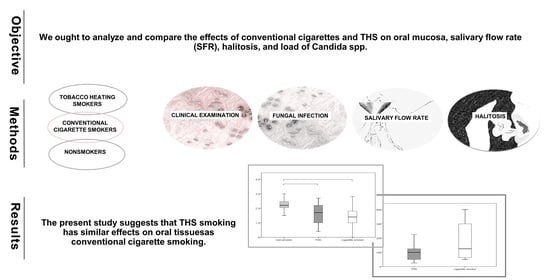
Impact of the Tobacco Heating System and Cigarette Smoking on the Oral Cavity: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Selection Criteria
2.2. Questionnaire and Clinical Examination
2.3. Salivary Flow Rate
2.4. Halitosis
2.5. Cultivation and Identification of Candida spp.
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Participants and Demographic Data
3.2. Self-Reported Oral Lesions and Symptoms
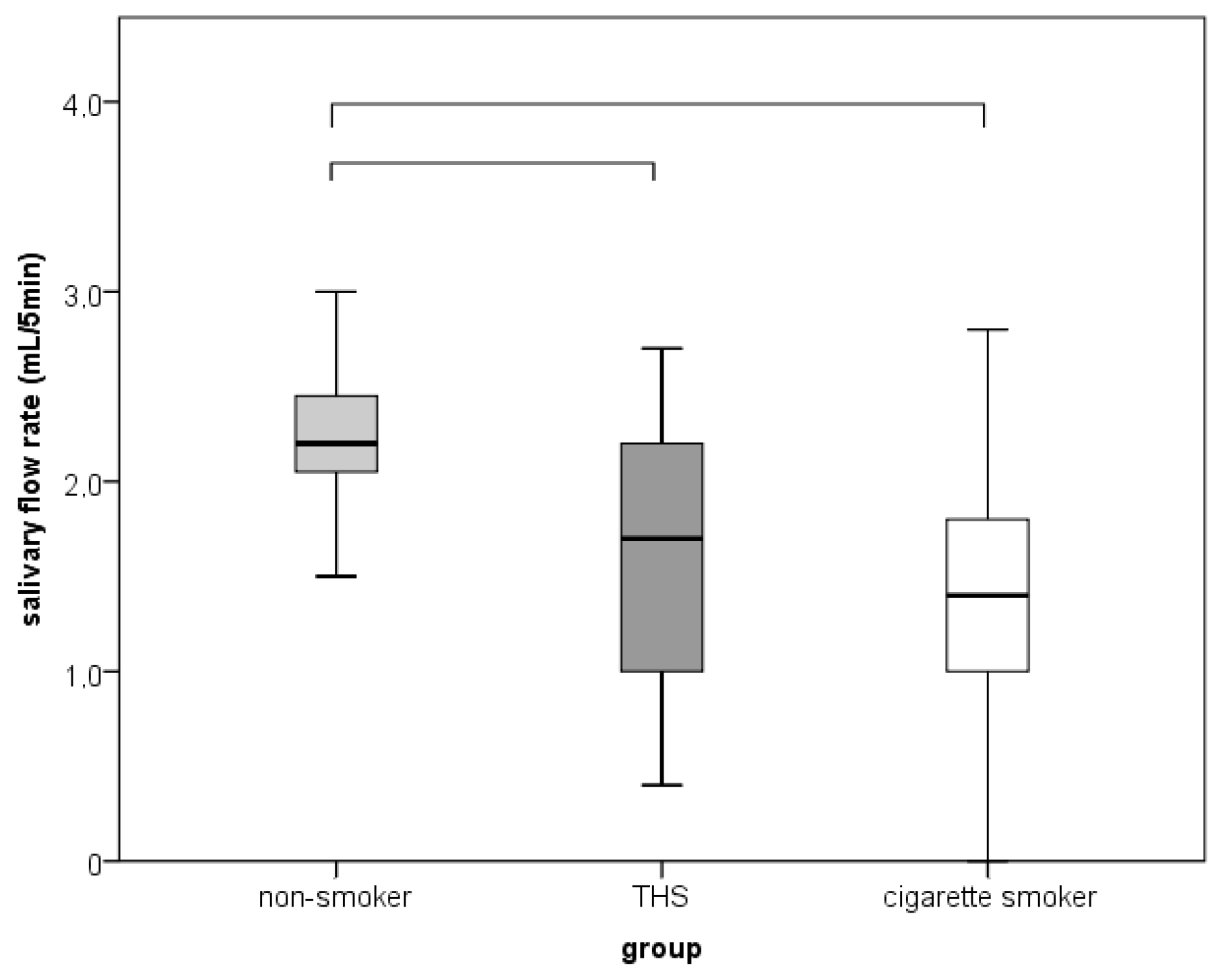
3.3. Halitosis, Oral Lesions, SFR and Oral Candidiasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopa, P.N.; Pawliczak, R. IQOS—A heat-not-burn (HnB) tobacco product-chemical composition and possible impact on oxidative stress and inflammatory response: A systematic review. Toxicol. Mech. Methods 2020, 30, 81–87. [Google Scholar] [CrossRef]
- Kaur, G.; Muthumalage, T.; Rahman, I. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol. Lett. 2018, 288, 143–155. [Google Scholar] [CrossRef]
- O’Connell, G.; Wilkinson, P.; Burseg, K.M.M.; Stotesbury, S.J.; Pritchard, J.D. Heated tobacco products create side-stream emissions: Implications for regulation. J. Environ. Anal. Chem. 2015, 2, 163. [Google Scholar] [CrossRef]
- Zanetti, F.; Titz, B.; Sewer, A.; Lo Sasso, G.; Scotti, E.; Schlage, W.K.; Mathis, C.; Leroy, P.; Majeed, S.; Torres, L.O.; et al. Comparative systems toxicology analysis of cigarette smoke and aerosol from a candidate modified risk tobacco product in organotypic human gingival epithelial cultures: A 3-day repeated exposure study. Food Chem. Toxicol. 2017, 101, 15–35. [Google Scholar] [CrossRef]
- Nosratzehi, T. Salivary Chemical Factors in Relation with Oral Cancer in Smokers and NonSmokers: A Literature Review. J. Dent. 2017, 18, 237–243. [Google Scholar]
- Taybos, G. Oral changes associated with tobacco use. Am. J. Med. Sci. 2003, 26, 179–182. [Google Scholar] [CrossRef]
- Michalak, E.; Halko-Gąsior, A.; Chomyszyn-Gajewska, M. The impact of tobacco on oral health-based on literature. Prz. Lek. 2016, 73, 516–519. [Google Scholar]
