Differences in Supragingival Microbiome in Patients with and without Full-Crown Prostheses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical-Sample Collection and DNA Isolation
2.3. High-Throughput Sequencing
2.4. Statistical and Bioinformatics Analyses
3. Results
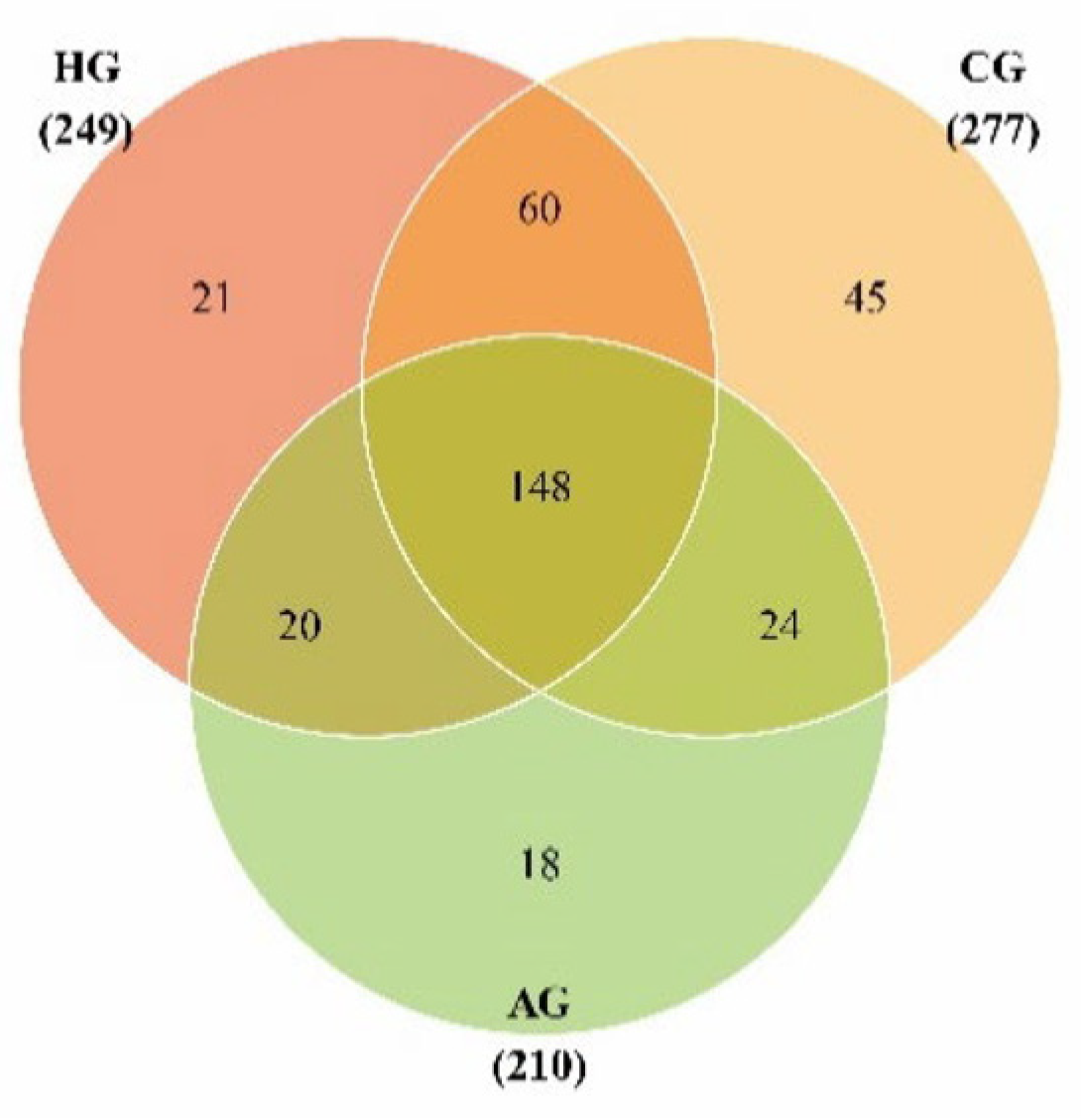
3.1. General Outline
3.2. Species Annotation and Diversity in Samples from Porcelain-Fused-to-Metal Crowns, All-Ceramic Crowns, and No Prostheses
3.3. Species-Difference Analysis of Samples from Porcelain-Fused-to-Metal Crowns, All-Ceramic Crowns, and No Prostheses
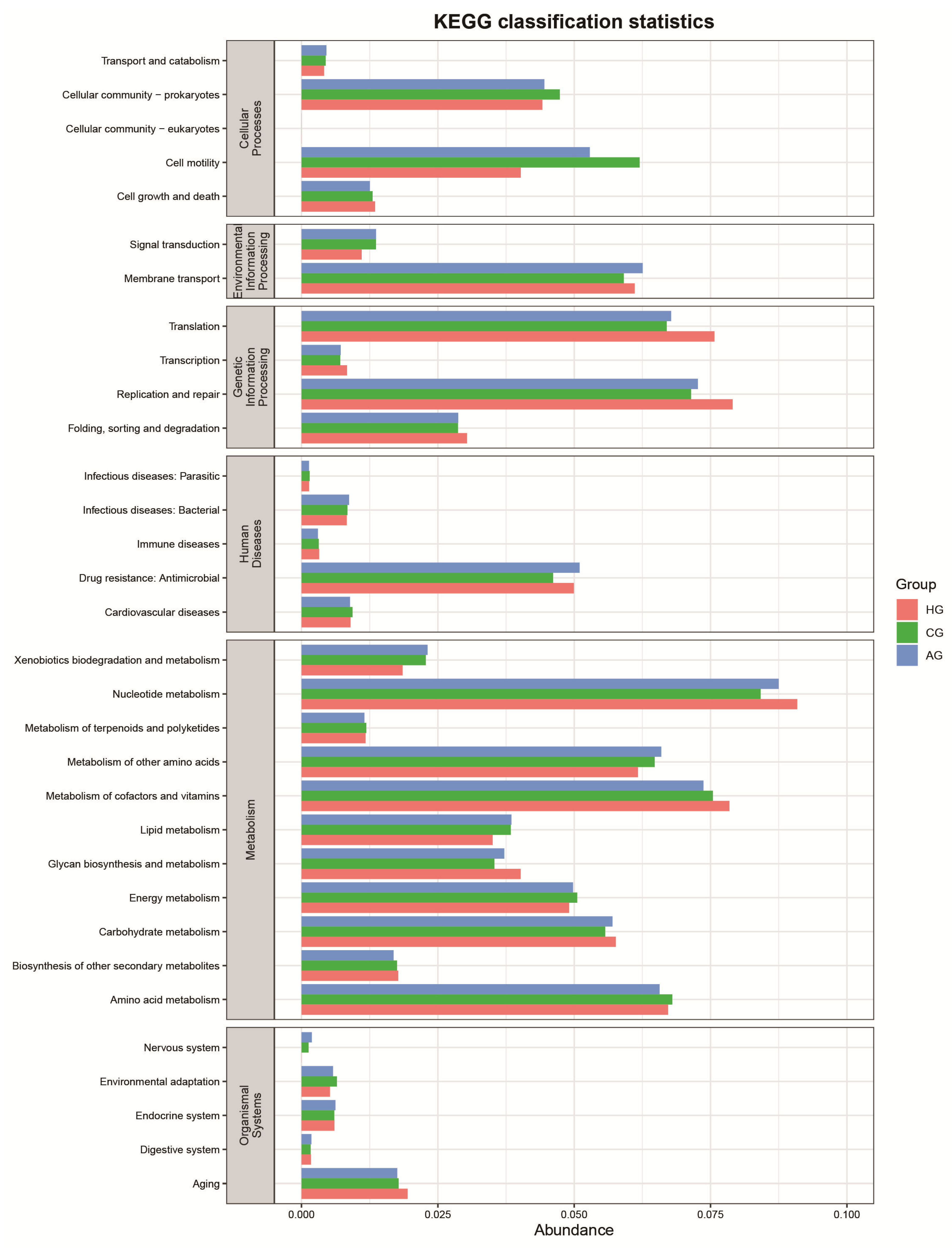
3.4. Function-Predictive Analysis of Microbial Community
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HP1, HP2, HP3: | The three samples from patients with no prostheses. |
| CPH, CPR, CPF: | The three samples from patients with porcelain crowns. |
| APH, APR, APF: | The three samples from patients with all-ceramic crowns. |
References
- McBain, A.J.; O’Neill, C.A.; Amezquita, A.; Price, L.J.; Faust, K.; Tett, A.; Segata, N.; Swann, J.R.; Smith, A.M.; Murphy, B.; et al. Consumer Safety Considerations of Skin and Oral Microbiome Perturbation. Clin. Microbiol. Rev. 2019, 32, e00051-19. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; Pippin, D.J. Implications of resource-ratio theory for oral microbial ecology. Eur. J. Oral Sci. 1998, 106, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Nelson, K.E.; Edlund, A. The oral host-microbial interactome: An ecological chronometer of health? Trends Microbiol. 2021, 29, 551–561. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Kolodny, O.; Callahan, B.J.; Douglas, A.E. The role of the microbiome in host evolution. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190588. [Google Scholar] [CrossRef]
- Colombo, A.; Tanner, A. The Role of Bacterial Biofilms in Dental Caries and Periodontal and Peri-implant Diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2016, 23, 276–286. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef]
