Bioengineering Tools Applied to Dentistry: Validation Methods for In Vitro and In Silico Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Source Selection
2.2. Data Source
3. Results
4. Literature Review
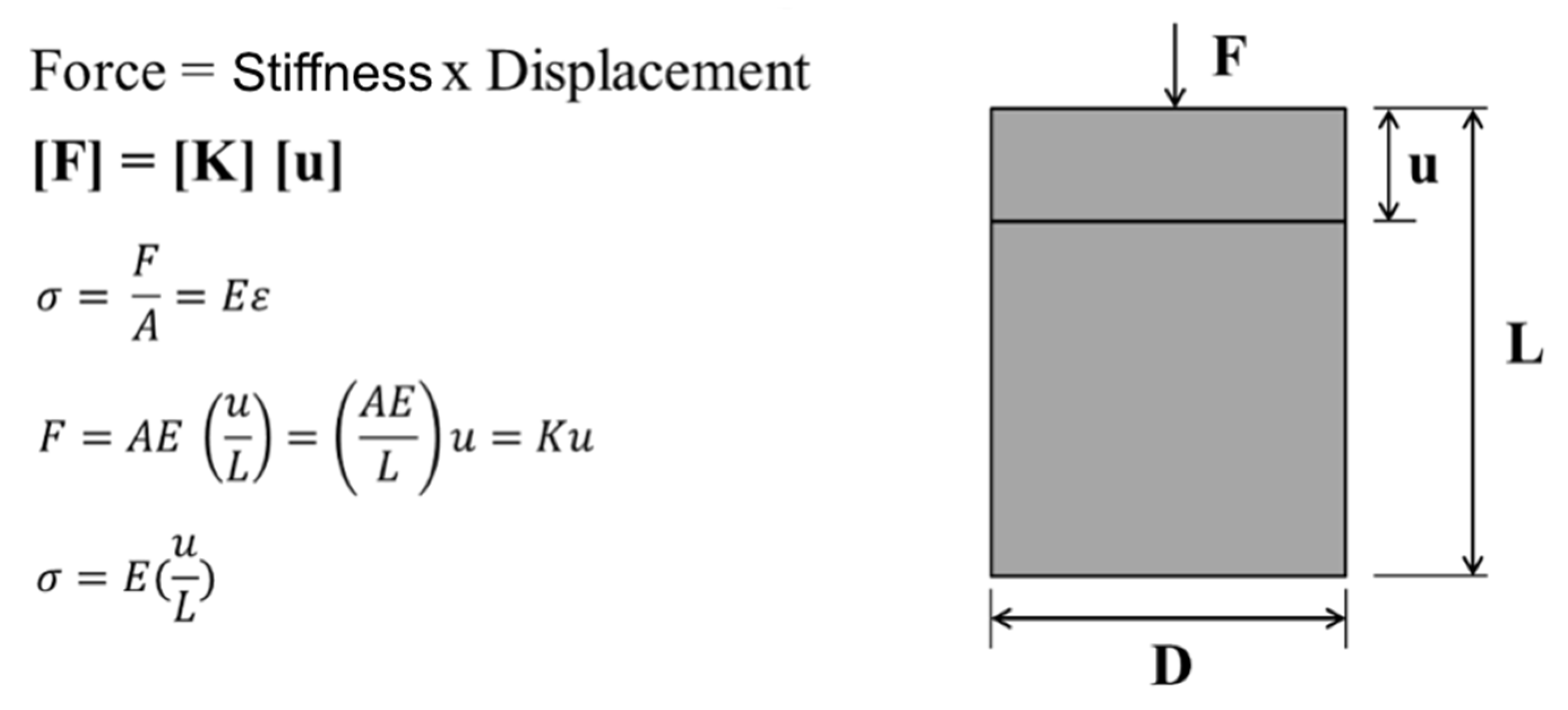
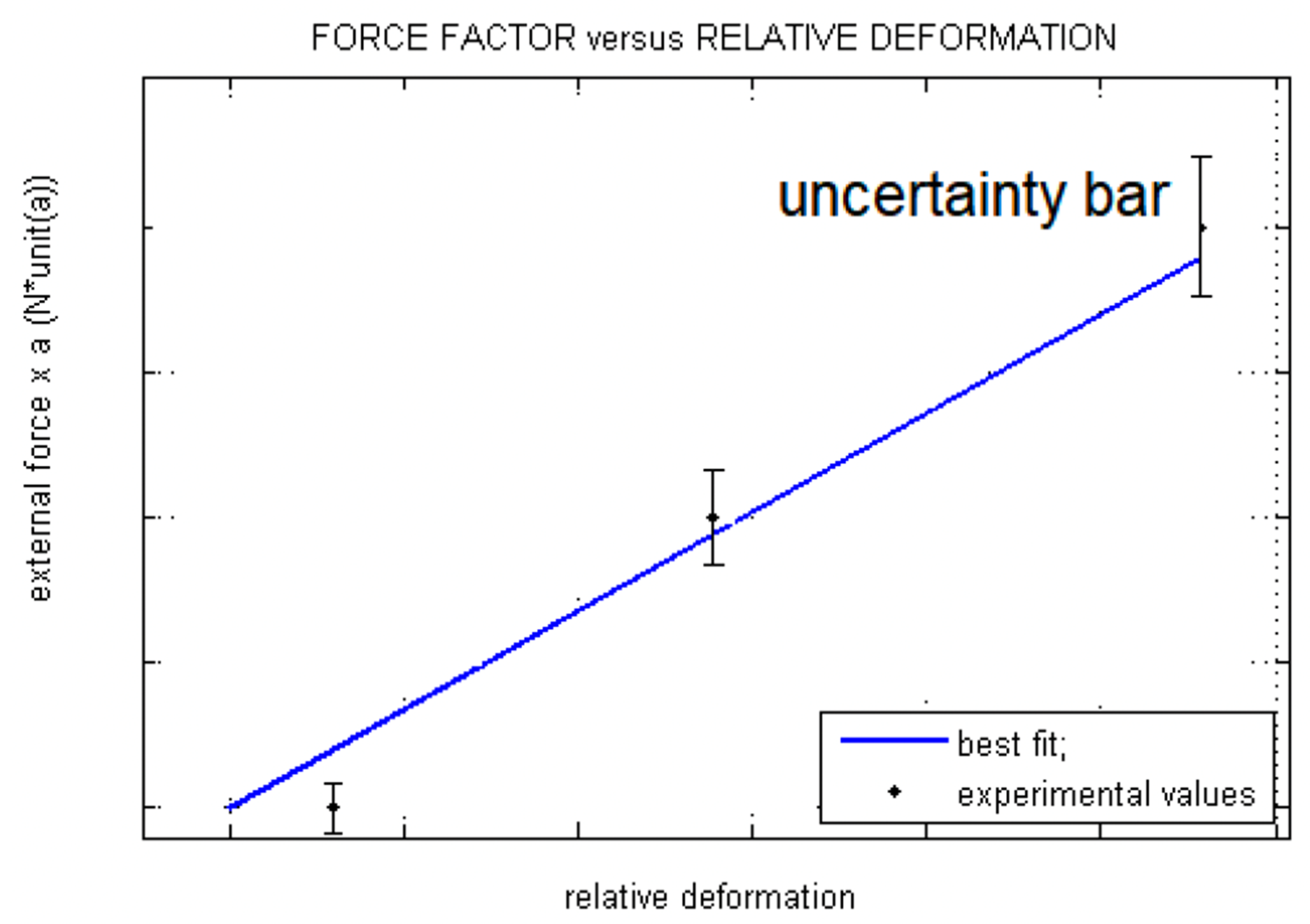