- Petrušić, N.; Posavac, M.; Sabol, I.; Mravak-Stipetić, M. The Effect of Tobacco Smoking on Salivation. Acta Stomatol. Croat. 2015, 49, 309–315. [Google Scholar] [CrossRef]
- Kanwar, A.; Sah, K.; Grover, N.; Chandra, S.; Singh, R.R. Long-term effect of tobacco on resting whole mouth salivary flow rate and pH: An institutional based comparative study. Eur. J. Gen. Dent. 2013, 2, 296–299. [Google Scholar] [CrossRef]
- Saputri, D.; Abdillah, N.; Mutiara, S.; Basri, G. The correlation between pH and flow rate of salivary smokers related to nicotine levels labelled on cigarettes. Dent. J. 2017, 50, 61–65. [Google Scholar] [CrossRef][Green Version]
- Kauss, A.R.; Antunes, M.; Zanetti, F.; Hankins, M.; Hoeng, J.; Heremans, A.; van der Plas, A. Influence of tobacco smoking on the development of halitosis. Toxicol. Rep. 2022, 9, 316–322. [Google Scholar] [CrossRef]
- Rad, M.; Kakoie, S.; Brojeni, F.N.; Pourdamghan, N. Effect of Long-term Smoking on Whole mouth Salivary Flow Rate and Oral Health. J. Dent. Res. Dent. Clin. Dent. Prospect. 2010, 4, 110–114. [Google Scholar] [CrossRef]
- Ye, P.; Chen, W.; Huang, F.; Liu, Q.; Zhu, Y.N.; Wang, X.; Han, X.D.; Wang, W.M. Smoking increases oral mucosa susceptibility to Candida albicans infection via the Nrf2 pathway: In vitro and animal studies. J. Cell Mol. Med. 2021, 25, 7948–7960. [Google Scholar] [CrossRef]
- Hanioka, T.; Ojima, M.; Tanaka, K.; Aoyama, H. Relationship between smoking status and tooth loss: Findings from national databases in Japan. J. Epidemiol. 2007, 17, 125–132. [Google Scholar] [CrossRef]
- Brinkman, G.L.; Coates, E.O., Jr. The effect of bronchitis, smoking, and occupation on ventilation. Am. Rev. Respir. Dis. 1963, 87, 684–693. [Google Scholar] [CrossRef]
- Glazar, I.; Muhvic Urek, M.; Kuis, D.; Prpić, J.; Misković, I.; Kovacevic Pavicic, D.; Pezelj-Ribaric, S. Salivary flow rate, oral yeast colonization and dental status in institutionalized and non-institutionalized elderly. Acta Clin. Croat. 2016, 55, 390–395. [Google Scholar] [CrossRef]