- Baldi, A.; Comba, A.; Tempesta, R.M.; Carossa, M.; Pereira, G.K.R.; Valandro, L.F.; Paolone, G.; Vichi, A.; Goracci, C.; Scotti, N. External Marginal Gap Variation and Residual Fracture Resistance of Composite and Lithium-Silicate CAD/CAM Overlays after Cyclic Fatigue over Endodontically-Treated Molars. Polymers 2021, 13, 3002. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Pfeifer, C.; Khajotia, S.; Ferracane, J. Interaction between the Oral Microbiome and Dental Composite Biomaterials: Where We Are and Where We Should Go. J. Dent. Res. 2020, 99, 1140–1149. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Do dental resin composites accumulate more oral biofilms and plaque than amalgam and glass ionomer materials? Materials 2016, 9, 888. [Google Scholar] [CrossRef]
- Conrads, G.; Wendt, L.K.; Hetrodt, F.; Deng, Z.-L.; Pieper, D.; Abdelbary, M.M.H.; Barg, A.; Wagner-Döbler, I.; Apel, C. Deep sequencing of biofilm microbiomes on dental composite materials. J. Oral Microbiol. 2019, 11, 1617013. [Google Scholar] [CrossRef]
- Overmann, J.; Abt, B.; Sikorski, J. Present and Future of Culturing Bacteria. Annu. Rev. Microbiol. 2017, 71, 711–730. [Google Scholar] [CrossRef]
- Anderson, I.C.; Cairney, J.W.G. Diversity and ecology of soil fungal communities: Increased understanding through the application of molecular techniques. Environ. Microbiol. 2004, 6, 769–779. [Google Scholar] [CrossRef]
- Jo, J.; Oh, J.; Park, C. Microbial community analysis using high-throughput sequencing technology: A beginner’s guide for microbiologists. J. Microbiol. 2020, 58, 176–192. [Google Scholar] [CrossRef]
- Sierra, M.A.; Li, Q.; Pushalkar, S.; Paul, B.; Sandoval, T.A.; Kamer, A.R.; Corby, P.; Guo, Y.; Ruff, R.R.; Alekseyenko, A.V.; et al. The Influences of Bioinformatics Tools and Reference Databases in Analyzing the Human Oral Microbial Community. Genes 2020, 11, 878. [Google Scholar] [CrossRef]
- Lawley, B.; Tannock, G.W. Analysis of 16S rRNA Gene Amplicon Sequences Using the QIIME Software Package. Methods Mol. Biol. 2016, 1537, 153–163. [Google Scholar] [CrossRef]
- Balvočiūtė, M.; Huson, D.H. SILVA, RDP, Greengenes, NCBI and OTT—How do these taxonomies compare? BMC Genom. 2017, 18, 114. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Q.; Cole, J.R.; Rosen, G.L. Using the RDP Classifier to Predict Taxonomic Novelty and Reduce the Search Space for Finding Novel Organisms. PLoS ONE 2012, 7, e32491. [Google Scholar] [CrossRef]
