Management of Oral Hygiene in Head-Neck Cancer Patients Undergoing Oncological Surgery and Radiotherapy: A Systematic Review
Abstract
1. Introduction
1.1. Introduction to Cancer Treatment, and the Main Problem with Prophylaxis during HNC Treatment
1.2. Radiotherapy
1.3. Surgery
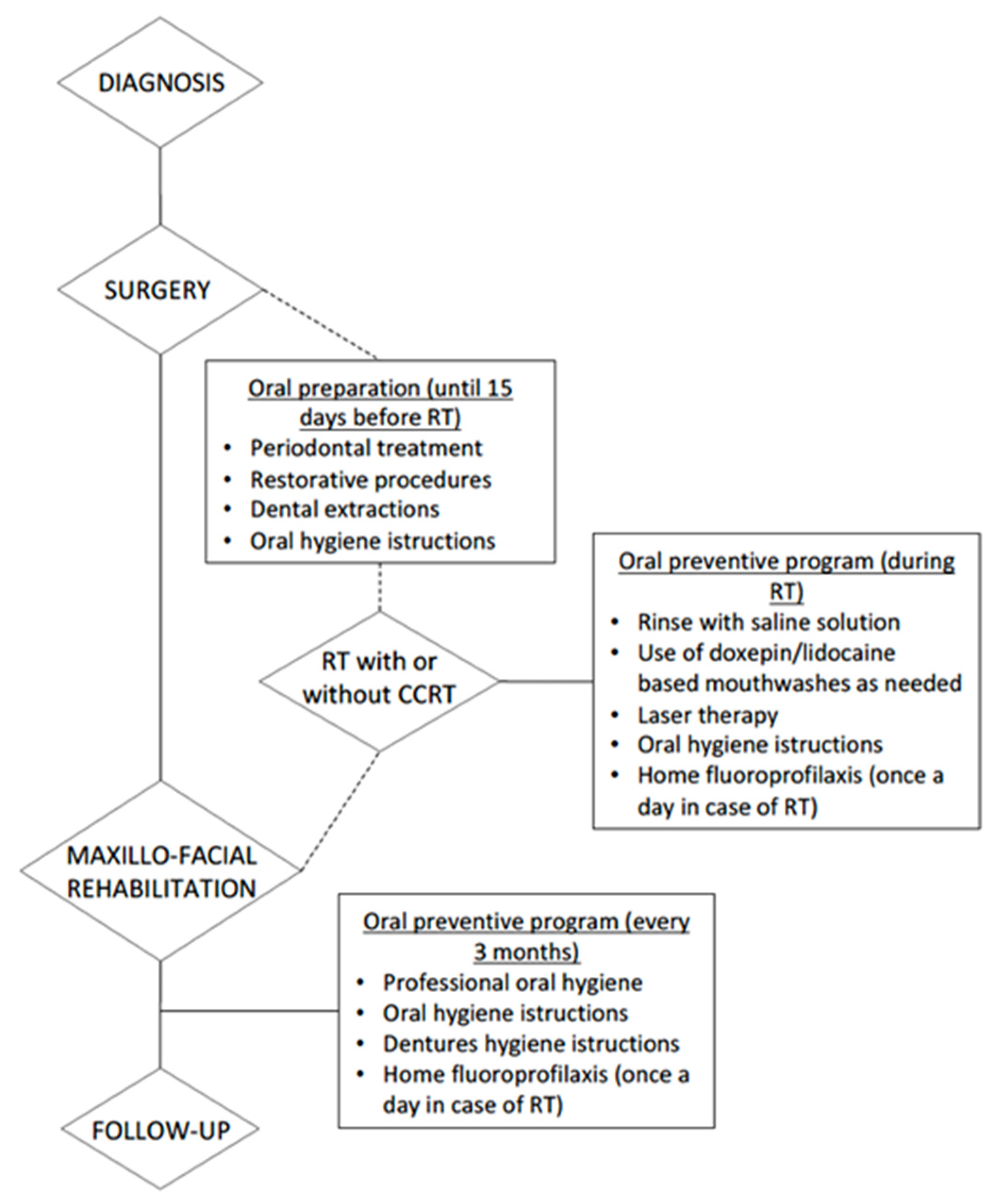
1.4. Management
1.5. Role of Dental Health Professionals
2. Materials and Methods
- Participants Patients diagnosed HNC and undergoing cancer ablation surgery and postoperative adjuvant RT with or without CCRT.
