Wearable Orofacial Technology and Orthodontics
Abstract
1. Introduction
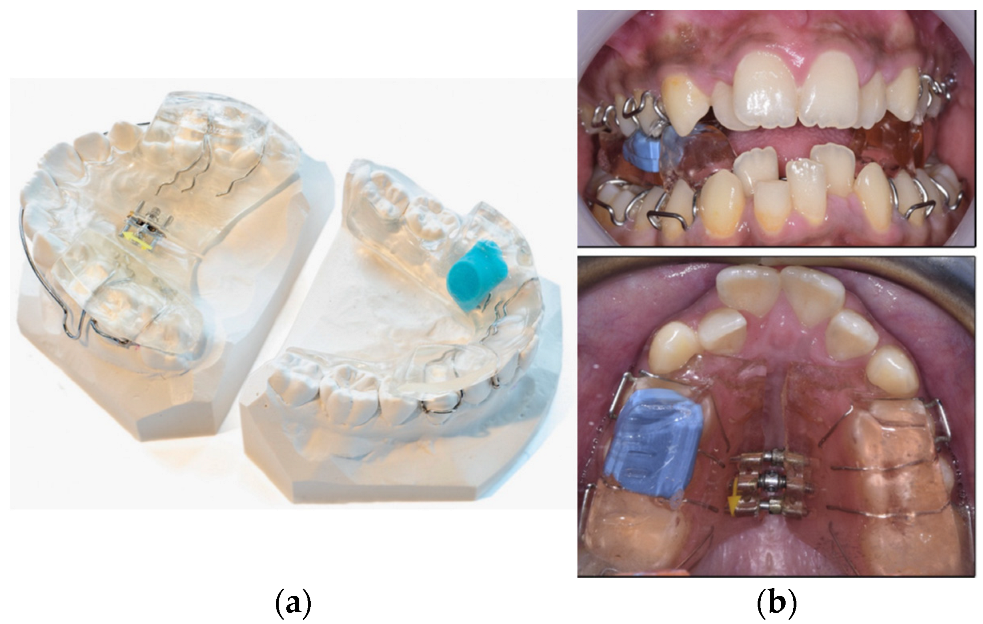
2. Compliance Monitoring
3. Sleep Measurements
4. Jaw Function
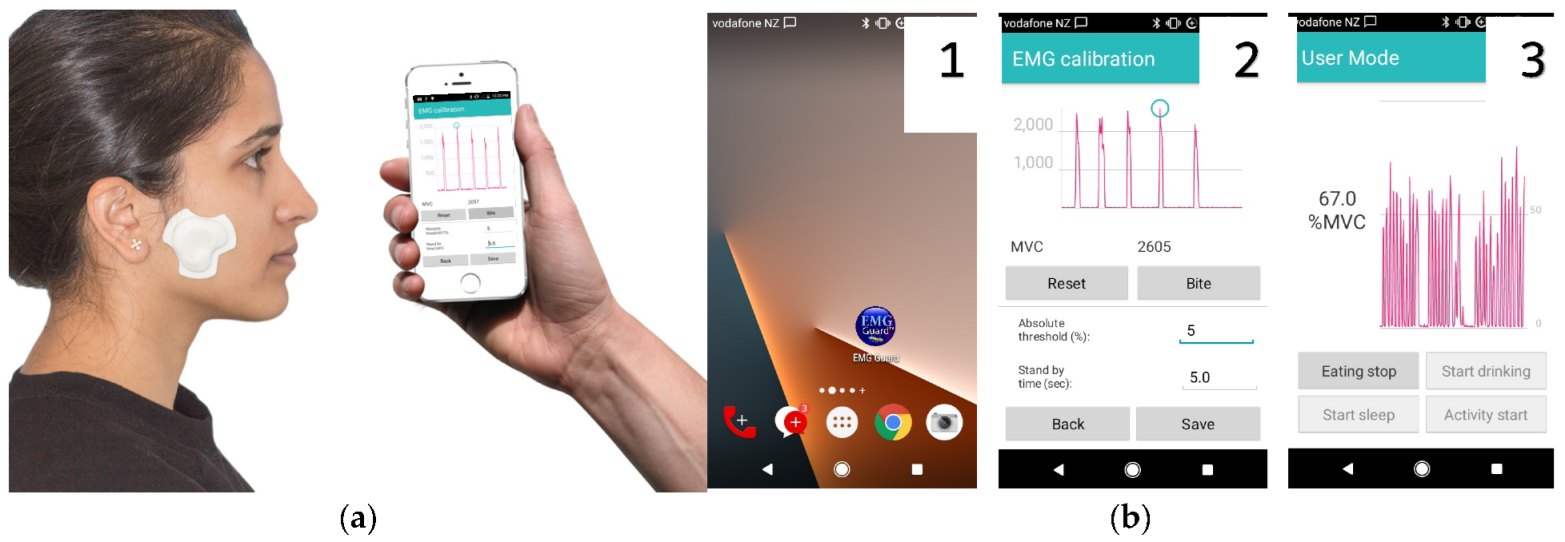
4.1. Masticatory Muscle sEMG
4.2. Tracking Mandibular Motion (TMM)
5. Research Impact
5.1. Intraoral Applications
5.2. Extraoral Applications
6. Risk Assessment and Regulatory Aspects
7. Futurology
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augarten, S. Bit by Bit: An Illustrated History of Computers; Houghton Mifflin Co.: Boston, MA, USA, 1984. [Google Scholar]
- Moore, G.E. Cramming more components onto integrated circuits, Reprinted from Electronics, volume 38, number 8, April 19, 1965, pp.114 ff. IEEE Solid-State Circuits Soc. Newsl. 2006, 11, 33–35. [Google Scholar] [CrossRef]
- Hilbert, M.; López, P. The world’s technological capacity to store, communicate, and compute information. Science 2011, 332, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Tosi, J.; Taffoni, F.; Santacatterina, M.; Sannino, R.; Formica, D. Performance Evaluation of Bluetooth Low Energy: A Systematic Review. Sensors 2017, 17, 2898. [Google Scholar] [CrossRef] [PubMed]
- Ometov, A.; Shubina, V.; Klus, L.; Skibińska, J.; Saafi, S.; Pascacio, P.; Flueratoru, L.; Gaibor, D.Q.; Chukhno, N.; Chukhno, O.; et al. A Survey on Wearable Technology: History, State-of-the-Art and Current Challenges. Comput. Netw. 2021, 193, 108074. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Khattula, V.; Moustakas, E. Use of Wearable Healthcare Devices by US adults: Patterns of Use and Key Predictors. JMIR 2020, 22, e22443. [Google Scholar]
- Slade, P.; Kochenderfer, M.J.; Delp, S.L.; Collins, S.H. Sensing leg movement enhances wearable monitoring of energy expenditure. Nat. Commun. 2021, 12, 4312. [Google Scholar] [CrossRef]
- Scalise, L.; Cosoli, G. Wearables for health and fitness: Measurement characteristics and accuracy. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–6. [Google Scholar]
- Dinh-Le, C.; Chuang, R.; Chokshi, S.; Mann, D. Wearable health technology and electronic health record integration: Scoping review and future directions. JMIR Mhealth Uhealth 2019, 7, e12861. [Google Scholar] [CrossRef]
- Channa, A.; Popescu, N.; Skibinska, J.; Burget, R. The rise of wearable devices during the COVID-19 pandemic: A systematic review. Sensors 2021, 21, 5787. [Google Scholar] [CrossRef]
- Voulgaris, A.; Ferini-Strambi, L.; Steiropoulos, P. Sleep medicine and COVID-19. Has a new era begun? Sleep Med. 2020, 73, 170–176. [Google Scholar] [CrossRef]
- Smith-Spangler, C.; Gienger, A.; Lin, N.; Lewis, R.; Stave, C.; Olkin, I. Using pedometers to increase physical activity: A systematic review. JAMA 2007, 298, 2296–2304. [Google Scholar]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Beauchamp, U.L.; Pappot, H.; Holländer-Mieritz, C. The use of wearables in clinical trials during cancer treatment: Systematic review. JMIR Mhealth Uhealth 2020, 8, e22006. [Google Scholar] [CrossRef]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A critical review of consumer wearables, mobile applications, and equipment for providing biofeedback, monitoring stress, and sleep in physically active populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef]
- Xu, Q.; Cheung, S.-c.S.; Soares, N. LittleHelper: An augmented reality glass application to assist individuals with autism in job interview. In Proceedings of the 2015 Asia-Pacific Signal and Information Processing Association Annual Summit and Conference (APSIPA), Hong Kong, China, 16–19 December 2015; pp. 1276–1279. [Google Scholar]
- Sahin, N.T.; Keshav, N.U.; Salisbury, J.P.; Vahabzadeh, A. Second version of google glass as a wearable socio-affective aid: Positive school desirability, high usability, and theoretical framework in a sample of children with autism. JMIR Hum. Factors 2018, 5, e8785. [Google Scholar] [CrossRef]
- Bos, A.; Hoogstraten, J.; Prahl-Andersen, B. Towards a comprehensive model for the study of compliance in orthodontics. Eur. J. Orthod. 2005, 27, 296–301. [Google Scholar] [CrossRef]
- Mavreas, D.; Athanasiou, A.E. Factors affecting the duration of orthodontic treatment: A systematic review. Eur. J. Orthod. 2008, 30, 386–395. [Google Scholar] [CrossRef]
- Sergl, H.G.; Zentner, A. Predicting patient compliance in orthodontic treatment. Semin. Orthod. 2000, 6, 231–236. [Google Scholar] [CrossRef]
- Albino, J.E.N. Factors influencing adolescent cooperation in orthodontic treatment. Semin. Orthod. 2000, 6, 214–223. [Google Scholar] [CrossRef]
- Pratt, M.C.; Kluemper, G.T.; Lindstrom, A.F. Patient compliance with orthodontic retainers in the postretention phase. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 196–201. [Google Scholar] [CrossRef]
- Fleming, P.S.; Al-Moghrabi, D.; Fudalej, P.; Pandis, N. Orthodontic pain: The use of non-pharmacological adjuncts and its effect on compliance. Semin. Orthod. 2018, 24, 248–258. [Google Scholar] [CrossRef]
- Siddiqui, N.R.; Hodges, S.; Sharif, M.O. Availability of orthodontic smartphone apps. J. Orthod. 2019, 46, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. Orthodontic apps for smartphones. J. Orthod. 2013, 40, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Baheti, M.J.; Toshniwal, N. Orthodontic apps at fingertips. Prog. Orthod. 2014, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Z.-R.; Tang, N.; Ye, C.; Zhu, X.-L.; Zhou, T.; Zhao, Z.-H. Effect of intervention using a messaging app on compliance and duration of treatment in orthodontic patients. Clin. Oral Investig. 2016, 20, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Ahn, S.J.; Kim, T.W. Patient compliance and locus of control in orthodontic treatment: A prospective study. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 354–358. [Google Scholar] [CrossRef]
- Kacer, K.; Valiathan, M.; Narendran, S.; Hans, M. Retainer wear and compliance in the first 2 years after active orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 592–598. [Google Scholar] [CrossRef]
- Al-Moghrabi, D.; Salazar, F.C.; Pandis, N.; Fleming, P.S. Compliance with removable orthodontic appliances and adjuncts: A systematic review and meta-analysis. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 117–132. [Google Scholar] [CrossRef]
- Tüfekçi, E.; Allen, S.B.; Best, A.M.; Lindauer, S.J. Current trends in headgear use for the treatment of Class II malocclusions. Angle Orthod. 2016, 86, 584–589. [Google Scholar] [CrossRef]
- Northcutt, M. The timing headgear. J. Clin. Orthod. 1974, 8, 321–324. [Google Scholar]
- Clemmer, E.J.; Hayes, E.W. Patient cooperation in wearing orthodontic headgear. Am. J. Orthod. 1979, 75, 517–524. [Google Scholar] [CrossRef]