4.1. Measure Strain and Microstrain
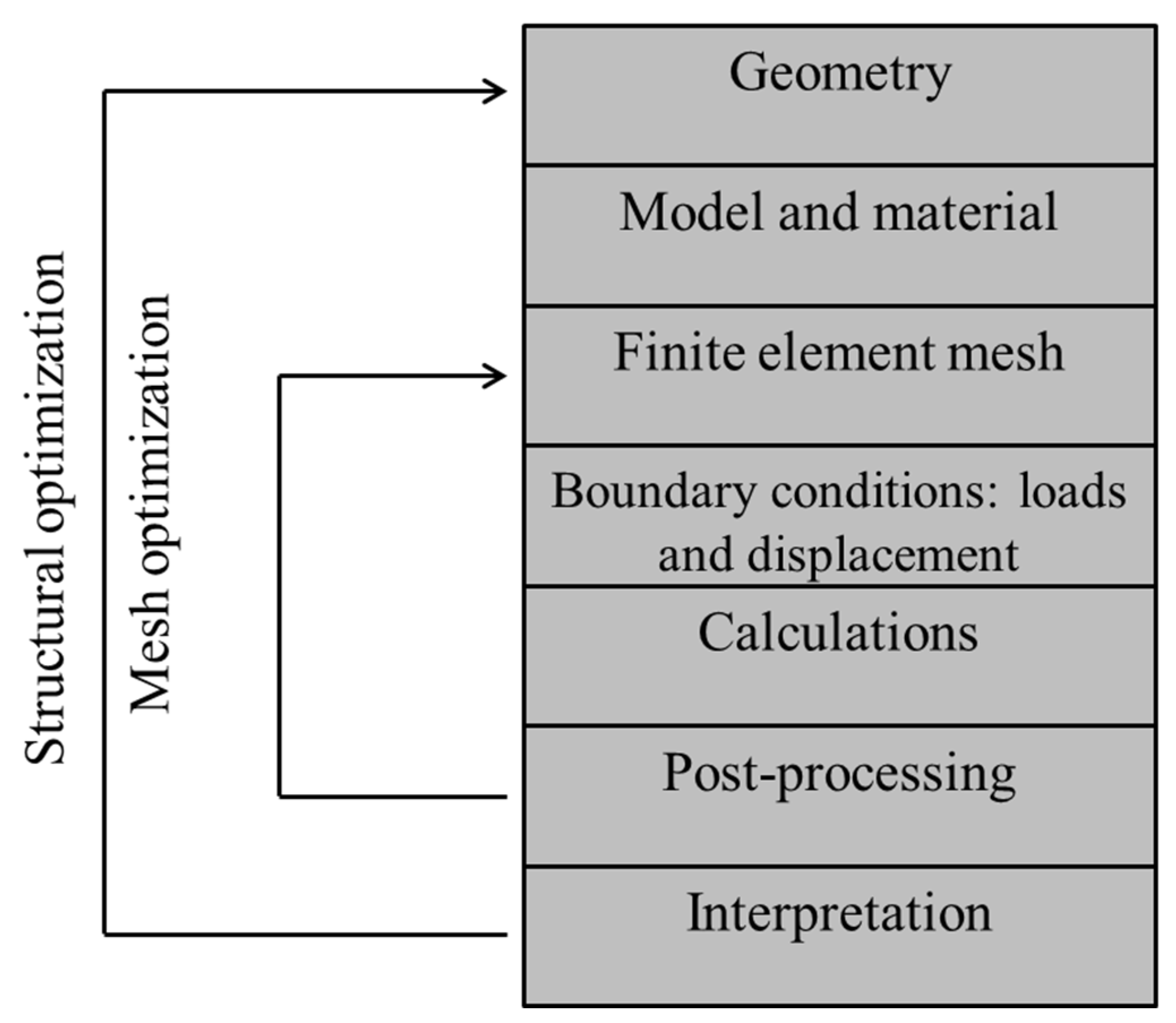
