Oral Lichen Planus and Mutated TP53—A Road to Cancer?
Abstract
1. Introduction
2. Summary of Three Studies on OLP and OSCC in Iceland
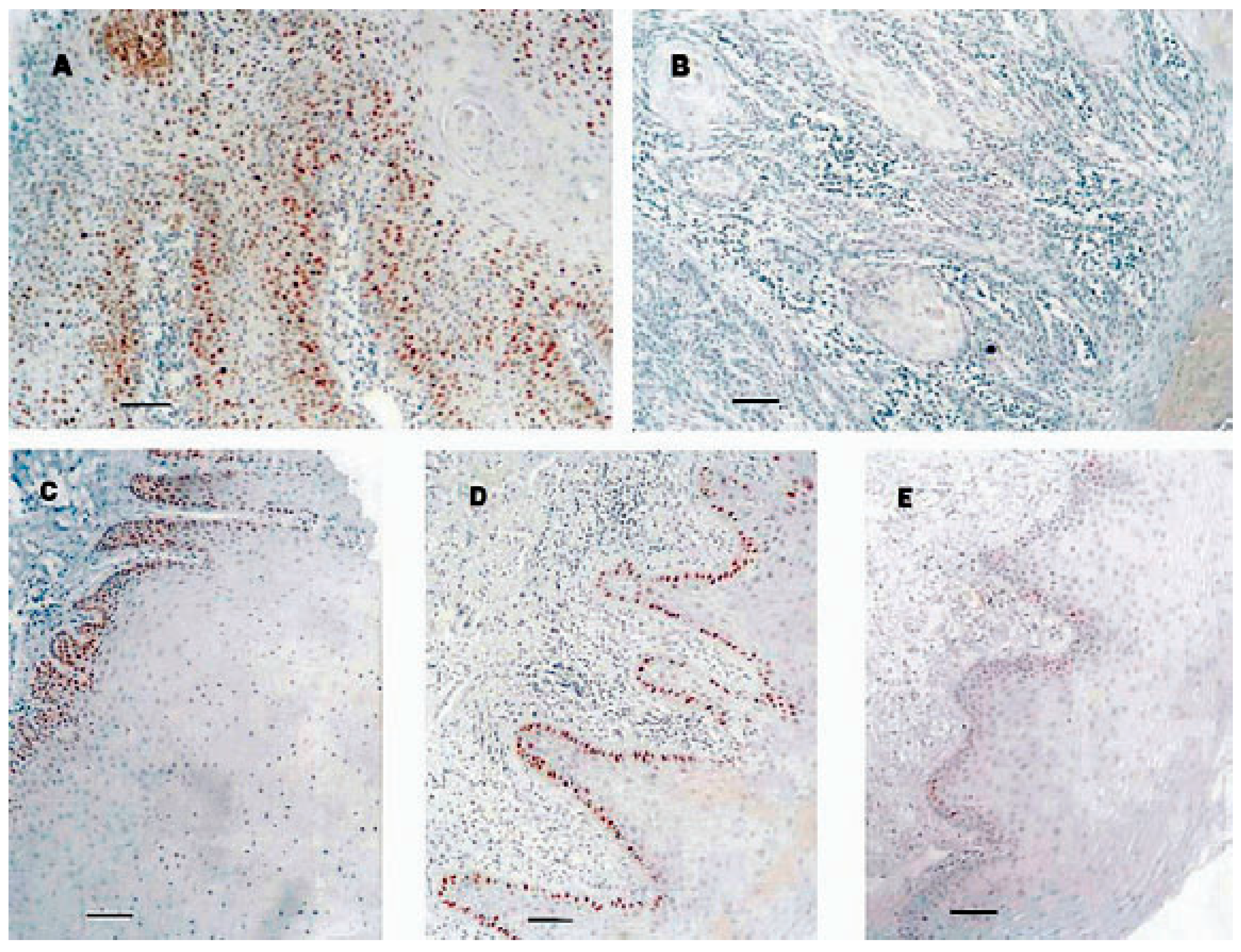
2.1. The Original Study of TP53 in OSCC and Premalignant Oral Lesions
2.2. Longitudinal Study of Eight Patients with OSCC and Premalignant Oral Lesions
2.3. Follow-Up Study and Current Follow-Up of Original Group
3. Discussion
3.1. The Malignant Potential of OLP
3.2. Developing Knowledge on TP53
3.3. Searching for Risk Factors That Contribute to Malignant Transformation of OLP
3.4. Field Cancerization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aghbari, S.M.H.; Abushouk, A.I.; Attia, A.; Elmaraezy, A.; Menshawy, A.; Ahmed, M.S.; Elsaadany, B.A.; Ahmed, E.M. Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20,095 patient data. Oral Oncol. 2017, 68, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Muzio, L.L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019, 25, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Halonen, P.; Jakobsson, M.; Heikinheimo, O.; Riska, A.; Gissler, M.; Pukkala, E. Cancer risk of Lichen planus: A cohort study of 13,100 women in Finland. Int. J. Cancer 2018, 142, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, F.; Sheykhbahaei, N.; SadrZadeh-Afshar, M.-S. Evaluation of Potential Risk Factors that contribute to Malignant Transformation of Oral Lichen Planus: A Literature Review. J. Contemp. Dent. Pract. 2016, 17, 692–701. [Google Scholar] [CrossRef]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef]
- Lane, D. P53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef]
- Amelio, I.; Melino, G. Context is everything: Extrinsic signalling and gain-of-function p53 mutants. Cell Death Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Thorlacius, S.; Thorgilsson, B.; Björnsson, J.; Tryggvadottir, L.; Börresen, A.-L.; Ögmundsdottir, H.; Eyfjörd, J. TP53 mutations and abnormal p53 protein staining in breast carcinomas related to prognosis. Eur. J. Cancer 1995, 31, 1856–1864. [Google Scholar] [CrossRef]
- Dahse, R.; Fiedler, W.; Eggeling, F.V.; Schimmel, B.; Koscielny, S.; Beleites, E.; Claussen, U.; Ernse, G. P53 genotyping–an effective concept for molecular testing of head and neck cancer? Int. J. Mol. Med. 1999, 4, 279–283. [Google Scholar] [CrossRef]