- Banks, P.; Read, M. An investigation into the reliability of the timing headgear. Br. J. Orthod. 1987, 14, 263–267. [Google Scholar] [CrossRef]
- Cureton, S.L.; Regennitter, F.J.; Orbell, M. An accurate, inexpensive headgear timer. J. Clin. Orthod. 1991, 25, 749–754. [Google Scholar]
- Güray, E.; Orhan, M. Selçuk type headgear-timer (STHT). Am. J. Orthod. Dentofac. Orthop. 1997, 111, 87–92. [Google Scholar] [CrossRef]
- Kyriacou, P.A.; Jones, D.P. Compliance monitor for use with removable orthodontic headgear appliances. Med. Biol. Eng. Comput. 1997, 35, 57–60. [Google Scholar] [CrossRef]
- Gungor, M.; Kyriacou, P.; Jones, D. A new micro-controller based wear-time monitor for use with removable orthodontic appliances. In Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Magnificent Milestones and Emerging Opportunities in Medical Engineering (Cat. No. 97CH36136), Chicago, IL, USA, 30 October–2 November 1997; pp. 2419–2421. [Google Scholar]
- Cureton, S.L.; Regennitter, F.J.; Yancey, J.M. The role of the headgear calendar in headgear compliance. Am. J. Orthod. Dentofac. Orthop. 1993, 104, 387–394. [Google Scholar] [CrossRef]
- Doruk, C.; Agar, U.; Babacan, H. The role of the headgear timer in extraoral co-operation. Eur. J. Orthod. 2004, 26, 289–291. [Google Scholar] [CrossRef]
- Trotman, C.-A.; McNamara, J.A.; Moyers, R.E. University of Michigan; Symposium on Craniofacial Growth. In Creating the Compliant Patient; Center for Human Growth and Development; University of Michigan: Ann Arbor, MI, USA, 1996; 198p, p. viii. [Google Scholar]
- Sahm, G.; Bartsch, A.; Witt, E. Reliability of patient reports on compliance. Eur. J. Orthod. 1990, 12, 438–446. [Google Scholar] [CrossRef]
- Sahm, G.; Bartsch, A.; Witt, E. Micro-electronic monitoring of functional appliance wear. Eur. J. Orthod. 1990, 12, 297–301. [Google Scholar] [CrossRef]
- Schott, T.C.; Göz, G. Wearing times of orthodontic devices as measured by the TheraMon® microsensor. J. Orofac. Orthop. 2011, 72, 103–110. [Google Scholar] [CrossRef]
- Frilund, E.; Sonesson, M.; Magnusson, A. Patient compliance with Twin Block appliance during treatment of Class II malocclusion: A randomized controlled trial on two check-up prescriptions. Eur. J. Orthod. 2022, cjac046, Epub ahead of print. [Google Scholar] [CrossRef]
- Parekh, J.; Counihan, K.; Fleming, P.S.; Pandis, N.; Sharma, P.K. Effectiveness of part-time vs. full-time wear protocols of a Twin Block appliance on dental and skeletal changes: A randomized controlled trial. Am. J. Orthod. Dentofacial Orthop. 2019, 155, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, K.; Lehmann, S.; Naterstad, I.; Berge, M.; Johansson, A. Reliability of an adherence monitoring sensor embedded in an oral appliance used for treatment of obstructive sleep apnoea. J. Oral Rehabil. 2018, 45, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, K.; Lehmann, S.; Bjorvatn, B.; Berge, M.; Thuen, F.; Berge, T.; Johansson, A. Partner perceptions are associated with objective sensor-measured adherence to oral appliance therapy in obstructive sleep apnea. J. Sleep Res. 2022, 31, e13462. [Google Scholar] [CrossRef] [PubMed]
- Vanderveken, O.M.; Dieltjens, M.; Wouters, K.; De Backer, W.A.; Van de Heyning, P.H.; Braem, M.J. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax 2013, 68, 91. [Google Scholar] [CrossRef]
- Dieltjens, M.; Verbruggen, A.E.; Braem, M.J.; Wouters, K.; Verbraecken, J.A.; De Backer, W.A.; Hamans, E.; Van de Heyning, P.H.; Vanderveken, O.M. Determinants of Objective Compliance During Oral Appliance Therapy in Patients With Sleep-Disordered Breathing: A Prospective Clinical Trial. JAMA Otolaryngol.–Head Neck Surg. 2015, 141, 894–900. [Google Scholar] [CrossRef]
- Stocker, B.; Willmann, J.H.; Wilmes, B.; Vasudavan, S.; Drescher, D. Wear-time recording during early Class III facemask treatment using TheraMon chip technology. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 533–540. [Google Scholar] [CrossRef]
- Arreghini, A.; Trigila, S.; Lombardo, L.; Siciliani, G. Objective assessment of compliance with intra- and extraoral removable appliances. Angle Orthod. 2017, 87, 88–95. [Google Scholar] [CrossRef]
- Schott, T.C.; Fritz, U.; Meyer-Gutknecht, H. Maxillary Expansion Therapy with Plates Featuring a Transverse Screw: Implications of Patient Compliance with Wear-Time and Screw Activation Requirements. J. Orofac. Orthop. 2014, 75, 107–117. [Google Scholar] [CrossRef]
- Pauls, A.; Nienkemper, M.; Panayotidis, A.; Wilmes, B.; Drescher, D. Effects of wear time recording on the patient’s compliance. Angle Orthod. 2013, 83, 1002–1008. [Google Scholar] [CrossRef]
- Schäfer, K.; Ludwig, B.; Meyer-Gutknecht, H.; Schott, T.C. Quantifying patient adherence during active orthodontic treatment with removable appliances using microelectronic wear-time documentation. Eur. J. Orthod. 2015, 37, 73–80. [Google Scholar] [CrossRef]
- Schott, T.C.; Schlipf, C.; Glasl, B.; Schwarzer, C.L.; Weber, J.; Ludwig, B. Quantification of patient compliance with Hawley retainers and removable functional appliances during the retention phase. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 533–540. [Google Scholar] [CrossRef]
- Hyun, P.; Preston, C.B.; Al-Jewair, T.S.; Park-Hyun, E.; Tabbaa, S. Patient compliance with Hawley retainers fitted with the SMART(®) sensor: A prospective clinical pilot study. Angle Orthod. 2015, 85, 263–269. [Google Scholar] [CrossRef]
- Vagdouti, G.; Karvouni, E.; Bitsanis, E.; Koletsi, D. Objective evaluation of compliance after orthodontic treatment using Hawley or vacuum-formed retainers: A 2-center randomized controlled trial over a 3-month period. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 717–726.e2. [Google Scholar] [CrossRef]
- Schott, T.C.; Meyer-Gutknecht, H.; Mayer, N.; Weber, J.; Weimer, K. A comparison between indirect and objective wear-time assessment of removable orthodontic appliances. Eur. J. Orthod. 2017, 39, 170–175. [Google Scholar] [CrossRef]
- Tsomos, G.; Ludwig, B.; Grossen, J.; Pazera, P.; Gkantidis, N. Objective assessment of patient compliance with removable orthodontic appliances: A cross-sectional cohort study. Angle Orthod. 2014, 84, 56–61. [Google Scholar] [CrossRef]
- Inoko, Y.; Yoshimura, K.; Kato, C.; Morita, O.; Kohno, M. Efficacy and safety of temperature data loggers in measuring compliance with the use of oral appliances. Sleep Biol. Rhythm. 2009, 7, 188–192. [Google Scholar] [CrossRef]
- Sutherland, K.; Almeida, F.R.; Kim, T.; Brown, E.C.; Knapman, F.; Ngiam, J.; Yang, J.; Bilston, L.E.; Cistulli, P.A. Treatment usage patterns of oral appliances for obstructive sleep apnea over the first 60 days: A cluster analysis. J. Clin. Sleep Med. 2021, 17, 1785–1792. [Google Scholar] [CrossRef]
