. 2013, 41, 313–320. [Google Scholar] [CrossRef]
- Liu, H.; Tu, J. Reduction of extrinsic tooth stain by a toothpaste containing 10% high cleaning silica, 0.5% sodium phytate and 0.5% sodium pyrophosphate: An 8-week randomised clinical trial. BMC Oral Health 2021, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Putt, M.S.; Milleman, J.L.; Ghassemi, A. Extrinsic tooth stain removal efficacy of a sodium bicarbonate dual-phase dentifrice containing calcium and phosphate in a six-week clinical trial. J. Clin. Dent. 2004, 15, 71–75. [Google Scholar]
- Dursun, M.N.; Ergin, E.; Tekce, A.U.; Gurgan, S. Which whitening toothpaste with different contents is more effective on color and bond strength of enamel? J. Esthet. Restor. Dent. 2023, 35, 397–405. [Google Scholar] [CrossRef]
- Vertuan, M.; da Silva, J.F.; de Oliveira, A.C.M.; da Silva, T.T.; Justo, A.P.; Zordan, F.L.S.; Magalhães, A.C. The In Vitro Effect of Dentifrices with Activated Charcoal on Eroded Teeth. Int. Dent. J. 2022; in press. [Google Scholar] [CrossRef]
- Machla, F.; Mulic, A.; Bruzell, E.; Valen, H.; Stenhagen, I.S.R. In vitro abrasivity and chemical properties of charcoal-containing dentifrices. Biomater. Investig. Dent. 2020, 7, 167–174. [Google Scholar] [CrossRef]
- Tomás, D.B.M.; Pecci-Lloret, M.P.; Guerrero-Gironés, J. Effectiveness and abrasiveness of activated charcoal as a whitening agent: A systematic review of in vitro studies. Ann. Anat. 2023, 245, 151998. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
- Shanbhag, S.; Stavropoulos, A.; Bolstad, A.I.; Suliman, S.; Mustafa, K. Ectopic Bone Tissue Engineering in Mice Using Human Gingiva or Bone Marrow-Derived Stromal/Progenitor Cells in Scaffold-Hydrogel Constructs. Front. Bioeng. Biotechnol. 2021, 9, 783468. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]
| Grade | Intensity | Extension |
|---|---|---|
| 0 | Absence of pigmentations | Absence of pigmentations |
| 1 | Light spot ranging from yellow to light brown–grey | Pigmentations on more than one third of the area |
| 2 | Moderate brown spot | Persistence of the pigmentations from one third and two thirds of the assessed area |
| 3 | Dark spot ranging from brown to dark brown | Pigmentations on more than two thirds of the area evaluated |
| Gel | Manufacturer | Composition |
|---|---|---|
| Blanx Black® | Coswell S.p.A., Funo di Argelato, BO, Italy | Limonene, aqua, sodium lauryl sulfate, glycerin, sorbitol, silica, cellulose gum, charcoal powder, sodium monofluorophosphate, hydrated silica, aroma, xylitol, cetraria islandica extract, papain, maltodextrin, benzyl alcohol, sodium saccharin, usnea barbata extract, sodium benzoate, phenoxyethanol. |
| Colgate Sensation White® | Colgate-Palmolive, New York, NY, USA | Aqua, aroma, hydrated silica, sodium fluoride, sorbitol, sodium saccharin, polyethyleneglycol-12, sodium bicarbonate, sodium lauryl sulfate, cellulose gum, limonene, CI 77891, xanthan gum, CI 74160. |
| Appointment | Procedures |
|---|---|
| |
| |
| |
| Baseline (T0) |
|
| After 10 days (T1) After 1 month (T2) After 3 months (T3) |
|
| Group | Time | Mean | St. Dev. | Min. | Max. | Median | Interquartile Range | Significance * |
|---|---|---|---|---|---|---|---|---|
| Control (crystals) | T0 | 78.75 | 25.07 | 25.00 | 100.00 | 80.00 | 20.00 | A |
| T1 | 63.50 | 31.71 | 15.00 | 100.00 | 65.00 | 70.00 | A, B, C | |
| T2 | 49.75 | 24.14 | 10.00 | 80.00 | 50.00 | 42.50 | B, C, D | |