- Chan, B.K.C. Data Analysis Using R Programming. Adv. Exp. Med. Biol. 2018, 1082, 47–122. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Abu-Izze, F.; Ramos, G.; Borges, A.; Anami, L.; Bottino, M. Fatigue behavior of ultrafine tabletop ceramic restorations. Dent. Mater. 2018, 34, 1401–1409. [Google Scholar] [CrossRef]
- Falahchai, M.; Babaee, H.Y.; Neshandar, A.H.; Neshandar, A.M. Marginal adaptation of zirconia-reinforced lithium silicate overlays with different preparation designs. J. Esthet. Restor. Dent. 2020, 32, 823–830. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Zhang, Y.-L.; Geng, F.-H. Clinical efficacy and effects of CAD/CAM zirconia all-ceramic crown and metal-ceramic crown restoration on periodontal tissues. Shanghai Kou Qiang Yi Xue Shanghai J. Stomatol. 2017, 26, 331–335. [Google Scholar]
- Peraire, M.; Martinez-Gomis, J.; Anglada, J.M.; Bizar, J.; Salsench, J.; Gil, F.J. Effects of recasting on the chemical composition, microstructure, microhardness, and ion release of 3 dental casting alloys and titanium. Int. J. Prosthodont. 2007, 20, 286–288. [Google Scholar]
- Devlin, R.D.; Reddy, S.V.; Savino, R.; Ciliberto, G.; Roodman, G.D. IL-6 Mediates the Effects of IL-1 or TNF, but Not PTHrP or 1,25(OH)2D3, on Osteoclast-like Cell Formation in Normal Human Bone Marrow Cultures. J. Bone Miner. Res. 1998, 13, 393–399. [Google Scholar] [CrossRef]
- Graves, D.; Cochran, D. The Contribution of Interleukin-1 and Tumor Necrosis Factor to Periodontal Tissue Destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Deinzer, R.; Mossanen, B.S.; Herforth, A. Methodological considerations in the assessment of gingival crevicular fluid volume. J. Clin. Periodontol. 2000, 27, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, A.R.A.; Idris, G.; Hajeer, M.Y.; Alawer, K.; Cannon, R.D. Efficacy of removing Candida albicans from orthodontic acrylic bases: An in vitro study. BMC Oral Health 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the healthy "core microbiome" of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Yu, W.-Q.; Tian, F.; Guo, X.-K.; Zhang, F.-Q.; Ye, D.-X. Changes of oral bacteria flora after wearing complete denture. Shanghai Kou Qiang Yi Xue Shanghai J. Stomatol. 2018, 27, 56–60. [Google Scholar]