- Castle, E.; Chung, P.; Behfar, M.H.; Chen, M.; Gao, J.; Chiu, N.; Nelson, G.; Roy, S.; Oberoi, S. Compliance monitoring via a Bluetooth-enabled retainer: A prospective clinical pilot study. Orthod. Craniofac. Res. 2019, 22, 149–153. [Google Scholar] [CrossRef]
- Brierley, C.A.; Benson, P.E.; Sandler, J. How accurate are TheraMon® microsensors at measuring intraoral wear-time? Recorded vs. actual wear times in five volunteers. J. Orthod. 2017, 44, 241–248. [Google Scholar] [CrossRef]
- Schott, T.C.; Göz, G. Applicative Characteristics of New Microelectronic Sensors Smart Retainer® and TheraMon® for Mea suring Wear Time. J. Orofac. Orthop. 2010, 71, 339–347. [Google Scholar] [CrossRef]
- Ackerman, M.B.; Thornton, B. Posttreatment compliance with removable maxillary retention in a teenage population: A short-term randomized clinical trial. Orthodontics (Chic) 2011, 12, 22–27. [Google Scholar] [PubMed]
- Al-Moghrabi, D.; Pandis, N.; McLaughlin, K.; Johal, A.; Donos, N.; Fleming, P.S. Evaluation of the effectiveness of a tailored mobile application in increasing the duration of wear of thermoplastic retainers: A randomized controlled trial. Eur. J. Orthod. 2020, 42, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, S.J.; Dalci, O.; Dolce, C.; Holliday, L.S.; Naraghi, S. Orthodontic retention: What’s on the horizon? Br. Dent. J. 2021, 230, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Thirumoorthy, S.N.; Gopal, S. Is remote monitoring a reliable method to assess compliance in clear aligner orthodontic treatment? Evid.-Based Dent. 2021, 22, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Hansa, I.; Katyal, V.; Ferguson, D.J.; Vaid, N. Outcomes of clear aligner treatment with and without Dental Monitoring: A retrospective cohort study. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Schott, T.C.; Göz, G. Color fading of the blue compliance indicator encapsulated in removable clear Invisalign Teen® aligners. Angle Orthod. 2011, 81, 185–191. [Google Scholar] [CrossRef]
- Tuncay, O.C.; Bowman, S.J.; Nicozisis, J.L.; Amy, B.D. Effectiveness of a compliance indicator for clear aligners. J. Clin. Orthod. 2009, 43, 263–268. [Google Scholar]
- Al-Moghrabi, D.; Colonio Salazar, F.B.; Johal, A.; Fleming, P.S. Factors influencing adherence to vacuum-formed retainer wear: A qualitative study. J. Orthod. 2019, 46, 212–219. [Google Scholar] [CrossRef]
- Brandão, M.; Pinho, H.S.; Urias, D. Clinical and quantitative assessment of headgear compliance: A pilot study. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 239–244. [Google Scholar] [CrossRef]
- Bos, A.; Kleverlaan, C.J.; Hoogstraten, J.; Prahl-Andersen, B.; Kuitert, R. Comparing subjective and objective measures of headgear compliance. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 801–805. [Google Scholar] [CrossRef]
- Arponen, H.; Hirvensalo, R.; Lindgren, V.; Kiukkonen, A. Treatment compliance of adolescent orthodontic patients with headgear activator and twin-block appliance assessed prospectively using microelectronic wear-time documentation. Eur. J. Orthod. 2020, 42, 180–186. [Google Scholar] [CrossRef]
- Bianchi, M. Sleep Devices: Wearables and nearables, informational and interventional, consumer and clinical. Metab. Clin. Exp. 2017, 84, 99–108. [Google Scholar] [CrossRef]
- Behrents, R.G.; Shelgikar, A.V.; Conley, R.S.; Flores-Mir, C.; Hans, M.; Levine, M.; McNamara, J.A.; Palomo, J.M.; Pliska, B.; Stockstill, J.W.; et al. Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White Paper. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 13–28. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Zhang, X.L.; Xiao, Y. Sleep health service in China during the coronavirus disease outbreak. J. Clin. Sleep Med. 2020, 16, 1221–1222. [Google Scholar] [CrossRef]
- Penzel, T.; Schöbel, C.; Fietze, I. New technology to assess sleep apnea: Wearables, smartphones, and accessories. F1000Research 2018, 7, 413. [Google Scholar] [CrossRef]
- Pépin, J.L.; Letesson, C.; Le-Dong, N.N.; Dedave, A.; Denison, S.; Cuthbert, V.; Martinot, J.B.; Gozal, D. Assessment of Mandibular Movement Monitoring With Machine Learning Analysis for the Diagnosis of Obstructive Sleep Apnea. JAMA Netw. Open 2020, 3, e1919657. [Google Scholar] [CrossRef]
- Depner, C.M.; Cheng, P.C.; Devine, J.K.; Khosla, S.; de Zambotti, M.; Robillard, R.; Vakulin, A.; Drummond, S.P.A. Wearable technologies for developing sleep and circadian biomarkers: A summary of workshop discussions. Sleep 2020, 43, zsz254. [Google Scholar] [CrossRef]
- Weaver, T.E.; Grunstein, R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef]
- Turino, C.; Benítez, I.D.; Rafael-Palou, X.; Mayoral, A.; Lopera, A.; Pascual, L.; Vaca, R.; Cortijo, A.; Moncusí-Moix, A.; Dalmases, M.; et al. Management and Treatment of Patients With Obstructive Sleep Apnea Using an Intelligent Monitoring System Based on Machine Learning Aiming to Improve Continuous Positive Airway Pressure Treatment Compliance: Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e24072. [Google Scholar] [CrossRef]
- Lowe, A.A.; Sjöholm, T.T.; Ryan, C.F.; Fleetham, J.A.; Ferguson, K.A.; Remmers, J.E. Treatment, airway and compliance effects of a titratable oral appliance. Sleep 2000, 23, S172–S178. [Google Scholar] [PubMed]
- Kirshenblatt, S.J. Microsensor Technology to Evaluate Patient Adherence with Removable Oral Appliances. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2014. [Google Scholar]
- Devine, J.; Schwartz, L.; Choynowski, J.; Hursh, S. Expert Demand for Consumer Sleep Technology Features and Wearable Devices: A Case Study. IoT 2022, 3, 315–331. [Google Scholar] [CrossRef]
- Pepin, J.L.; Le-Dong, N.N.; Cuthbert, V.; Coumans, N.; Tamisier, R.; Malhotra, A.; Martinot, J.B. Mandibular Movements are a Reliable Noninvasive Alternative to Esophageal Pressure for Measuring Respiratory Effort in Patients with Sleep Apnea Syndrome. Nat. Sci. Sleep 2022, 14, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.L.; Ben Messaoud, R.; Joyeux-Faure, M.; Terrail, R.; Tamisier, R.; Martinot, J.-B.; Le-Dong, N.-N.; Morrell, M.J.; Pépin, J.-L. Diagnosis of Sleep Apnoea Using a Mandibular Monitor and Machine Learning Analysis: One-Night Agreement Compared to in-Home Polysomnography. Front. Neurosci. 2022, 16, 333. [Google Scholar] [CrossRef] [PubMed]
- Le-Dong, N.N.; Martinot, J.B.; Coumans, N.; Cuthbert, V.; Tamisier, R.; Bailly, S.; Pépin, J.L. Machine Learning-based Sleep Staging in Patients with Sleep Apnea Using a Single Mandibular Movement Signal. Am. J. Respir. Crit. Care Med. 2021, 204, 1227–1231. [Google Scholar] [CrossRef]