| T3 | 44.50 | 27.76 | 10.00 | 80.00 | 50.00 | 51.25 | B, C, D | |
| Trial (charcoal) | T0 | 72.75 | 29.67 | 20.00 | 100.00 | 80.00 | 46.25 | A, B |
| T1 | 43.25 | 20.98 | 10.00 | 80.00 | 50.00 | 36.25 | B, C | |
| T2 | 31.75 | 17.49 | 10.00 | 80.00 | 25.00 | 21.25 | C, D | |
| T3 | 17.75 | 10.19 | 5.00 | 50.00 | 20.00 | 10.00 | D |
| Group | Time | Mean | St. Dev. | Min. | Max. | Median | Interquartile Range | Significance * |
|---|---|---|---|---|---|---|---|---|
| Control (crystals) | T0 | 13.50 | 9.33 | 0.00 | 30.00 | 10.00 | 10.00 | A, C |
| T1 | 8.50 | 7.96 | 0.00 | 20.00 | 5.00 | 16.25 | A, B, D | |
| T2 | 5.00 | 5.13 | 0.00 | 15.00 | 5.00 | 10.00 | B, D | |
| T3 | 6.75 | 3.73 | 0.00 | 10.00 | 7.50 | 5.00 | A, D | |
| Trial (charcoal) | T0 | 28.25 | 27.92 | 0.00 | 80.00 | 20.00 | 26.25 | C |
| T1 | 11.50 | 10.14 | 0.00 | 30.00 | 10.00 | 12.50 | A, B, C | |
| T2 | 6.75 | 6.34 | 0.00 | 20.00 | 7.50 | 10.00 | A, B, D | |
| T3 | 3.25 | 4.38 | 0.00 | 10.00 | 0.00 | 6.25 | D |
| Group | Time | Mean | St. Dev. | Min. | Median | Max. | Significance * |
|---|---|---|---|---|---|---|---|
| Control (crystals) | T0 | 1.85 | 0.67 | 1.00 | 2.00 | 3.00 | A, B |
| T1 | 1.70 | 0.66 | 1.00 | 2.00 | 3.00 | A, B | |
| T2 | 1.15 | 0.75 | 0.00 | 1.00 | 2.00 | B, C, D, E | |
| T3 | 0.85 | 0.49 | 0.00 | 1.00 | 2.00 | D, E | |
| Trial (charcoal) | T0 | 2.25 | 0.64 | 1.00 | 2.00 | 3.00 | A |
| T1 | 1.85 | 0.81 | 0.00 | 2.00 | 3.00 | A, B, C | |
| T2 | 1.30 | 0.66 | 0.00 | 1.00 | 3.00 | B, C, D | |
| T3 | 0.35 | 0.49 | 0.00 | 0.00 | 1.00 | E |
| Group | Time | Mean | St. Dev. | Min. | Median | Max. | Significance * |
|---|---|---|---|---|---|---|---|
| Control (crystals) | T0 | 1.90 | 0.72 | 1.00 | 2.00 | 3.00 | A, B |
| T1 | 1.60 | 0.68 | 1.00 | 1.50 | 3.00 | A, B | |
| T2 | 1.15 | 0.49 | 0.00 | 1.00 | 2.00 | B, C, D | |
| T3 | 0.80 | 0.52 | 0.00 | 1.00 | 2.00 | D, E | |
| Trial (charcoal) | T0 | 2.05 | 0.60 | 1.00 | 2.00 | 3.00 | A |
| T1 | 1.60 | 0.68 | 1.00 | 1.50 | 3.00 | A, B, C | |
| T2 | 1.25 | 0.44 | 1.00 | 1.00 | 2.00 | B, C, D | |
| T3 | 0.25 | 0.44 | 0.00 | 0.00 | 1.00 | E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butera, A.; Pascadopoli, M.; Gallo, S.; Pardo, A.; Stablum, G.; Lelli, M.; Pandolfi, A.; Scribante, A. Evaluation of the Efficacy of Low-Particle-Size Toothpastes against Extrinsic Pigmentations: A Randomized Controlled Clinical Trial. Dent. J. 2023, 11, 82. https://doi.org/10.3390/dj11030082
Butera A, Pascadopoli M, Gallo S, Pardo A, Stablum G, Lelli M, Pandolfi A, Scribante A. Evaluation of the Efficacy of Low-Particle-Size Toothpastes against Extrinsic Pigmentations: A Randomized Controlled Clinical Trial. Dentistry Journal. 2023; 11(3):82. https://doi.org/10.3390/dj11030082
Chicago/Turabian StyleButera, Andrea, Maurizio Pascadopoli, Simone Gallo, Alessia Pardo, Giulia Stablum, Marco Lelli, Anna Pandolfi, and Andrea Scribante. 2023. "Evaluation of the Efficacy of Low-Particle-Size Toothpastes against Extrinsic Pigmentations: A Randomized Controlled Clinical Trial" Dentistry Journal 11, no. 3: 82. https://doi.org/10.3390/dj11030082
APA StyleButera, A., Pascadopoli, M., Gallo, S., Pardo, A., Stablum, G., Lelli, M., Pandolfi, A., & Scribante, A. (2023). Evaluation of the Efficacy of Low-Particle-Size Toothpastes against Extrinsic Pigmentations: A Randomized Controlled Clinical Trial. Dentistry Journal, 11(3), 82. https://doi.org/10.3390/dj11030082