- Intervention Professional and home oral hygiene techniques.
- Comparator Comparison with different professional and home oral hygiene techniques.
- Outcome Improvement of the condition of the patient undergoing RT.
3. Results
3.1. Radio-Induced Mucositis
3.2. Prevention or Management of Xerostomia Due to Radiation Therapy
3.3. Prevention of Postoperative Infection and Oral Health Improvement
3.4. Radio-Induced Caries Prevention
3.5. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abed, H.; Reilly, D.; Burke, M.; Daly, B. Patients with head and neck cancers’ oral health knowledge, oral health-related quality of life, oral health status, and adherence to advice on discharge to primary dental care: A prospective observational study. Spec. Care Dentist. 2019, 39, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Levi, L.E.; Lalla, R.V. Dental treatment planning for the patient with oral cancer. Dent. Clin. N. Am. 2018, 62, 121–130. [Google Scholar] [CrossRef]
- Phasuk, K.; Haug, S.P. Maxillofacial Prosthetics. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Ottaviani, G.; Treister, N.S.; Hanna, G.J. An overview of clinical oncology and impact on oral health. Front. Oral Health 2022, 3, 874332. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.H.; Zafar, K.; Ghafoor, R. Management of oral complications in irradiated head and neck cancer patients: Literature review. IJS Short Rep. 2018, 3, 15–21. [Google Scholar] [CrossRef]
- Matsuda, Y.; Jayasinghe, R.D.; Zhong, H.; Arakawa, S.; Kanno, T. Oral Health Management and Rehabilitation for Patients with Oral Cancer: A Narrative Review. Healthcare 2022, 10, 960. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; Kataoka, T.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Mohe, D. Standards & Guidelines PRISMA Statement per il reporting di revisioni sistematiche e meta-analisi degli studi che valutano gli interventi sanitari: Spiegazione ed elaborazione. Ann. Intern. Med. 2009, 151, 65–94. [Google Scholar]
- Cooke, A.; Smith, D.; Booth, A. Beyond PICO: The SPIDER tool for qualitative evidence synthesis. Qual. Health Res. 2012, 22, 1435–1443. [Google Scholar] [CrossRef]
- Huang, B.S.; Wu, S.C.; Lin, C.Y.; Fan, K.H.; Chang, J.T.; Chen, S.C. The effectiveness of a saline mouth rinse regimen and education programme on radiation-induced oral mucositis and quality of life in oral cavity cancer patients: A randomised controlled trial. Eur. J. Cancer Care 2018, 27, e12819. [Google Scholar] [CrossRef]
- Kongwattanakul, S.; Petchann, N.; Petroch, P.; Thanthong, S.; Tungfung, S.; Chamchod, S.; Pitiporn, S.; Nantajit, D. Prophylactic management of radiation-induced mucositis using herbal mouthwash in patients with head and neck cancer: An assessor-blinded randomized controlled trial. J. Complement. Integr. Med. 2022, 19, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, A.; Moslemi, D.; Akbari, J.; Ghasemi, A.; Azadbakht, M.; Asgharpour, A.; Hosseinimehr, S.J. The effectiveness of Zataria extract mouthwash for the management of radiation-induced oral mucositis in patients: A randomized placebo-controlled double-blind study. Clin. Oral Investig. 2018, 22, 2263–2272. [Google Scholar] [CrossRef]
- Dodd, M.J.; Cho, M.H.; Cooper, B.A.; MacPhail, L.; Miaskowski, C. A randomized clinical trial of granulocyte macrophage colony stimulating factor mouthwash for oral mucositis in head and neck cancer. Eur. J. Oncol Nurs. 2022, 56, 102093. [Google Scholar] [CrossRef]
- Lalla, R.V.; Solé, S.; Becerra, S.; Carvajal, C.; Bettoli, P.; Letelier, H.; Santini, A.; Vargas, L.; Cifuentes, A.; Larsen, F.; et al. Efficacy and safety of Dentoxol® in the prevention of radiation-induced oral mucositis in head and neck cancer patients (ESDOM): A randomized, multicenter, double-blind, placebo-controlled, phase II trial. Support. Care Cancer 2020, 28, 5871–5879. [Google Scholar] [CrossRef] [PubMed]