- Markiewicz, M.R.; Ohrbach, R.; McCall, W., Jr. Oral behaviors checklist: Reliability of performance in targeted waking-state behaviors. J. Orofac. Pain 2006, 20, 306–316. [Google Scholar]
- Kaplan, S.E.; Ohrbach, R. Self-Report of Waking-State Oral Parafunctional Behaviors in the Natural Environment. J. Oral Facial Pain Headache 2016, 30, 107–119. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Palla, S.; Erni, S.; Sieber, M.; Gallo, L.M. Nonfunctional tooth contact in healthy controls and patients with myogenous facial pain. J. Orofac. Pain 2007, 21, 185–193. [Google Scholar]
- Zani, A.; Lobbezoo, F.; Bracci, A.; Ahlberg, J.; Manfredini, D. Ecological momentary assessment and intervention principles for the study of awake bruxism behaviors, Part 1: General principles and preliminary data on healthy young Italian adults. Front. Neurol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Pedroni-Pereira, A.; Marquezin, M.C.S.; Araujo, D.S.; Pereira, L.J.; Bommarito, S.; Castelo, P.M. Lack of agreement between objective and subjective measures in the evaluation of masticatory function: A preliminary study. Physiol. Behav. 2018, 184, 220–225. [Google Scholar] [CrossRef]
- Almotairy, N.; Kumar, A.; Trulsson, M.; Grigoriadis, A. Development of the jaw sensorimotor control and chewing - a systematic review. Physiol. Behav. 2018, 194, 456–465. [Google Scholar] [CrossRef]
- Ottenhoff, F.A.; van der Bilt, A.; van der Glas, H.W.; Bosman, F. Control of human jaw elevator muscle activity during simulated chewing with varying bolus size. Exp. Brain Res. 1993, 96, 501–512. [Google Scholar] [CrossRef]
- Peyron, M.A.; Lassauzay, C.; Woda, A. Effects of increased hardness on jaw movement and muscle activity during chewing of visco-elastic model foods. Exp. Brain Res. 2002, 142, 41–51. [Google Scholar] [CrossRef]
- Piancino, M.G.; Farina, D.; Talpone, F.; Castroflorio, T.; Gassino, G.; Margarino, V.; Bracco, P. Surface EMG of jaw-elevator muscles and chewing pattern in complete denture wearers. J. Oral Rehabil. 2005, 32, 863–870. [Google Scholar] [CrossRef]
- Miles, T.S. Postural control of the human mandible. Arch. Oral Biol. 2007, 52, 347–352. [Google Scholar] [CrossRef]
- Neeman, H.; McCall, W.; Plesh, O.; Bishop, B. Analysis of jaw movements and masticatory muscle activity. Comput. Methods Programs Biomed. 1990, 31, 19–32. [Google Scholar] [CrossRef]
- Michelotti, A.; Farella, M.; Vollaro, S.; Martina, R. Mandibular rest position and electrical activity of the masticatory muscles. J. Prosthet. Dent. 1997, 78, 48–53. [Google Scholar] [CrossRef]
- John, D.; Ruge, S.; Kordass, B. Analysis of jaw movements and muscle activity during mastication with JawReports Software. Int. J. Comput. Dent. 2011, 14, 227–231. [Google Scholar]
- Grigoriadis, A.; Trulsson, M. Excitatory drive of masseter muscle during mastication with dental implants. Sci. Rep. 2018, 8, 8597. [Google Scholar] [CrossRef]
- Miles, T.S.; Flavel, S.C.; Nordstrom, M.A. Control of human mandibular posture during locomotion. J. Physiol. 2004, 554, 216–226. [Google Scholar] [CrossRef]
- Okura, K.; Shigemoto, S.; Suzuki, Y.; Noguchi, N.; Omoto, K.; Abe, S.; Matsuka, Y. Mandibular movement during sleep bruxism associated with current tooth attrition. J. Prosthodont. Res. 2017, 61, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sforza, C.; Tartaglia, G.M.; Lovecchio, N.; Ugolini, A.; Monteverdi, R.; Giannì, A.B.; Ferrario, V.F. Mandibular movements at maximum mouth opening and EMG activity of masticatory and neck muscles in patients rehabilitated after a mandibular condyle fracture. J. Craniomaxillofac. Surg. 2009, 37, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Sgolastra, F.; Ciarrocchi, I.; Cattaneo, R. Effects of transcutaneous electrical nervous stimulation on electromyographic and kinesiographic activity of patients with temporomandibular disorders: A placebo-controlled study. J. Electromyogr. Kinesiol. 2012, 22, 463–468. [Google Scholar] [CrossRef] [PubMed]
- De Felício, C.M.; Mapelli, A.; Sidequersky, F.V.; Tartaglia, G.M.; Sforza, C. Mandibular kinematics and masticatory muscles EMG in patients with short lasting TMD of mild-moderate severity. J. Electromyogr. Kinesiol. 2013, 23, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Amft, O. Monitoring Chewing and Eating in Free-Living Using Smart Eyeglasses. IEEE J. Biomed. Health Inform. 2018, 22, 23–32. [Google Scholar] [CrossRef]
- Alex, J.; Turner, D.; Thomas, D.M.; McDougall, A.; Halawani, M.W.; Heymsfield, S.B.; Martin, C.K.; Scisco, J.L.; Salley, J.; Muth, E.; et al. Bite count rates in free-living individuals: New insights from a portable sensor. BMC Nutr. 2018, 4, 23. [Google Scholar] [CrossRef]
- Claude, A.; Robin, O.; Gehin, C.; Massot, B. Design and evaluation of a novel technology for ambulatory monitoring of bruxism events. Sens. Actuators A Phys. 2019, 295, 532–540. [Google Scholar] [CrossRef]
- Kinjo, R.; Wada, T.; Churei, H.; Ohmi, T.; Hayashi, K.; Yagishita, K.; Uo, M.; Ueno, T. Development of a Wearable Mouth Guard Device for Monitoring Teeth Clenching during Exercise. Sensors 2021, 21, 1503. [Google Scholar] [CrossRef]
- Pollis, M.; Maoddi, P.; Letizia, M.; Manfredini, D. Customized Appliance Device for Force Detection in Bruxism Individuals: An Observational Study. Int. J. Dent. 2022, 2022, 2524327. [Google Scholar] [CrossRef]
- Hugger, S.; Schindler, H.J.; Kordass, B.; Hugger, A. Clinical relevance of surface EMG of the masticatory muscles. (Part 1): Resting activity, maximal and submaximal voluntary contraction, symmetry of EMG activity. Int. J. Comput. Dent. 2012, 15, 297–314. [Google Scholar]
- Campillo, B.; Martin, C.; Palma, J.C.; Fuentes, A.D.; Alarcon, J.A. Electromyographic activity of the jaw muscles and mandibular kinematics in young adults with theoretically ideal dental occlusion: Reference values. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e383–e391. [Google Scholar] [CrossRef]
- Szyszka-Sommerfeld, L.; Machoy, M.; Lipski, M.; Wozniak, K. The Diagnostic Value of Electromyography in Identifying Patients With Pain-Related Temporomandibular Disorders. Front. Neurol. 2019, 10, 180. [Google Scholar] [CrossRef]
- Van Eijden, T.M.; Blanksma, N.G.; Brugman, P. Amplitude and timing of EMG activity in the human masseter muscle during selected motor tasks. J. Dent. Res. 1993, 72, 599–606. [Google Scholar] [CrossRef]
- Blanksma, N.G.; Van Eijden, T.M.; Weijs, W.A. Electromyographic heterogeneity in the human masseter muscle. J. Dent. Res. 1992, 71, 47–52. [Google Scholar] [CrossRef]
- Lotzmann, U.; Kobes, L.W.; Hirschfeld, Z.A. Long-term electromyography as a possible diagnostic aid for detecting craniomandibular dysfunctions. Eur. J. Prosthodont. Restor. Dent. 1992, 1, 35–38. [Google Scholar]