- Ahomadegbe, J.C.; Barrois, M.; Fogel, S.; Le Bihan, M.L.; Douc-Rasy, S.; Duvillard, P.; Armand, J.P.; Riou, G. High incidence of p53 alterations (mutation, deletion, overexpression) in head and neck primary tumors and metastases; absence of correlation with clinical outcome. Frequent protein overexpression in normal epithelium and in early non-invasive lesions. Oncogene 1995, 10, 1217–1227. [Google Scholar]
- Nylander, K.; Nilsson, P.; Mehle, C.; Roos, G. p53 mutations, protein expression and cell proliferation in squamous cell carcinomas of the head and neck. Br. J. Cancer 1995, 71, 826–830. [Google Scholar] [CrossRef]
- Gmundsdóttir, H.M.; Hilmarsdóttir, H.; Ástvaldsdóttir, Á.; Jóhannsson, J.H.; Holbrook, W.P. Oral lichen planus has a high rate of TP53 mutations. A study of oral mucosa in Iceland. Eur. J. Oral Sci. 2002, 110, 192–198. [Google Scholar] [CrossRef]
- Gmundsdóttir, H.M.; Björnsson, J.; Holbrook, W. Role of TP53 in the progression of pre-malignant and malignant oral mucosal lesions. A follow-up study of 144 patients. J. Oral Pathol. Med. 2009, 38, 565–571. [Google Scholar] [CrossRef]
- Gmundsdóttir, H.M.; Hilmarsdóttir, H.; Björnsson, J.; Holbrook, W.P. Longitudinal study of TP53 mutations in eight patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2009, 38, 716–721. [Google Scholar] [CrossRef]
- Girod, S.C.; Krämer, C.; Knüfermann, R.; Krueger, G.R.F. p53 Expression in the carcinogenesis in the oral mucosa. J. Cell. Biochem. 1994, 56, 444–448. [Google Scholar] [CrossRef]
- Fitzpatrick, S.G.; Hirsch, S.A.; Gordon, S.C. The malignant transformation of oral Lichen planus and oral lichenoid lesions: A systematic review. J. Am. Dent. Assoc. 2014, 145, 45–56. [Google Scholar] [CrossRef]
- Richards, D. Malignant transformation rates in Oral Lichen Planus. Evid. Based Dent. 2018, 19, 104. [Google Scholar] [CrossRef]
- Bombeccari, G.P.; Guzzi, G.; Tettamanti, M.; Giannì, A.B.; Baj, A.; Pallotti, F.; Spadar, F. Oral lichen planus and malignant transfor-mation: A longitudinal cohort study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 328–334. [Google Scholar] [CrossRef]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef]
- Soussi, T. The history of pA perfect example of the drawbacks of scientific paradigms. EMBO Rep. 2010, 11, 822–826. [Google Scholar] [CrossRef]
- Baker, S.J.; Preisinger, A.C.; Jessup, M.; Paraskeva, C.; Markowitz, S.; Willson, J.K.V.; Hamilton, S.; Vogelstein, B. P53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990, 50, 7717–7722. [Google Scholar]
- Atai, Z.; Khodadadi-Bohlouli, Z.; Navabi, N. Molecular markers as an indicator in the malignant potential of oral lichen planus: A systematic review. J. Oral Health Oral Epidemiol. 2017, 6, 54–62. [Google Scholar]