- Ebert, N.; Kensche, A.; Löck, S.; Hadiwikarta, W.W.; Hänsch, A.; Dörr, W.; Krause, M.; Hannig, C.; Baumann, M. Results of a randomized controlled phase III trial: Efficacy of polyphenol-containing cystus® tea mouthwash solution for the reduction of mucositis in head and neck cancer patients undergoing external beam radiotherapy. Strahlenther. Onkol. 2021, 197, 63–73. [Google Scholar] [CrossRef]
- Liao, Y.C.; Hsu, L.F.; Hsieh, L.Y.; Luo, Y.Y. Effectiveness of green tea mouthwash for improving oral health status in oral cancer patients: A single-blind randomized controlled trial. Int. J. Nurs. Stud. 2021, 121, 103985. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Belgioia, L.; Cante, D.; LA Porta, M.R.; Caspiani, O.; Guarnaccia, R.; Argenone, A.; Muto, P.; Musio, D.; DE Felice, F.; et al. Lactobacillus brevis CD2 for Prevention of Oral Mucositis in Patients With Head and Neck Tumors: A Multicentric Randomized Study. Anticancer Res. 2019, 39, 1935–1942. [Google Scholar] [CrossRef]
- Hamzah, M.H.; Mohamad, I.; Musa, M.Y.; Abd Mutalib, N.S.; Siti-Azrin, A.H.; Wan Omar, W.A. Propolis mouthwash for preventing radiotherapy-induced mucositis in patients with nasopharyngeal carcinoma. Med. J. Malaysia 2022, 77, 462–467. [Google Scholar]
- Lozano, A.; Marruecos, J.; Rubió, J.; Farré, N.; Gómez-Millán, J.; Morera, R.; Planas, I.; Lanzuela, M.; Vázquez-Masedo, M.G.; Cascallar, L.; et al. Randomized placebo-controlled phase II trial of high-dose melatonin mucoadhesive oral gel for the prevention and treatment of oral mucositis in patients with head and neck cancer undergoing radiation therapy concurrent with systemic treatment. Clin. Transl. Oncol. 2021, 23, 1801–1810. [Google Scholar] [CrossRef]
- Sio, T.T.; Le-Rademacher, J.G.; Leenstra, J.L.; Loprinzi, C.L.; Rine, G.; Curtis, A.; Singh, A.K.; Martenson, J.A., Jr.; Novotny, P.J.; Tan, A.D.; et al. Effect of Doxepin Mouthwash or Diphenhydramine-Lidocaine-Antacid Mouthwash vs Placebo on Radiotherapy-Related Oral Mucositis Pain: The Alliance A221304 Randomized Clinical Trial. JAMA 2019, 321, 1481–1490. [Google Scholar] [CrossRef]
- Shah, S.; Rath, H.; Sharma, G.; Senapati, S.N.; Mishra, E. Effectiveness of curcumin mouthwash on radiation-induced oral mucositis among head and neck cancer patients: A triple-blind, pilot randomised controlled trial. Indian J. Dent. Res. 2020, 31, 718–727. [Google Scholar] [CrossRef]
- Morais, M.O.; Martins, A.F.L.; de Jesus, A.P.G.; de Sousa Neto, S.S.; da Costa, A.W.F.; Pereira, C.H.; Oton-Leite, A.F.; de Freitas, N.M.A.; Leles, C.R.; Mendonça, E.F. A prospective study on oral adverse effects in head and neck cancer patients submitted to a preventive oral care protocol. Support. Care Cancer 2020, 28, 4263–4273. [Google Scholar] [CrossRef]
- Yin, J.; Xie, J.; Lin, J.; Weng, C.; Lu, S.; Xu, P.; Zhang, S.; Luo, C.; Huang, Y.; Li, L.; et al. Evaluation of the efficacy of the anti-ulcer oral mucosal protective agent RADoralex® in the prevention and treatment of radiation-induced oral mucosal reactions induced during treatment of nasopharyngeal carcinoma. Cancer Biol. Ther. 2022, 23, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Manifar, S.; Koopaie, M.; Jahromi, Z.M.; Kolahdooz, S. Effect of synbiotic mouthwash on oral mucositis induced by radiotherapy in oral cancer patients: A double-blind randomized clinical trial. Support. Care Cancer 2022, 31, 31. [Google Scholar] [CrossRef]
- Nuchit, S.; Lam-Ubol, A.; Paemuang, W.; Talungchit, S.; Chokchaitam, O.; Mungkung, O.O.; Pongcharoen, T.; Trachootham, D. Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: A randomized controlled trial. Support. Care Cancer 2020, 28, 2817–2828. [Google Scholar] [CrossRef]
- Jiang, N.; Zhao, Y.; Stensson, M.; Mårtensson, J. Effects of an integrated supportive program on xerostomia and saliva characteristics in patients with head and neck cancer radiated with a low dose to the major salivary glands: A randomized controlled trial. BMC Oral Health 2022, 22, 199. [Google Scholar] [CrossRef]