- Manfredini, D.; Ahlberg, J.; Wetselaar, P.; Svensson, P.; Lobbezoo, F. The bruxism construct: From cut-off points to a continuum spectrum. J. Oral Rehabil. 2019, 46, 991–997. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Mikami, S.; Maeda, M.; Saito, T.; Nakajima, T.; Yachida, W.; Gotouda, A. Portable and wearable electromyographic devices for the assessment of sleep bruxism and awake bruxism: A literature review. Cranio 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Jack, H.C.; Kieser, J.; Antoun, J.S.; Farella, M. The effect of incremental lower lip advancement on oral pressure and EMG activity of the lower lip. Eur. J. Orthod. 2014, 36, 672–677. [Google Scholar] [CrossRef]
- Shochat, T.; Gavish, A.; Arons, E.; Hadas, N.; Molotsky, A.; Lavie, P.; Oksenberg, A. Validation of the BiteStrip screener for sleep bruxism. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 104, e32–e39. [Google Scholar] [CrossRef]
- Mainieri, V.C.; Saueressig, A.C.; Pattussi, M.P.; Fagondes, S.C.; Grossi, M.L. Validation of the Bitestrip versus polysomnography in the diagnosis of patients with a clinical history of sleep bruxism. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 612–617. [Google Scholar] [CrossRef]
- Mainieri, V.C.; Saueressig, A.C.; Fagondes, S.C.; Teixeira, E.R.; Rehm, D.D.; Grossi, M.L. Analysis of the effects of a mandibular advancement device on sleep bruxism using polysomnography, the BiteStrip, the sleep assessment questionnaire, and occlusal force. Int. J. Prosthodont. 2014, 27, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Baad-Hansen, L.; Jadidi, F.; Castrillon, E.; Thomsen, P.B.; Svensson, P. Effect of a nociceptive trigeminal inhibitory splint on electromyographic activity in jaw closing muscles during sleep. J. Oral Rehabil. 2007, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, F.; Nørregaard, O.; Baad-Hansen, L.; Arendt-Nielsen, L.; Svensson, P. Assessment of sleep parameters during contingent electrical stimulation in subjects with jaw muscle activity during sleep: A polysomnographic study. Eur. J. Oral Sci. 2011, 119, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Stuginski-Barbosa, J.; Porporatti, A.L.; Costa, Y.M.; Svensson, P.; Conti, P.C. Diagnostic validity of the use of a portable single-channel electromyography device for sleep bruxism. Sleep Breath. 2016, 20, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Yachida, W.; Arima, T.; Castrillon, E.E.; Baad-Hansen, L.; Ohata, N.; Svensson, P. Diagnostic validity of self-reported measures of sleep bruxism using an ambulatory single-channel EMG device. J. Prosthodont. Res. 2016, 60, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.; Lobbezoo, F.; Castrillon, E.; Svensson, P.; Santamaria, A.; Alvarez, C.; Manrique, R.; Manfredini, D. Agreement between jaw-muscle activity measurement with portable single-channel electromyography and polysomnography in children. Int. J. Paediatr. Dent. 2018, 28, 33–42. [Google Scholar] [CrossRef]
- Needham, R.; Davies, S.J. Use of the Grindcare® device in the management of nocturnal bruxism: A pilot study. Br. Dent. J. 2013, 215, E1. [Google Scholar] [CrossRef]
- Fujisawa, M.; Kanemura, K.; Tanabe, N.; Gohdo, Y.; Watanabe, A.; Iizuka, T.; Sato, M.; Ishibashi, K. Determination of daytime clenching events in subjects with and without self-reported clenching. J. Oral Rehabil. 2013, 40, 731–736. [Google Scholar] [CrossRef]
- Endo, H.; Kanemura, K.; Tanabe, N.; Takebe, J. Clenching occurring during the day is influenced by psychological factors. J Prosthodont. Res. 2011, 55, 159–164. [Google Scholar] [CrossRef]
- Watanabe, A.; Kanemura, K.; Tanabe, N.; Fujisawa, M. Effect of electromyogram biofeedback on daytime clenching behavior in subjects with masticatory muscle pain. J. Prosthodont. Res. 2011, 55, 75–81. [Google Scholar] [CrossRef]
- Castroflorio, T.; Mesin, L.; Tartaglia, G.M.; Sforza, C.; Farina, D. Use of electromyographic and electrocardiographic signals to detect sleep bruxism episodes in a natural environment. IEEE J. Biomed. Health Inform. 2013, 17, 994–1001. [Google Scholar] [CrossRef]
- Deregibus, A.; Castroflorio, T.; Bargellini, A.; Debernardi, C. Reliability of a portable device for the detection of sleep bruxism. Clin. Oral Investig. 2014, 18, 2037–2043. [Google Scholar] [CrossRef]
- Castroflorio, T.; Bargellini, A.; Rossini, G.; Cugliari, G.; Deregibus, A.; Manfredini, D. Agreement between clinical and portable EMG/ECG diagnosis of sleep bruxism. J. Oral Rehabil. 2015, 42, 759–764. [Google Scholar] [CrossRef]
- Walters, T.J.; Kaschinske, K.A.; Strath, S.J.; Swartz, A.M.; Keenan, K.G. Validation of a portable EMG device to assess muscle activity during free-living situations. J. Electromyogr. Kinesiol. 2013, 23, 1012–1019. [Google Scholar] [CrossRef]
- Kawakami, S.; Kumazaki, Y.; Manda, Y.; Oki, K.; Minagi, S. Specific diurnal EMG activity pattern observed in occlusal collapse patients: Relationship between diurnal bruxism and tooth loss progression. PLoS ONE 2014, 9, e101882. [Google Scholar] [CrossRef]
- Mude, A.H.; Kawakami, S.; Kato, S.; Minagi, S. Properties of tonic episodes of masseter muscle activity during waking hours and sleep in subjects with and without history of orofacial pain. J. Prosthodont. Res. 2018, 62, 234–238. [Google Scholar] [CrossRef]
- Kumazaki, Y.; Naito, M.; Kawakami, S.; Hirata, A.; Oki, K.; Minagi, S. Development of a speech-discriminating electromyogram system for routine ambulatory recordings for the low-level masseter muscle activity. J. Oral Rehabil. 2014, 41, 266–274. [Google Scholar] [CrossRef]
- Omoto, K.; Shigemoto, S.; Suzuki, Y.; Nakamura, M.; Okura, K.; Nishigawa, K.; Goto, N.; Rodis, O.M.; Matsuka, Y. A preliminary investigation of reproducibility of EMG signals during daytime masticatory muscle activity using a portable EMG logging device. J. Electromyogr. Kinesiol. 2015, 25, 603–611. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Mikami, S.; Saito, M.; Okada, K.; Gotouda, A. A newly developed ultraminiature wearable electromyogram system useful for analyses of masseteric activity during the whole day. J. Prosthodont. Res. 2018, 62, 110–115. [Google Scholar] [CrossRef]
- Maeda, M.; Yamaguchi, T.; Mikami, S.; Yachida, W.; Saito, T.; Sakuma, T.; Nakamura, H.; Saito, M.; Mizuno, M.; Yamada, K.; et al. Validity of single-channel masseteric electromyography by using an ultraminiature wearable electromyographic device for diagnosis of sleep bruxism. J. Prosthodont. Res. 2020, 64, 90–97. [Google Scholar] [CrossRef]