- Dayma, A.; Jain, M.; Saxena, V.; Torwane, N.; Vishu, V.; Khare, A. Validation of organoleptics and instrumental measurement for halitosis among patient with malodor. J. Dent. Health Oral Disord. Ther. 2020, 11, 6–10. [Google Scholar] [CrossRef]
- Olsen, I. Denture stomatitis. Occurrence and distribution of fungi. Acta Odontol. Scand. 1974, 32, 329–333. [Google Scholar] [CrossRef]
- Tooyama, H.; Matsumoto, T.; Hayashi, K.; Kurashina, K.; Kurita, H.; Uchida, M.; Kasuga, E.; Honda, T. Candida concentrations determined following concentrated oral rinse culture reflect clinical oral signs. BMC Oral Health 2015, 15, 150. [Google Scholar] [CrossRef]
- Myagmar-Ochir, E.; Kaneko, M.; Tomiyama, K.; Zaitsu, M.; Watanabe, S.; Nishino, Y.; Takahashi, K.; Haruyama, Y.; Kobashi, G. Occupational difference in use of heated tobacco products: A cross-sectional analysis of retail workers in Japan. BMJ Open 2021, 11, 049395. [Google Scholar] [CrossRef]
- Patil, P.B.; Bathi, R.; Chaudhari, S. Prevalence of oral mucosal lesions in dental patients with tobacco smoking, chewing, and mixed habits: A cross-sectional study in South India. J. Fam. Community Med. 2013, 20, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.; Sewer, A.; Mathis, C.; Iskandar, A.R.; Kostadinova, R.; Schlage, W.K.; Leroy, P.; Majeed, S.; Guedj, E.; Trivedi, K.; et al. Systems Toxicology Assessment of the Biological Impact of a Candidate Modified Risk Tobacco Product on Human Organotypic Oral Epithelial Cultures. Chem. Res. Toxicol. 2016, 29, 1252–1269. [Google Scholar] [CrossRef] [PubMed]
- Shcheglov, A.V.; Oskolski, G.I.; Lushnikova, E.L.; Nepomnyashchikh, L.M. Morphological and immunological analysis of the oral mucosa in tobacco smoking and odontopreparation. Bull. Exp. Biol. Med. 2006, 142, 628–632. [Google Scholar] [CrossRef]
- Mori, Y.; Tanaka, M.; Kozai, H.; Aoyama, Y.; Shigeno, Y.; Hotta, K.; Aoike, M.; Kawamura, H.; Tsurudome, M.; Ito, M. Effects of Heat-Not-Burn Cigarette Smoking on the Secretion of Saliva and Its Innate Immune System Components. Healthcare 2022, 11, 132. [Google Scholar] [CrossRef]