- Sio, T.T.; Blanchard, M.J.; Novotny, P.J.; Patel, S.H.; Rwigema, J.M.; Pederson, L.D.; McGee, L.A.; Gamez, M.E.; Seeger, G.R.; Martenson, J.A.; et al. N-Acetylcysteine Rinse for Thick Secretion and Mucositis of Head and Neck Chemoradiotherapy (Alliance MC13C2): A Double-Blind Randomized Clinical Trial. Mayo. Clin Proc. 2019, 94, 1814–1824. [Google Scholar] [CrossRef]
- Apperley, O.; Medlicott, N.; Rich, A.; Hanning, S.; Huckabee, M.L. A clinical trial of a novel emulsion for potential use as a saliva substitute in patients with radiation-induced xerostomia. J. Oral Rehabil. 2017, 44, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Lam-Ubol, A.; Matangkasombut, O.; Trachootham, D.; Tarapan, S.; Sattabanasuk, V.; Talungchit, S.; Paemuang, W.; Phonyiam, T.; Chokchaitam, O.; Mungkung, O.O. Efficacy of gel-based artificial saliva on Candida colonization and saliva properties in xerostomic post-radiotherapy head and neck cancer patients: A randomized controlled trial. Clin. Oral Investig. 2021, 25, 1815–1827. [Google Scholar] [CrossRef]
- Marimuthu, D.; Han, K.M.; Mohamad, M.S.F.; Azman, M. Saliva substitute mouthwash in nasopharyngeal cancer survivors with xerostomia: A randomized controlled trial. Clin. Oral Investig. 2021, 25, 3105–3115. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Han, D.H.; Kim, J.H.; Wu, H.G. The effect of comprehensive oral care program on oral health and quality of life in patients undergoing radiotherapy for head and neck cancer: A quasi-experimental case-control study. Medicine 2021, 100, e25540. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Walker, G.D.; Manton, D.J.; Soong, Y.L.; Wee, J.; Adams, G.G.; Reynolds, E.C. Anticariogenic efficacy of a saliva biomimetic in head-and-neck cancer patients undergoing radiotherapy. Aust. Dent. J. 2019, 64, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, M.; Matsui, H.; Ono, S.; Hagiwara, Y.; Morita, K.; Yasunaga, H. Preoperative oral care and effect on postoperative complications after major cancer surgery. Br. J. Surg. 2018, 105, 1688–1696. [Google Scholar] [CrossRef]
- Gondo, T.; Fujita, K.; Nagafuchi, M.; Obuchi, T.; Ikeda, D.; Yasumatsu, R.; Nakagawa, T. The effect of preventive oral care on postoperative infections after head and neck cancer surgery. Auris Nasus Larynx 2020, 47, 643–649. [Google Scholar] [CrossRef]
- Sohn, H.O.; Park, E.Y.; Jung, Y.S.; Lee, E.K.; Kim, E.K. Effects of professional oral hygiene care in patients with head-and-neck cancer during radiotherapy: A randomized clinical trial. Indian J. Dent. Res. 2018, 29, 700–704. [Google Scholar] [CrossRef]
- Bhandari, S.; Soni, B.W.; Jamwal, A.; Ghoshal, S. Oral and dental care before radiotherapy: Guidelines and development of a time-bound protocol. Indian J. Cancer 2022, 59, 159–169. [Google Scholar]
- Haynes, D.A.; Vanison, C.C.; Gillespie, M.B. The Impact of Dental Care in Head and Neck Cancer Outcomes: A Systematic Review and Meta-Analysis. Laryngoscope 2022, 132, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Chavarri-Guerra, Y.; Corrêa, L.B.C.; Dean, D.R.; Epstein, J.B.; Fregnani, E.R.; Lee, J.; Matsuda, Y.; Mercadante, V.; Monsen, R.E.; et al. MASCC/ISOO expert opinion on the management of oral problems in patients with advanced cancer. Support. Care Cancer 2022, 30, 8761–8773. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Hu, S.; Haverman, T.; Stokman, M.; Napeñas, J.J.; Braber, J.B.; Gerber, E.; Geuke, M.; Vardas, E.; Waltimo, T.; et al. A systematic review of dental disease management in cancer patients. Support. Care Cancer 2018, 26, 155–174. [Google Scholar] [CrossRef]
- Lee, C.T.; Galloway, T.J. Pathogenesis and Amelioration of Radiation-Induced Oral Mucositis. Curr. Treat. Options Oncol. 2022, 23, 311–324. [Google Scholar] [CrossRef]
- Singh, V.; Singh, A.K. Oral mucositis. Natl. J. Maxillofac. Surg. 2020, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Purohit, B.; Ravi, P.; Priya, H.; Kumar, V. Effectiveness of topical fluorides in prevention of radiation caries in adults: A systematic review and meta- analysis. Oral Oncol. 2022, 129, 105869. [Google Scholar] [CrossRef] [PubMed]