- Pasinato, F.; Santos-Couto-Paz, C.C.; Zeredo, J.L.; Macedo, S.B.; Corrêa, E.C. Experimentally induced masseter-pain changes masseter but not sternocleidomastoid muscle-related activity during mastication. J. Electromyogr. Kinesiol. 2016, 31, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, M.; Bataglion, C.; de Luca Canto, G.; Machado Camolezi, N.; Theodoro, G.T.; Siéssere, S.; Semprini, M.; Regalo, S.C. Impact of sleep bruxism on masseter and temporalis muscles and bite force. Cranio 2016, 34, 309–315. [Google Scholar] [CrossRef]
- Prasad, S.; Paulin, M.; Cannon, R.D.; Palla, S.; Farella, M. Smartphone-assisted monitoring of masticatory muscle activity in freely moving individuals. Clin. Oral Investig. 2019, 23, 3601–3611. [Google Scholar] [CrossRef] [PubMed]
- Idris, G.; Smith, C.; Galland, B.; Taylor, R.; Christopher; Robertson, C.; Bennani, H.; Farella, M. Relationship between chewing features and body mass index in young adolescents. Pediatr. Obes. 2020, 16, e12743. [Google Scholar] [CrossRef] [PubMed]
- Idris, G.; Smith, C.; Galland, B.; Taylor, R.; Robertson, C.J.; Farella, M. Home-Based Monitoring of Eating in Adolescents: A Pilot Study. Nutrients 2021, 13, 4354. [Google Scholar] [CrossRef]
- Prasad, S.; Ramanan, D.; Bennani, H.; Paulin, M.; Cannon, R.D.; Palla, S.; Farella, M. Associations among masticatory muscle activity, physical activity and self-reported oral behaviours in adult women. Clin. Oral Investig. 2021, 25, 5049–5059. [Google Scholar] [CrossRef]
- Ramanan, D.; Palla, S.; Bennani, H.; Polonowita, A.; Farella, M. Oral behaviours and wake-time masseter activity in patients with masticatory muscle pain. J. Oral Rehabil. 2021, 48, 979–988. [Google Scholar] [CrossRef]
- Posselt, U. Movement areas of the mandible. J. Prosthet. Dent. 1957, 7, 375–385. [Google Scholar] [CrossRef]
- Wood, G.D. Recording the opening and closing cycle of the mandible. Br. Dent. J. 1979, 146, 305–309. [Google Scholar] [CrossRef]
- Knap, F.J.; Richardson, B.L.; Bogstad, J. Study of mandibular motion in six degrees of freedom. J. Dent. Res. 1970, 49, 289–292. [Google Scholar] [CrossRef]
- Honée, G.L.; Meijer, A.A. A method for jaw movement registration. J. Oral Rehabil. 1974, 1, 217–221. [Google Scholar] [CrossRef]
- Hickey, J.C.; Allison, M.L.; Woelfel, J.B.; Boucher, C.O.; Stacy, R.W. Mandibular movements in three dimensions. J. Prosthet. Dent. 1963, 13, 72–92. [Google Scholar] [CrossRef]
- Ostry, D.J.; Vatikiotis-Bateson, E.; Gribble, P.L. An examination of the degrees of freedom of human jaw motion in speech and mastication. J. Speech Lang. Hear. Res. 1997, 40, 1341–1351. [Google Scholar] [CrossRef]
- Mah, J.; Hatcher, D. Current status and future needs in craniofacial imaging. Orthod. Craniofac. Res. 2003, 6, 10–16. [Google Scholar] [CrossRef]
- Yoon, H.J.; Zhao, K.D.; Rebellato, J.; An, K.N.; Keller, E.E. Kinematic study of the mandible using an electromagnetic tracking device and custom dental appliance: Introducing a new technique. J. Biomech. 2006, 39, 2325–2330. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Piancino, M.G.; Dellavia, C.; Castroflorio, T.; Sforza, C.; Bracco, P. Quantitative analysis of the variability of unilateral chewing movements in young adults. Cranio 2006, 24, 274–282. [Google Scholar] [CrossRef]
- Buschang, P.H.; Hayasaki, H.; Throckmorton, G.S. Quantification of human chewing-cycle kinematics. Arch. Oral Biol. 2000, 45, 461–474. [Google Scholar] [CrossRef]
- Jankelson, B.; Swain, C.W.; Crane, P.F.; Radke, J.C. Kinesiometric instrumentation: A new technology. J. Am. Dent. Assoc. 1975, 90, 834–840. [Google Scholar] [CrossRef]
- Naeije, M.; Van der Weijden, J.J.; Megens, C.C. OKAS-3D: Optoelectronic jaw movement recording system with six degrees of freedom. Med. Biol. Eng. Comput. 1995, 33, 683–688. [Google Scholar] [CrossRef]
- Furtado, D.A.; Pereira, A.A.; Andrade Ade, O.; Bellomo, D.P., Jr.; da Silva, M.R. A specialized motion capture system for real-time analysis of mandibular movements using infrared cameras. Biomed. Eng. Online 2013, 12, 17. [Google Scholar] [CrossRef]
- Lin, Z.; Zecca, M.; Sessa, S.; Bartolomeo, L.; Ishii, H.; Takanishi, A. Development of the wireless ultra-miniaturized inertial measurement unit WB-4: Preliminary performance evaluation. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6927–6930. [Google Scholar] [CrossRef]
- Martinot, J.B.; Borel, J.C.; Cuthbert, V.; Guénard, H.J.; Denison, S.; Silkoff, P.E.; Gozal, D.; Pepin, J.L. Mandibular position and movements: Suitability for diagnosis of sleep apnoea. Respirology 2017, 22, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Cheliout-Heraut, F.; Senny, F.; Djouadi, F.; Ouayoun, M.; Bour, F. Obstructive sleep apnoea syndrome: Comparison between polysomnography and portable sleep monitoring based on jaw recordings. Neurophysiol. Clin. 2011, 41, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ozbek, M.M.; Lowe, A.A.; Sjöholm, T.T.; Love, L.L.; Fleetham, J.A.; Ryan, C.F. Mandibular posture during sleep in patients with obstructive sleep apnoea. Arch. Oral Biol. 1999, 44, 657–664. [Google Scholar] [CrossRef]
- Flavel, S.C.; Nordstrom, M.A.; Miles, T.S. A simple and inexpensive system for monitoring jaw movements in ambulatory humans. J. Biomech. 2002, 35, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Yabukami, S.; Kanetaka, H.; Tsuji, N.; Itagaki, A.; Yamaguchi, M.; Arai, K.I.; Mitani, H. A new tracking system of jaw movement using two magnets. In Proceedings of the 2002 IEEE International Magnetics Conference (INTERMAG), Amsterdam, The Netherlands, 28 April–2 May 2002; p. 8. [Google Scholar]
- Lin, Z.; Zecca, M.; Sessa, S.; Ishii, H.; Takanishi, A. Development of an Ultra-Miniaturized Inertial Measurement Unit for Jaw Movement Analysis during Free Chewing. J. Comput. Sci. 2010, 6, 896. [Google Scholar] [CrossRef]
- Martinot, J.-B.; Borel, J.-C.; Le-Dong, N.-N.; Silkoff, P.E.; Denison, S.; Gozal, D.; Pépin, J.-L. Bruxism Relieved Under CPAP Treatment in a Patient With OSA Syndrome. Chest 2020, 157, e59–e62. [Google Scholar] [CrossRef]
- Rues, S.; Panchaphongsaphak, B.; Gieschke, P.; Paul, O.; Lapatki, B.G. An analysis of the measurement principle of smart brackets for 3D force and moment monitoring in orthodontics. J. Biomech. 2011, 44, 1892–1900. [Google Scholar] [CrossRef]
- Olson, J.E.; Liu, Y.; Nickel, J.C.; Walker, M.P.; Iwasaki, L.R. Archwire vibration and stick-slip behavior at the bracket-archwire interface. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 314–322. [Google Scholar] [CrossRef]
- Quadir, N.A.; Albasha, L.; Taghadosi, M.; Qaddoumi, N.; Hatahet, B. Low-Power Implanted Sensor for Orthodontic Bond Failure Diagnosis and Detection. IEEE Sens. J. 2018, 18, 3003–3009. [Google Scholar] [CrossRef]
- Lazaro, E.; Yetisen, A.; Vega, K. BraceIO: Biosensing through Hydrogel Dental Ligatures. In Proceedings of the 2020 ACM International Symposium on Wearable Computers, Online, 12–17 September 2020. [Google Scholar]