- Ghulam Jillani, K.; Rashid, M.; ud Din, S.; ul Hag, I. Effects of long-term use of tobacco on taste receptors and salivary secretion. J. Ayub Med. Coll. Abbottabad 2003, 15, 37–39. [Google Scholar]
- Iida, T.; Ono, K.; Inagaki, T.; Hosokawa, R.; Inenaga, K. Nicotinic receptor agonist-induced salivation and its cellular mechanism in parotid acini of rats. Auton. Neurosci. 2011, 161, 81–86. [Google Scholar] [CrossRef]
- Jiun, I.L.; Siddik, S.N.; Malik, S.N.; Tin-Oo, M.M.; Alam, M.K.; Khan, M.M. Association Between Oral Hygiene Status and Halitosis Among Smokers and Nonsmokers. Oral Health Prev. Dent. 2015, 13, 395–405. [Google Scholar] [CrossRef]
- Gavazova, G.; Pechalova, P. The effect of mouthwash containing chlorhexidine digluconate 0.2% on halitosis in smokers and non-smokers. J. Dent. Sci. 2019, 13, 31–35. [Google Scholar] [CrossRef]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral candidiasis: A disease of opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef]
- de Azevedo Izidoro, A.C.S.; Semprebom, A.M.; Baboni, F.B.; Rosa, R.T.; Machado, M.A.N.; Samaranayake, L.P.; Rosa, E.A. Low virulent oral Candida albicans strains isolated from smokers. Arch. Oral Biol. 2012, 57, 148–153. [Google Scholar] [CrossRef]
- Bouquot, D.J.; Schroeder, K. Oral effects of tobacco abuse. J. Am. Dent. Inst. Cont. Educ. 1992, 43, 3–17. [Google Scholar]
| Sociodemographic Characteristics | N-S a | THS | CC-S | b (p) |
|---|---|---|---|---|
| Age | ||||
| Median | 29.5 | 27 | 29 | |
| Minimum | 20 | 21 | 22 | |
| Maximum | 55 | 56 | 56 | 0.632 |
| Gender | 1.00 | |||
| Male/n (%) | 3 (15%) | 3 (15%) | 3 (15%) | |
| Female/n (%) | 17 (85%) | 17 (85%) | 17 (85%) | |
| Education | ||||
| Magister degree | 11 (55%) | 8 (40%) | 4 (20%) | |
| Bachelor’s degree | 1 (5%) | 4 (20%) | 1 (5%) | |
| High school | 8 (40%) | 8 (40%) | 15 (75%) | 0.055 |
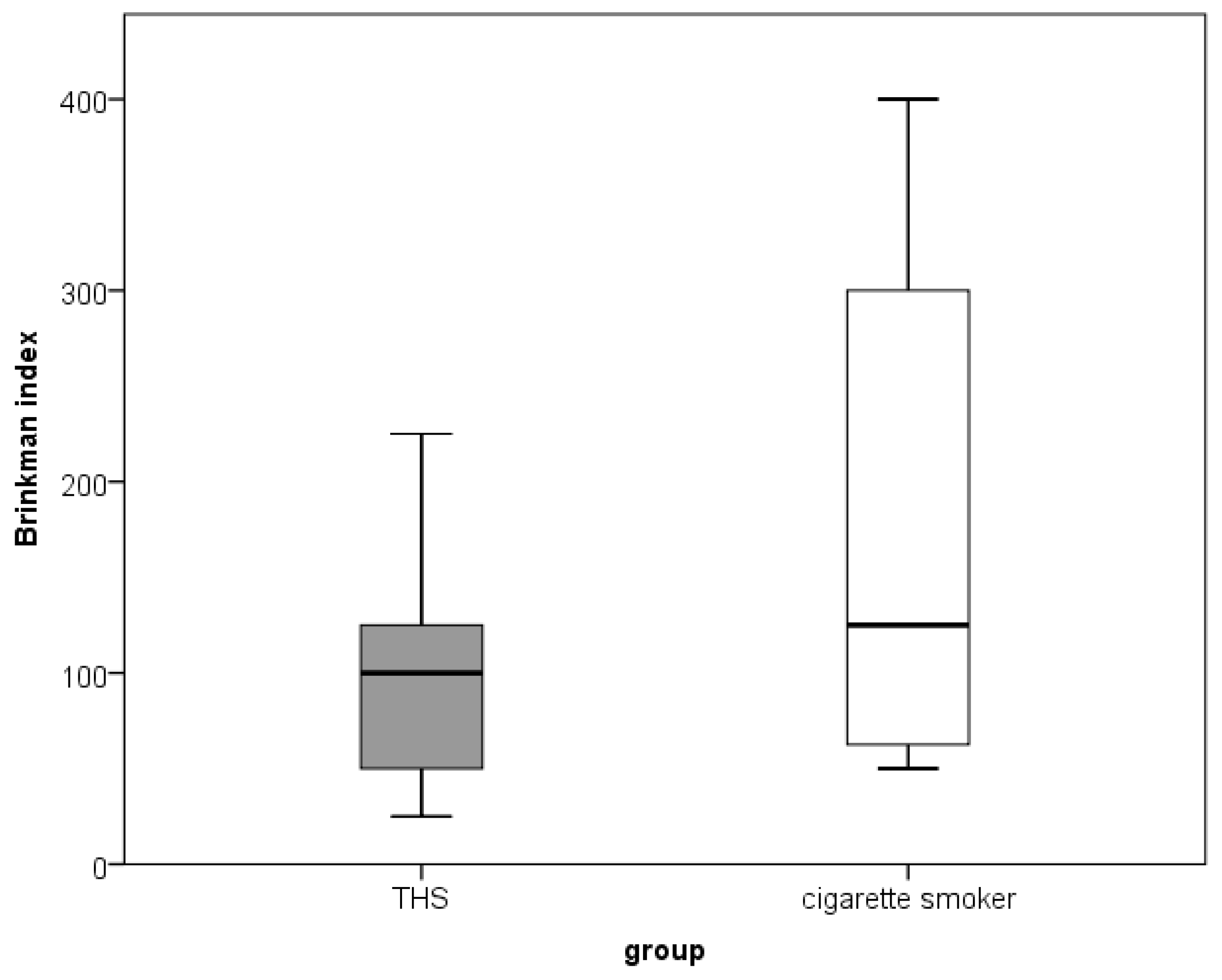
| Brinkman index | ||||
| Mean (Minimum–Maximum) | 0 | 100 (25–225) | 125 (50–400) | |
| Number of cigarettes/per day | 0 | 10 (5–20) | 15 (5–20) | |
| Duration of smoking/years | 0 | 10 (5–15) | 10 (5–20) |
| c | N-S a | THS | CC-S | b (p) | Effect b | |
|---|---|---|---|---|---|---|