| Topic | Authors | Study Design | Aim of the Study | Sample | Conclusions |
|---|---|---|---|---|---|
| Oral mucositis | B.-S. Huang et al. (2018) [10] | RCT with two groups | To analyze the effectiveness of a saline mouthrinse and the efficacy of an education programme | 91 patients diagnosed oral cavity cancer and undergoing postoperative adjuvant RT and CCRT | Saline mouth rinses together with an education programme were found effective in increasing physical and social-emotional QOL by improving the radiation-induced OM symptoms and promoting oral comfort |
| Oral mucositis | S. Kongwattanakul et al. (2022) [11] | RCT with three groups | To compare the efficacy of two mouthwashes containing extracts of payayor and fingerroot with the standard care of saline solution (0.9%) with sodium bicarbonate in preventing OM in head and neck cancer patients receiving radiotherapy | 121 patients with HNC undergoing RT | Payayor and fingerroot mouthwashes were found as having the same efficacy in reducing the severity of OM and could cause a slight delay in the onset of the symptoms |
| Oral mucositis | A. Aghamohammadi et al. (2018) [12] | Double-blind RCT | To assess the efficacy of Zataria Multiflora mouthwash in reducing the incidence of OM in HNC patients undergoing radiotherapy | 52 patients with HNC undergoing RT | After 6 weeks of treatment with Zataria Multiflora mouthwash, OM affected significantly less patients than in the placebo group |
| Oral mucositis | M.J. Dodd (2022) [13] | Double-blind RCT | To compare the effectiveness of the granulocyte macrophage colony stimulating factor mouthwash to Salt and Soda mouthwash for both the prevention (prior to onset of OM) and treatment (from onset of OM to its healing) of OM | 91 patients with HNC receiving a cumulative dose of RT of between 5500 and 7000 cGy (cGys) over 6–7 weeks | The granulocyte macrophage colony stimulating factor mouthwash was not found to be more effective than Salt and Soda mouthwash neither in the prevention nor in the treatment of OM |
| Oral mucositis | R.V. Lalla et al. (2020) [14] | Parallel-group, double-blind RCT | To assess the efficacy of Dentoxol® in reducing the severity of OM | 108 patients with HNC undergoing RT at least 5000 cGy, with or without CCRT | Dentoxol used 5 times/day caused significantly fewer time-points with severe OM and caused a delay in the onset of severe OM, compared with a control rinse |
| Oral mucositis | N. Ebert et al. (2021) [15] | Prospective, single-center, randomised phase III trial | To compare Cystus® tea effects as mouthwash to sage tea effects on OM in patients undergoing RT and CCRT for HNC | 57 patients with histologically confirmed HNC | Cystus® and sage tea effects were found not statistically different. This mouthwash can be applied in addition to accurate oral care and hygiene along with the application of fluorides |
| Oral mucositis | Y.C. Liao et al. (2021) [16] | Prospective, single-blind RCT | To evaluate the effectiveness of green tea mouthwash in the improvement of oral health in oral cancer patients undergoing cancer treatment | 61 HNC patients treated with oral surgery and RT with and without CCRT | The prolonged use of green tea mouthwash was effective in improving and mantaining the oral health status |
| Oral mucositis | V. De Sanctis et al. (2019) [17] | Multicentric, phase III, open-label RCT | To investigate the effects of Lactobacillus Brevis CD2 in preventing OM onset during RT | 68 patients with histologically diagnosed HNC, except larynx, parotid and other salivary glands under RT and CCRT | Lactobacillus Brevis CD2 lozenges were not demonstrated to be effective in preventing radiation-induced mucositis in patients with HNC |
| Oral mucositis | M.H. Hamzah et al. (2022) [18] | Prospective, double-arm, RCT with intervention | To evaluate the effects of a 2.5% propolis mouthwash in the prevention of RT-induced OM in patients with nasopharyngeal carcinoma | 17 patients with nasopharyngeal carcinoma who underwent HNC surgery | Propolis mouthwash was found statistically effective in reducing the severity of OM following RT |