- Sarti, A.; Tubaro, S. Smart Toothbrushes: Inertial Measurement Sensors Fusion with Visual Tracking. In Proceedings of the 14th European Conference on Computer Vision, Amsterdam, The Netherlands, 8–16 October 2016; Volume 9914, pp. 480–494. [Google Scholar]
- Patel, K.; Patel, S.; Scholar, P.; Salazar, C. Internet of Things-IOT: Definition, Characteristics, Architecture, Enabling Technologies, Application & Future Challenges. Int. J. Eng. Sci. Comput. 2016, 6, 6122–6131. [Google Scholar]
- Ackerman, M.B.; Ackerman, J.L. Smile analysis and design in the digital era. J. Clin. Orthod. 2002, 36, 221–236. [Google Scholar]
- Schmidt, K.L.; Ambadar, Z.; Cohn, J.F.; Reed, L.I. Movement Differences Between Deliberate And Spontaneous Facial Expressions: Zygomaticus Major Action in Smiling. J. Nonverbal Behav. 2006, 30, 37–52. [Google Scholar] [CrossRef]
- Ekman, P. Facial Expressions of Emotion: New Findings, New Questions. Psychol. Sci. 1992, 3, 34–38. [Google Scholar] [CrossRef]
- Cohn, J.; Schmidt, K. The timing of facial motion in posed and spontaneous smiles. Int. J. Wavelets Multiresolution Inf. Process. 2011, 2, 121–132. [Google Scholar] [CrossRef]
- McLaren, E.A.; Culp, L. Smile analysis. J. Cosmet. Dent. 2013, 29, 94–108. [Google Scholar]
- Morley, J.; Eubank, J. Macroesthetic elements of smile design. J. Am. Dent. Assoc. 2001, 132, 39–45. [Google Scholar] [CrossRef]
- Durgekar, S.G.; Nagaraj, K.; Naik, V. The ideal smile and its orthodontic implications. World J. Orthod. 2010, 11, 211–220. [Google Scholar]
- Sarver, D.M.; Ackerman, M.B. Dynamic smile visualization and quantification: Part 1. Evolution of the concept and dynamic records for smile capture. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 4–12. [Google Scholar] [CrossRef]
- Sarver, D.M.; Ackerman, M.B. Dynamic smile visualization and quantification: Part 2. Smile analysis and treatment strategies. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 116–127. [Google Scholar] [CrossRef]
- Popat, H.; Richmond, S.; Benedikt, L.; Marshall, D.; Rosin, P.L. Quantitative analysis of facial movement—A review of three-dimensional imaging techniques. Comput. Med. Imaging Graph. 2009, 33, 377–383. [Google Scholar] [CrossRef]
- Dindaroğlu, F.; Duran, G.S.; Görgülü, S.; Yetkiner, E. Social smile reproducibility using 3-D stereophotogrammetry and reverse engineering technology. Angle Orthod. 2016, 86, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, C.; Takada, K. Test-retest reliability of smile tasks using three-dimensional facial topography. Angle Orthod. 2018, 88, 319–328. [Google Scholar] [CrossRef]
- Husain, A.; Makhija, P.G.; Ummer, A.A.; Kuijpers-Jagtman, A.M.; Kuijpers, M.A. Three-camera setup to record simultaneously standardized high-definition video for smile analysis. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Teeters, A.; El Kaliouby, R.; Picard, R. Self-Cam: Feedback from what would be your social partner. In ACM SIGGRAPH 2006 Research Posters; Association for Computing Machinery: New York, NY, USA, 2006. [Google Scholar]
- Gruebler, A.; Suzuki, K. A wearable interface for reading facial expressions based on bioelectrical signals. In Proceedings of the International Conference on Kansei Engineering and Emotion Research, Paris, France, 2–4 March 2010. [Google Scholar]
- Gruebler, A.; Suzuki, K. Design of a wearable device for reading positive expressions from facial emg signals. IEEE Trans. Affect. Comput. 2014, 5, 227–237. [Google Scholar] [CrossRef]
- Scheirer, J.; Fernandez, R.; Picard, R.W. Expression glasses: A wearable device for facial expression recognition. In Proceedings of the CHI99: Conference on Human Factors in Computing Systems, Pittsburgh, PA, USA, 15–20 May 1999; pp. 262–263. [Google Scholar]
- Wei, N.J.; Dougherty, B.; Myers, A.; Badawy, S.M. Using Google Glass in Surgical Settings: Systematic Review. JMIR Mhealth Uhealth 2018, 6, e54. [Google Scholar] [CrossRef]
- Starner, T. Project glass: An extension of the self. IEEE Pervasive Comput. 2013, 12, 14–16. [Google Scholar] [CrossRef]
- Cohn, J.F.; Ambadar, Z.; Ekman, P. Observer-based measurement of facial expression with the Facial Action Coding System. Handb. Emot. Elicitation Assess. 2007, 1, 203–221. [Google Scholar]
- Girard, J.M.; Cohn, J.F.; Jeni, L.A.; Sayette, M.A.; De la Torre, F. Spontaneous facial expression in unscripted social interactions can be measured automatically. Behav. Res. Methods 2015, 47, 1136–1147. [Google Scholar] [CrossRef]
- Hamm, J.; Kohler, C.G.; Gur, R.C.; Verma, R. Automated facial action coding system for dynamic analysis of facial expressions in neuropsychiatric disorders. J. Neurosci. Methods 2011, 200, 237–256. [Google Scholar] [CrossRef]
- Fridlund, A.J.; Cacioppo, J.T. Guidelines for human electromyographic research. Psychophysiology 1986, 23, 567–589. [Google Scholar] [CrossRef]
- Hess, U.; Kappas, A.; McHugo, G.J.; Kleck, R.E.; Lanzetta, J.T. An analysis of the encoding and decoding of spontaneous and posed smiles: The use of facial electromyography. J. Nonverbal Behav. 1989, 13, 121–137. [Google Scholar] [CrossRef]
- Ilves, M.; Lylykangas, J.; Rantanen, V.; Mäkelä, E.; Vehkaoja, A.; Verho, J.; Lekkala, J.; Rautiainen, M.; Surakka, V. Facial muscle activations by functional electrical stimulation. Biomed. Signal Process. Control. 2019, 48, 248–254. [Google Scholar] [CrossRef]
- Cohn, J.F. Advances in behavioral science using automated facial image analysis and synthesis [social sciences]. IEEE Signal Process. Mag. 2010, 27, 128–133. [Google Scholar] [CrossRef]
- Perusquía-Hernández, M.; Dollack, F.; Tan, C.K.; Namba, S.; Ayabe-Kanamura, S.; Suzuki, K. Facial movement synergies and Action Unit detection from distal wearable Electromyography and Computer Vision. arXiv 2020, arXiv:2008.08791. [Google Scholar]
- Kwon, J.; Ha, J.; Kim, D.-H.; Choi, J.W.; Kim, L. Emotion recognition using a glasses-type wearable device via multi-channel facial responses. IEEE Access 2021, 9, 146392–146403. [Google Scholar] [CrossRef]
- Zhuang, M.; Yin, L.; Wang, Y.; Bai, Y.; Zhan, J.; Hou, C.; Yin, L.; Xu, Z.; Tan, X.; Huang, Y. Highly Robust and Wearable Facial Expression Recognition via Deep-Learning-Assisted, Soft Epidermal Electronics. Research 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Wipperman, M.; Pogoncheff, G.; Mateo, K.; Wu, X.; Chen, Y.; Levy, O.; Avbersek, A.; Deterding, R.; Hamon, S.; Vu, T.; et al. A pilot study of the Earable device to measure facial muscle and eye movement tasks among healthy volunteers. arXiv 2022, arXiv:2202.00206. [Google Scholar] [CrossRef]