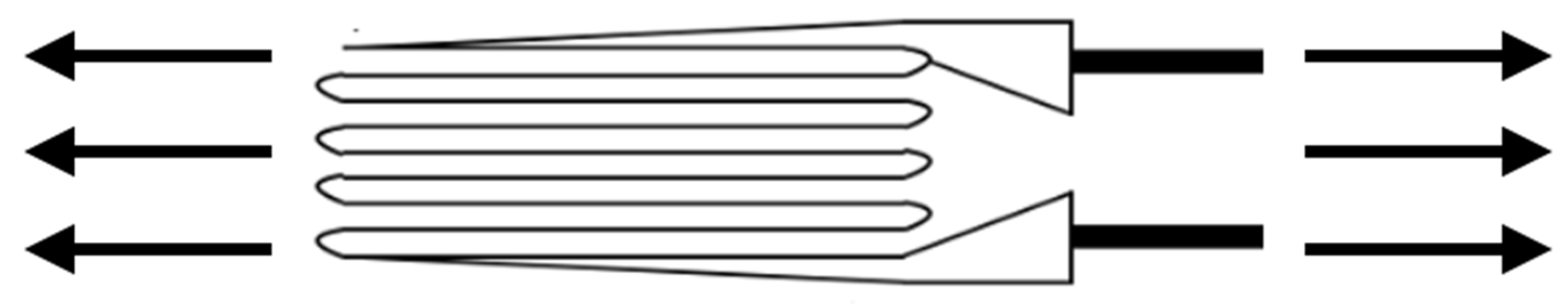
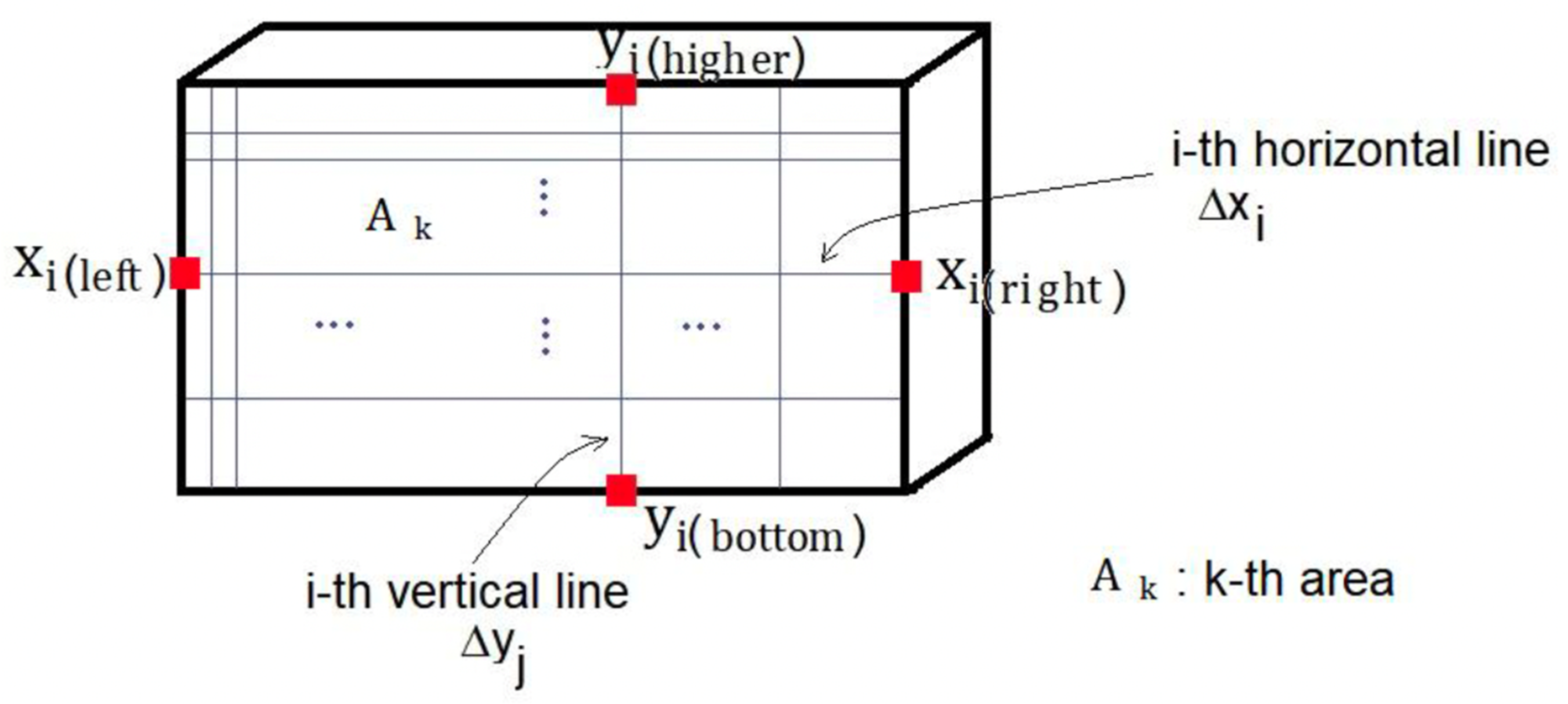
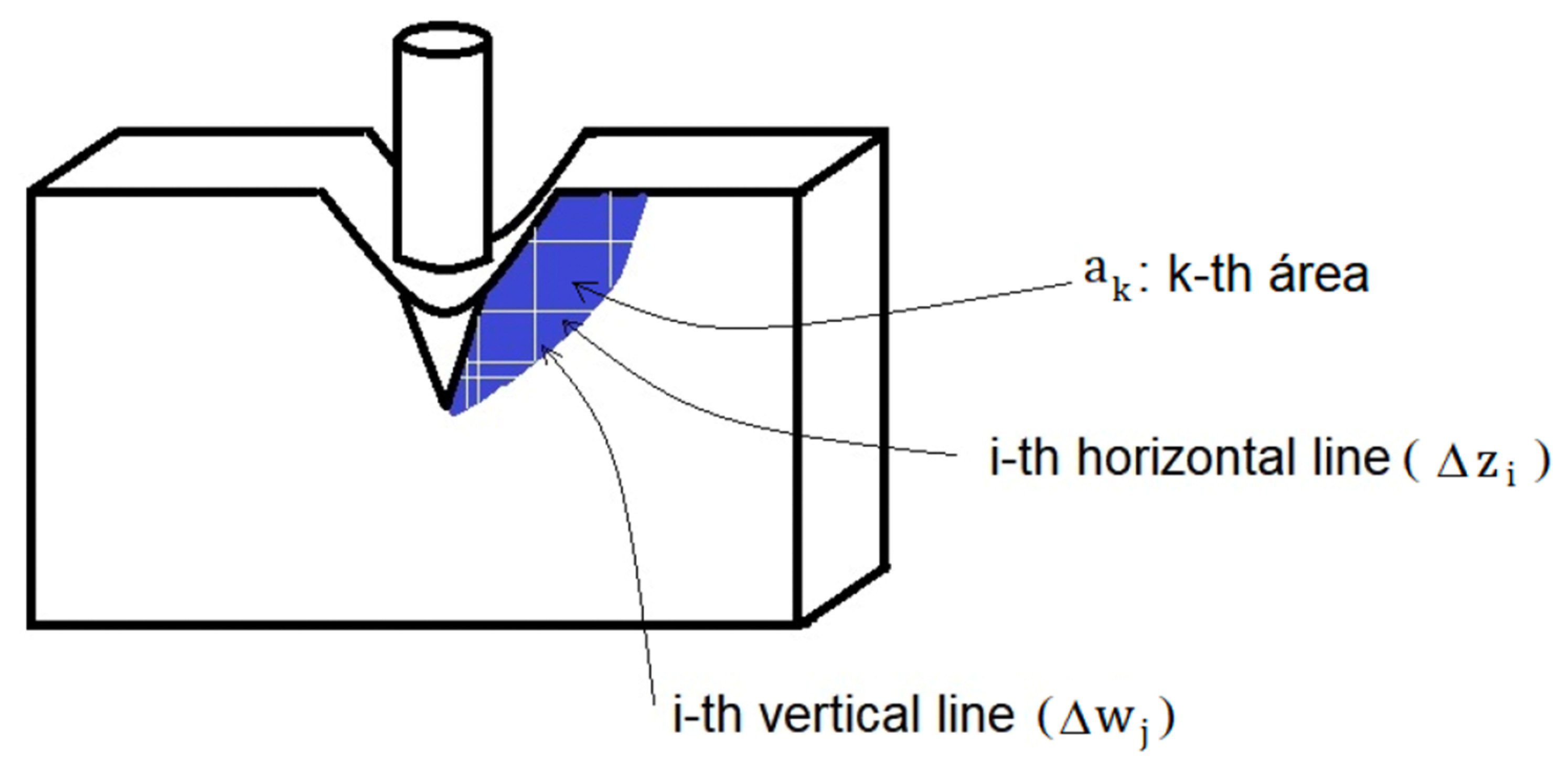