| Oral mucositis | A. Lozano et al. (2021) [19] | Multicentric, phase IIa, prospective, double-blind RCT | To evaluate the effectiveness of melatonin oral gel mouthwashes in preventing and treating OM in patients in treatment for HNC | 79 patients with HNC treated RT and CCRT | 3% melatonin oral gel caused a lower incidence and a shorter duration of OM |
| Oral mucositis | T.T. Sio et al. (2019) [20] | 3-group, double-blind, phase 3, RCT | To assess the efficacy of doxepin mouthwash or diphenhydramine–lidocaine–antacid mouthwash on OM-related pain | 230 patients with HNC treated RT | Patients undergoing head and neck radiotherapy, and using doxepin mouthwash or diphenhydramine–lidocaine–antacid mouthwash compared with placebo showed a significant reduction in OM-related pain during the first 4 h after administration |
| Oral mucositis | S. Shah et al. (2020) [21] | Parallel arm, triple-blinded RCT | To compare the effectiveness of 0.1% curcumin and 0.15% benzydamine mouthwash on radio-induced OM | 74 patients with histological confirmation of HNC, scheduled to receive RT | 0.1% curcumin mouthwash significantly delayed the onset of OM |
| Oral mucositis | M.O. Morais et al. (2020) [22] | Prospective observational study | To assess oral complications and quality of life in HNC patients undergoing a preventive oral care program and photobiomodulation therapy | 61 patients diagnosed with HNC undergoing RT and CCRT | The photobiomodulation therapy reduced quality of life impacts and interruption of RT due to severe OM |
| Oral mucositis | Y. Jun et al. (2022) [23] | RCT | To assess the efficacy and safety RADoralex® in preventing and treating radiation-induced oral mucosal reactions | 90 patients with locally advanced Nasopharyngeal carcinoma who received RT and CCRT and showed post-treatment grade 1 oral mucositis | OM incidence and severity was reduced and the progression was delayed using RADoralex® |
| Oral mucositis | S. Manifar et al. (2023) [24] | Double-blind RCT | To assess and compare the effects of a synbiotic mouthwash with a saline mouthwash on preventing and controlling radiotherapy-induced OM in oral cancer patients | 64 patients with oral cancer, who received 6000 cGY of RT in 34 fractions | Synbiotic mouthwash caused a significant reduction in OM intensity and prevented its onset in oral cancer patients undergoing RT |
| Xerostomia | S. Nuchit et al. (2020) [25] | Single-blinded RCT | To evaluate the effectiveness of an edible saliva substitute, on dry mouth, swallowing ability, and nutritional status in post-RT HNC patients | 62 patients with HNC who have completed RT at least 1 month earlier | Using saliva substitutes (OMJ or GC) continuously for at least a month improved dry mouth condition and enhanced swallowing ability |
| Xerostomia | N. Jiang et al. (2022) [26] | RCT | To investigate the effects of an integrated supportive program on xerostomia and saliva characteristics in patients with HNC 1 year post-RT | 92 patients with histologically diagnosis of HNC who received a low dose RT to the major salivary glands | Patients with HNC with a low dose RT to the major salivary glands who were followed up for 12 months post-RT in an integrated supportive program with good adherence experienced a relief in xerostomia and an increase in unstimulated saliva flow rate |
| Xerostomia | T.T. Sio et al. (2019) [27] | Prospective, double-blind RCT | To evaluate the effectiveness of N-acetylcysteine rinse in improving thickened secretions and dry mouth during and after RT | 32 patients undergoing CCRT for HNC | After using N-acetylcysteine rinse patients reported an improvement in thickened saliva and xerostomia |
| Xerostomia | O. Apperley et al. (2017) [28] | RCT | To compare a novel oily emulsion to a currently available saliva substitute | 29 patients with xerostomia after RT to the HNC | Patients reported no clinically significant difference between the novel oily formulation, methylcellulose, and water |