- Chen, T.; Li, Y.; Tao, S.; Lim, H.; Sakashita, M.; Zhang, R.; Guimbretiere, F.; Zhang, C. NeckFace: Continuously Tracking Full Facial Expressions on Neck-mounted Wearables. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2021, 5, 1–31. [Google Scholar] [CrossRef]
- Valstar, M.F.; Gunes, H.; Pantic, M. How to distinguish posed from spontaneous smiles using geometric features. In Proceedings of the 9th International Conference on Multimodal Interfaces, Nagoya, Japan, 12–15 November 2007; pp. 38–45. [Google Scholar]
- Guihard, M.; Gracies, J.M.; Baude, M. Three-Dimensional Quantification of Facial Morphology and Movements Using a Wearable Helmet. BioMed Res. Int. 2022, 2022, 2774713. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef]
- Khatsenko, K.; Khin, Y.; Maibach, H. Allergic Contact Dermatitis to Components of Wearable Adhesive Health Devices. Dermatitis 2020, 31, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Farella, M.; Paulin, M.; Yao, S.; Zhu, Y.; van Vuuren, L.J. Effect of electrode characteristics on electromyographic activity of the masseter muscle. J. Electromyogr. Kinesiol. 2021, 56, 102492. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.; Zomaya, A. Handbook of Large-Scale Distributed Computing in Smart Healthcare; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Li, M.; Lou, W.; Ren, K. Data security and privacy in wireless body area networks. IEEE Wirel. Commun. 2010, 17, 51–58. [Google Scholar] [CrossRef]
- Kotz, D.; Gunter, C.A.; Kumar, S.; Weiner, J.P. Privacy and Security in Mobile Health: A Research Agenda. Computer 2016, 49, 22–30. [Google Scholar] [CrossRef]
- Dimitriou, T.; Ioannis, K. Security issues in biomedical wireless sensor networks. In Proceedings of the 2008 First International Symposium on Applied Sciences on Biomedical and Communication Technologies, Aalborg, Denmark, 25–28 October 2008; pp. 1–5. [Google Scholar]
- Digital Health Software Precertification (Pre-Cert) Program. Available online: https://www.fda.gov/medical-devices/digital-health-center-excellence/digital-health-software-precertification-pre-cert-program (accessed on 31 August 2022).
- Framework for FDA’s Real-World Evidence Program. Available online: https://www.fda.gov/media/120060/download (accessed on 31 August 2022).
- Bastos, D.; Giubilo, F.; Shackleton, M.; El-Mousa, F. GDPR Privacy Implications for the Internet of Things. In Proceedings of the 4th Annual IoT Security Foundation Conference, London, UK, 4 December 2018. [Google Scholar]
- Spender, A.; Bullen, C.; Altmann-Richer, L.; Cripps, J.; Duffy, R.; Falkous, C.; Farrell, M.; Horn, T.; Wigzell, J.; Yeap, W. Wearables and the internet of things: Considerations for the life and health insurance industry. Br. Actuar. J. 2019, 24, e22. [Google Scholar] [CrossRef]
- Kang, H.S.; Exworthy, M. Wearing the Future—Wearables to Empower Users to Take Greater Responsibility for Their Health and Care: Scoping Review. JMIR Mhealth Uhealth 2022, 10, e35684. [Google Scholar] [CrossRef]
- Jacox, L.A.; Mihas, P.; Cho, C.; Lin, F.C.; Ko, C.C. Understanding technology adoption by orthodontists: A qualitative study. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 432–442. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef]
- Hashem, M.; Al Kheraif, A.A.; Fouad, H. Design and development of wireless wearable bio-tooth sensor for monitoring of tooth fracture and its bio metabolic components. Comput. Commun. 2020, 150, 278–285. [Google Scholar] [CrossRef]
- Li, C.-Y.; Chen, Y.-C.; Chen, W.-J.; Huang, P.; Chu, H.-H. Sensor-embedded teeth for oral activity recognition. In Proceedings of the 2013 International Symposium on Wearable Computers, Zurich, Switzerland, 9–12 September 2013; pp. 41–44. [Google Scholar]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-Integrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef]
- Nagele, A.N.; Hough, J.; Dinnen, Z. The Subjectivities of Wearable Sleep-Trackers-A Discourse Analysis. In Proceedings of the CHI Conference on Human Factors in Computing Systems Extended Abstracts, New Orleans, LA, USA, 30 April–5 May 2022; pp. 1–8. [Google Scholar]

| Monitoring Mechanism | Orthodontic Appliance | Study | |
|---|---|---|---|
| Extra-oral | Aledyne timer | Cervical pull headgear | Clemmer et al. 1979 [33] |
| Commercial wrist-watch | Cervical pull headgear | Cureton et al. 1993 [39] | |
| Compliance science system | Cervical pull headgear | Brandao et al. 2006 [74] | |
| Thermochron i-Button | Cervical pull headgear | Bos et al. 2007 [75] | |
| TheraMon-microsensors | Facemask (forehead rest) | Arreghini et al. 2017 [52] | |
| HG and functional appliance | Arponen et al. 2020 [76] | ||
| Intra-oral | Thermochron i-Button | Oral appliances for sleep apnea | Inoko et al. 2009 [61] |
| TheraMon-microsensors | Removable appliances | Schott et al. 2011 [44] | |
| Oral appliance for sleep-disordered breathing | Vanderveken et al. 2012 [49] | ||
| Removable appliances/retainers | Pauls et al. 2013 [54] | ||
| Hawley retainers/functional appliance retainers | Schott et al. 2013 [56] | ||
| Removable maxillary expanders | Schott et al. 2014 [53] | ||
| Active/passive orthodontic removable appliances | Tsomos et al. 2014 [60] | ||
| Mandibular advancement device | Dieltjens et al. 2015 [50] | ||
| Functional/active removable appliances | Schäfer et al. 2015 [55] | ||
| Functional appliances | Arreghini et al. 2017 [52] | ||
| Expansion plates, functional appliances, and retention plates. | Schott et al. 2017 [59] | ||
| Hawley and vacuum-formed retainers | Vagdouti et al. 2019 [58] | ||
| Twin Block | Frilund et al. 2022 [45] | ||
| SMART Microsensor | Maxillary Hawley retainer | Hyun et al. 2015 [57] | |
| DentiTrac thermal sensor | Mandibular advancement device | Gjerde et al. 2018 [47] | |
| Food-grade dye | Clear aligners | Tuncay et al. 2009 [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, S.; Arunachalam, S.; Boillat, T.; Ghoneima, A.; Gandedkar, N.; Diar-Bakirly, S. Wearable Orofacial Technology and Orthodontics. Dent. J. 2023, 11, 24. https://doi.org/10.3390/dj11010024
Prasad S, Arunachalam S, Boillat T, Ghoneima A, Gandedkar N, Diar-Bakirly S. Wearable Orofacial Technology and Orthodontics. Dentistry Journal. 2023; 11(1):24. https://doi.org/10.3390/dj11010024
Chicago/Turabian StylePrasad, Sabarinath, Sivakumar Arunachalam, Thomas Boillat, Ahmed Ghoneima, Narayan Gandedkar, and Samira Diar-Bakirly. 2023. "Wearable Orofacial Technology and Orthodontics" Dentistry Journal 11, no. 1: 24. https://doi.org/10.3390/dj11010024
APA StylePrasad, S., Arunachalam, S., Boillat, T., Ghoneima, A., Gandedkar, N., & Diar-Bakirly, S. (2023). Wearable Orofacial Technology and Orthodontics. Dentistry Journal, 11(1), 24. https://doi.org/10.3390/dj11010024