- Jaeger, N.; McDonough, R.T.; Rosen, A.L.; Hernandez-Leyva, A.; Wilson, N.G.; Lint, M.A.; Russler-Germain, E.V.; Chai, J.N.; Bacharier, L.B.; Hsieh, C.S.; et al. Airway microbiota-host interactions regulate secretory leukocyte protease inhibitor levels and influence allergic airway inflammation. Cell Rep. 2020, 33, 108331. [Google Scholar] [CrossRef]
- Campos, M.S.; Marchini, L.; Bernardes, L.A.S.; Paulino, L.C.; Nobrega, F.G. Biofilm microbial communities of denture stomatitis. Oral Microbiol. Immunol. 2008, 23, 419–424. [Google Scholar] [CrossRef]
- Zhang, S.-M.; Qiu, J.; Tian, F.; Guo, X.-K.; Zhang, F.-Q.; Huang, Q.-F. Corrosion behavior of pure titanium in the presence of Actinomyces naeslundii. J. Mater. Sci. Mater. Med. 2013, 24, 1229–1237. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Gill, S.R.; Iobst, S.E.; Vickerman, M.M.; Kolenbrander, P.E. Regulation of Gene Expression in a Mixed-Genus Community: Stabilized Arginine Biosynthesis in Streptococcus gordonii by Coaggregation with Actinomyces naeslundii. J. Bacteriol. 2008, 190, 3646–3657. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Belda-Ferre, P.; Simón-Soro, A.; Mira, A. Microbiota diversity and gene expression dynamics in human oral biofilms. BMC Genom. 2014, 15, 311. [Google Scholar] [CrossRef]
- Barken, K.B.; Pamp, S.J.; Yang, L.; Gjermansen, M.; Bertrand, J.J.; Klausen, M.; Givskov, M.; Whitchurch, C.B.; Engel, J.N.; Tolker-Nielsen, T. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2008, 10, 2331–2343. [Google Scholar] [CrossRef]
- Tatullo, M.; Genovese, F.; Aiello, E.; Amantea, M.; Makeeva, I.; Zavan, B.; Rengo, S.; Fortunato, L. Phosphorene Is the New Graphene in Biomedical Applications. Materials 2019, 12, 2301. [Google Scholar] [CrossRef]