| Xerostomia | A. Lam-ubol et al. (2021) [29] | Single-blind RCT | To assess the effectiveness of an edible artificial saliva gel, an oral moisturizing jelly, and a topical commercial gel on Candida colonization and saliva properties | 56 post-RT HNC patients with xerostomia | Both the gels reduced the number of Candida species and improved saliva properties in post-RT patients with xerostomia |
| Xerostomia | D. Marimuthu et al. (2021) [30] | Prospective, double-blind RCT | To investigate the effects of a saliva substitute mouthwash in patients with nasopharyngeal cancer and xerostomia | 94 patients patients diagnosed with nasopharyngeal carcinoma who underwent either RT or CCRT | Xerostomia scores were reduced and salivary flow improved after using an immunologically-active saliva substitute mouthwash |
| Radio-induced caries | H.J Lee et al. (2021) [31] | Quasi-experimental, non-synchronized non-equivalent case-control study | To investigate the effect of the comprehensive oral care program on oral health status and symptoms in HNC patients undergoing RT | 61 Patients undergoing RT for non-metastatic HNC | Comprehensive oral care intervention is effective in preventing dental caries and increasing the quality of life in HNC patients |
| Radio-induced caries | C.P.C. Sim et al. (2019) [32] | Double-blind RCT | To evaluate the effects of treatment with the saliva biomimetic, casein phosphopeptide-amorphous calcium phosphate and SnF2/NaF compared with SnF2/NaF alone on the progression of coronal surface caries in HNC patients undergoing RT | 24 patients (2685 tooth surfaces) undergoing RT to the HNC | The progression of coronal surface caries was reduced by the use of the saliva biomimetic, casein phosphopeptide-amorphous calcium phosphate, and SnF2/NaF |
| Preventive oral care protocol | M. Ishimaru et al. (2018) [33] | Retrospective observational cohort study | To investigate the association between preoperative oral care and postoperative complications in patients undergoing major HNC surgery | 509,179 patients who underwent surgery for HNC and other cancers | The preoperative oral care, in patients with HNC, led to a significant decrease in postoperative pneumonia and all-cause 30-day mortality |
| Preventive oral care protocol | T. Gondo et al. (2020) [34] | Retrospective observational study | To evaluate the incidence of postoperative pneumonia and surgical site infection in HNC patients and investigate the link between oral care and postoperative infection | 209 patients who underwent HNC surgery | Oral care before and after surgery reduced postoperative infections in patients with HNC |
| Preventive oral care protocol | H.O. Sohn et al. (2018) [35] | RCT | To investigate the effects of professional oral hygiene care during RT in patients with HNC | 27 patients with HNC who underwent RT | Periodic dental visits, oral hygiene care, and instructions improved oral health in patients with HNC during RT |
| Authors (years) | Study’s Design | Randomization | Allocation Concealment | Assessor Blinding | Operators Blinding | Missing Outcome Data Reported | Missing Outcome Were Balanced among Groups | Reasons for Drop out | Selective Outcome Reporting | Statistical Method | Sample Size Estimation | Examiner Calibration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B.-S. Huang et al. (2018) [10] | RCT | Adequate | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | NR | NR |
| S. Kongwattanakul et al. (2022) [11] | RCT | Adequate | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| A. Aghamohammadi et al. (2018) [12] | RCT | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR | NR |
| M.J. Dodd (2022) [13] | RCT | Unclear | Unclear | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR | Adequate |
| R.V. Lalla et al. (2020) [14] | RCT | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate |
| N. Ebert et al. (2021) [15] | RCT | Unclear | NR | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Unclear | NR |
| Y.C. Liao et al. (2021) [16] | RCT | Adequate | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| V. De Sanctis et al. (2019) [17] | RCT | Adequate | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | NR | NR |
| M.H. Hamzah et al. (2022) [18] | RCT | NR | NR | NR | NR | Inadequate | Inadequate | NR | Adequate | NR | NR | NR |
| A. Lozano et al. (2021) [19] | RCT | Unclear | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| T.T. Sio et al. (2019) [20] | RCT | Adequate | Adequate | Unclear | Unclear | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| S. Shah et al. (2020) [21] | RCT | Unclear | Unclear | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate |
| Y. Jun et al. (2022) [23] | RCT | Unclear | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| S. Manifar et al. (2023) [24] | RCT | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR | NR |
| S. Nuchit et al. (2020) [25] | RCT | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| N. Jiang et al. (2022) [26] | RCT | Adequate | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Inadequate | NR |
| T.T. Sio et al. (2019) [27] | RCT | Adequate | Adequate | Unclear | Unclear | Adequate | Adequate | Adequate | Adequate | Adequate | NR | NR |
| O. Apperley et al. (2017) [28] | RCT | Unclear | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| A. Lam-ubol et al. (2021) [29] | RCT | Unclear | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| D. Marimuthu et al. (2021) [30] | RCT | Adequate | Adequate | NR | NR | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate |
| C.P.C. Sim et al. (2019) [32] | RCT | Adequate | Adequate | Unclear | Unclear | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | NR |
| H.O. Sohn et al. (2018) [35] | RCT | Unclear | Unclear | NR | NR | Inadequate | Inadequate | Inadequate | Adequate | Adequate | NR | NR |
| Authors (years) | Study’s Design | Representativeness of the Exposed Subjects | Selection of Non-Exposed Subjects | Ascertainment of Exposure | Ascertainment of Outcome | Comparability of Exposed and Non-Exposed Groups on the Basis of the Design or Analysis | Assessment of Outcome |
|---|---|---|---|---|---|---|---|
| M.O. Morais et al. (2020) [22] | Prospective observational study | Adequate | Inadequate | Adequate | Adequate | Adequate | Adequate |
| H.J Lee et al. (2021) [31] | Case-control study | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate |
| M. Ishimaru et al. (2018) [33] | Retrospective observational cohort study | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate |
| T. Gondo et al. (2020) [34] | Retrospective observational study | Adequate | Inadequate | Adequate | Adequate | Adequate | Adequate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzetti, J.; Finotti, F.; Savarino, M.; Gassino, G.; Dell’Acqua, A.; Erovigni, F.M. Management of Oral Hygiene in Head-Neck Cancer Patients Undergoing Oncological Surgery and Radiotherapy: A Systematic Review. Dent. J. 2023, 11, 83. https://doi.org/10.3390/dj11030083
Lanzetti J, Finotti F, Savarino M, Gassino G, Dell’Acqua A, Erovigni FM. Management of Oral Hygiene in Head-Neck Cancer Patients Undergoing Oncological Surgery and Radiotherapy: A Systematic Review. Dentistry Journal. 2023; 11(3):83. https://doi.org/10.3390/dj11030083
Chicago/Turabian StyleLanzetti, Jacopo, Federica Finotti, Maria Savarino, Gianfranco Gassino, Alessandro Dell’Acqua, and Francesco M. Erovigni. 2023. "Management of Oral Hygiene in Head-Neck Cancer Patients Undergoing Oncological Surgery and Radiotherapy: A Systematic Review" Dentistry Journal 11, no. 3: 83. https://doi.org/10.3390/dj11030083
APA StyleLanzetti, J., Finotti, F., Savarino, M., Gassino, G., Dell’Acqua, A., & Erovigni, F. M. (2023). Management of Oral Hygiene in Head-Neck Cancer Patients Undergoing Oncological Surgery and Radiotherapy: A Systematic Review. Dentistry Journal, 11(3), 83. https://doi.org/10.3390/dj11030083