| Subjective concern | 0 | 17 a | 10 ab | 9 b | 8.139 | 0.363 |
| 1 | 3 a | 10 ab | 11 b | (0.021) | ||
| Intraoral finding | 0 | 20 a | 9 b | 11 b | 18.257 | 0.507 |
| 1 | 0 a | 11 b | 9 b | (<0.001) | ||
| Bad breath | 0 | 20 | 15 | 16 | 6.115 | |
| 1 | 0 | 5 | 4 | (0.062) | ||
| Halitosis | 0 | 20 a | 10 b | 10 b | ||
| 1 | 0 a | 8 b | 3 ab | 20.987 | ||
| 2 | 0 a | 2 a | 5 a | (<0.001) | 0.624 | |
| 3 | 0 a | 0 a | 2 a | |||
| Dry mouth | 0 | 19 a | 16 ab | 12 b | 7.075 | 0.348 |
| 1 | 1 a | 4 ab | 8 b | (0.030) | ||
| Hyposalivation | 0 | 20 | 18 | 14 | 4.745 | |
| 1 | 0 | 2 | 4 | (0.069) |
| c | N-S a | THS | CC-S | b (p) | |
|---|---|---|---|---|---|
| Atrophy | 0 | 20 | 18 | 19 | 1.921 |
| 1 | 0 | 2 | 1 | (0.766) | |
| Inflammation | 0 | 20 | 20 | 18 | 2.765 |
| 1 | 0 | 0 | 2 | (0.322) | |
| Erosion/ulceration | 0 | 20 | 17 | 19 | 3.111 |
| 1 | 0 | 3 | 1 | (0.310) | |
| Morsication | 0 | 20 | 17 | 17 | 3.550 |
| 1 | 0 | 3 | 3 | (0.228) | |
| Coated tongue | 0 | 20 | 15 | 18 | 5.728 |
| 1 | 0 | 5 | 2 | (0.058) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sever, E.; Božac, E.; Saltović, E.; Simonić-Kocijan, S.; Brumini, M.; Glažar, I. Impact of the Tobacco Heating System and Cigarette Smoking on the Oral Cavity: A Pilot Study. Dent. J. 2023, 11, 251. https://doi.org/10.3390/dj11110251
Sever E, Božac E, Saltović E, Simonić-Kocijan S, Brumini M, Glažar I. Impact of the Tobacco Heating System and Cigarette Smoking on the Oral Cavity: A Pilot Study. Dentistry Journal. 2023; 11(11):251. https://doi.org/10.3390/dj11110251
Chicago/Turabian StyleSever, Ella, Elvis Božac, Ema Saltović, Sunčana Simonić-Kocijan, Martina Brumini, and Irena Glažar. 2023. "Impact of the Tobacco Heating System and Cigarette Smoking on the Oral Cavity: A Pilot Study" Dentistry Journal 11, no. 11: 251. https://doi.org/10.3390/dj11110251
APA StyleSever, E., Božac, E., Saltović, E., Simonić-Kocijan, S., Brumini, M., & Glažar, I. (2023). Impact of the Tobacco Heating System and Cigarette Smoking on the Oral Cavity: A Pilot Study. Dentistry Journal, 11(11), 251. https://doi.org/10.3390/dj11110251