- Tatullo, M.; Zavan, B.; Genovese, F.; Codispoti, B.; Makeeva, I.; Rengo, S.; Fortunato, L.; Spagnuolo, G. Borophene Is a Promising 2D Allotropic Material for Biomedical Devices. Appl. Sci. 2019, 9, 3446. [Google Scholar] [CrossRef]




| Characteristics | HG (n = 3) | CG (n = 3) | AG (n = 3) | p-Value |
|---|---|---|---|---|
| Gender (M/F) | 1/2 | 2/1 | 1/2 | |
| Age (Mean ± SD (years)) | 39.00 ± 8.72 | 43.67 ± 9.29 | 42.33 ± 11.93 | 0.847 |
| Number of missing teeth (Mean ± SD) | 24.33 ± 2.08 | 23.67 ± 1.53 | 23.33 ± 2.08 | 0.815 |
| DMFT (Mean ± SD) | 1.00 ± 1.00 | 1.33 ± 1.15 | 1.33 ± 1.53 | 0.932 |
| Number of detected gingival-bleeding teeth (Mean ± SD) | 3.00 ± 3.00 | 4.33 ± 2.08 | 4.00 ± 2.00 | 0.787 |
| Number of detected periodontal-pocket teeth (Mean ± SD) | 0.33 ± 0.58 | 0.67 ± 0.58 | 0.67 ± 1.15 | 0.850 |
| Number of detected attachment-loss teeth | 0 | 0 | 0 |
| Group | Valid CCS Sequences | OTUs | chao1 | Shannon |
|---|---|---|---|---|
| HG | 13,996 ± 2896 | 155 ± 41 | 186.5 ± 51.8 | 4.6 ± 0.8 |
| CG | 10,718 ± 891 | 187 ± 17 | 234.8 ± 53.2 | 4.4 ± 1.2 |
| AG | 10,718 ± 891 | 138 ± 23 | 170.9 ± 26.6 | 4.4 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Zhang, Z.; Lu, J.; Wang, D.; Yan, Y.; Zhang, S.; Yu, X.; Su, S.; Yuan, L.; Li, Z.; et al. Differences in Supragingival Microbiome in Patients with and without Full-Crown Prostheses. Dent. J. 2022, 10, 152. https://doi.org/10.3390/dj10080152
Guo M, Zhang Z, Lu J, Wang D, Yan Y, Zhang S, Yu X, Su S, Yuan L, Li Z, et al. Differences in Supragingival Microbiome in Patients with and without Full-Crown Prostheses. Dentistry Journal. 2022; 10(8):152. https://doi.org/10.3390/dj10080152
Chicago/Turabian StyleGuo, Manli, Zhidong Zhang, Jiyuan Lu, Di Wang, Yimin Yan, Shen Zhang, Xin Yu, Songhua Su, Lu Yuan, Zhige Li, and et al. 2022. "Differences in Supragingival Microbiome in Patients with and without Full-Crown Prostheses" Dentistry Journal 10, no. 8: 152. https://doi.org/10.3390/dj10080152
APA StyleGuo, M., Zhang, Z., Lu, J., Wang, D., Yan, Y., Zhang, S., Yu, X., Su, S., Yuan, L., Li, Z., & Zhang, B. (2022). Differences in Supragingival Microbiome in Patients with and without Full-Crown Prostheses. Dentistry Journal, 10(8), 152. https://doi.org/10.3390/dj10080152