- Németh, C.G.; Röcken, C.; Siebert, R.; Wiltfang, J.; Ammerpohl, O.; Gassling, V. Recurrent chromosomal and epigenetic alterations in oral squamous cell carcinoma and its putative premalignant condition oral lichen planus. PLoS ONE 2019, 14, e0215055. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Braakhuis, B.J.M.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef]
- Klein, A.M.; Brash, D.E.; Jones, P.H.; Simons, B.D. Stochastic fate of p53mutant epidermal progenitor cells is tilted toward prolif-eration by UV B during preneoplasia. Proc. Natl. Acad. Sci. USA 2010, 107, 270–275. [Google Scholar] [CrossRef]
- Galandiuk, S.; Rodriguez-Justo, M.; Jeffery, R.; Nicholson, A.M.; Cheng, Y.; Oukrif, D.; Elia, G.; Leedham, S.J.; McDonald, S.A.; Wright, N.; et al. Field Cancerization in the Intestinal Epithelium of Patients with Crohn’s Ileocolitis. Gastroenterology 2012, 142, 855–864. [Google Scholar] [CrossRef]
- Bird-Lieberman, E.; Dunn, J.M.; Coleman, H.; Lao–Sirieix, P.; Oukrif, D.; Moore, C.E.; Varghese, S.; Johnston, B.T.; Arthur, K.; McManus, D.T.; et al. Population-Based Study Reveals New Risk-Stratification Biomarker Panel for Barrett’s Esophagus. Gastroenterology 2012, 143, 927–935. [Google Scholar] [CrossRef]
- Van der Meij, E.H.; Van der Waal, I. Kack of clinicopathological correlation in the diagnsosi of oral lichen planus based on the presently available diagnostic criteria and suggestions for moifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
| TP53 Protein Expression | TP53 Mutation | OSCC/Site/Years Until Diagnosis |
|---|---|---|
| Positive: 16 | Yes: 4 | 1/lip/21 |
| No: 12 | ||
| Not done: 0 | ||
| Negative: 29 | Yes: 5 | 1/tonsil/6 |
| No: 9 | ||
| Not done: 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holbrook, W.P.; Ögmundsdottir, H.M. Oral Lichen Planus and Mutated TP53—A Road to Cancer? Dent. J. 2022, 10, 176. https://doi.org/10.3390/dj10090176
Holbrook WP, Ögmundsdottir HM. Oral Lichen Planus and Mutated TP53—A Road to Cancer? Dentistry Journal. 2022; 10(9):176. https://doi.org/10.3390/dj10090176
Chicago/Turabian StyleHolbrook, William Peter, and Helga M. Ögmundsdottir. 2022. "Oral Lichen Planus and Mutated TP53—A Road to Cancer?" Dentistry Journal 10, no. 9: 176. https://doi.org/10.3390/dj10090176
APA StyleHolbrook, W. P., & Ögmundsdottir, H. M. (2022). Oral Lichen Planus and Mutated TP53—A Road to Cancer? Dentistry Journal, 10(9), 176. https://doi.org/10.3390/dj10090176