4.2. Finite Element Method or Analysis
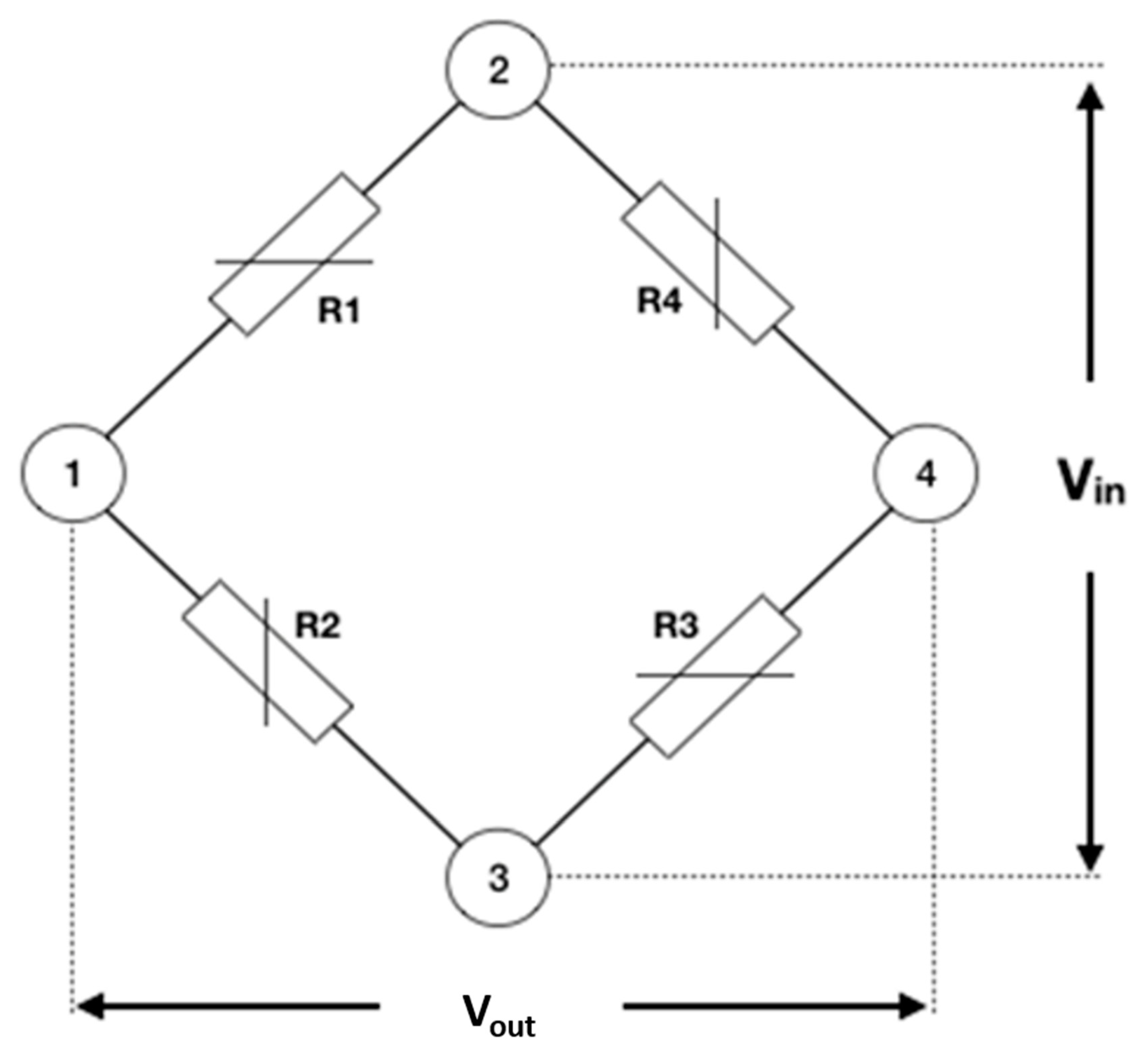
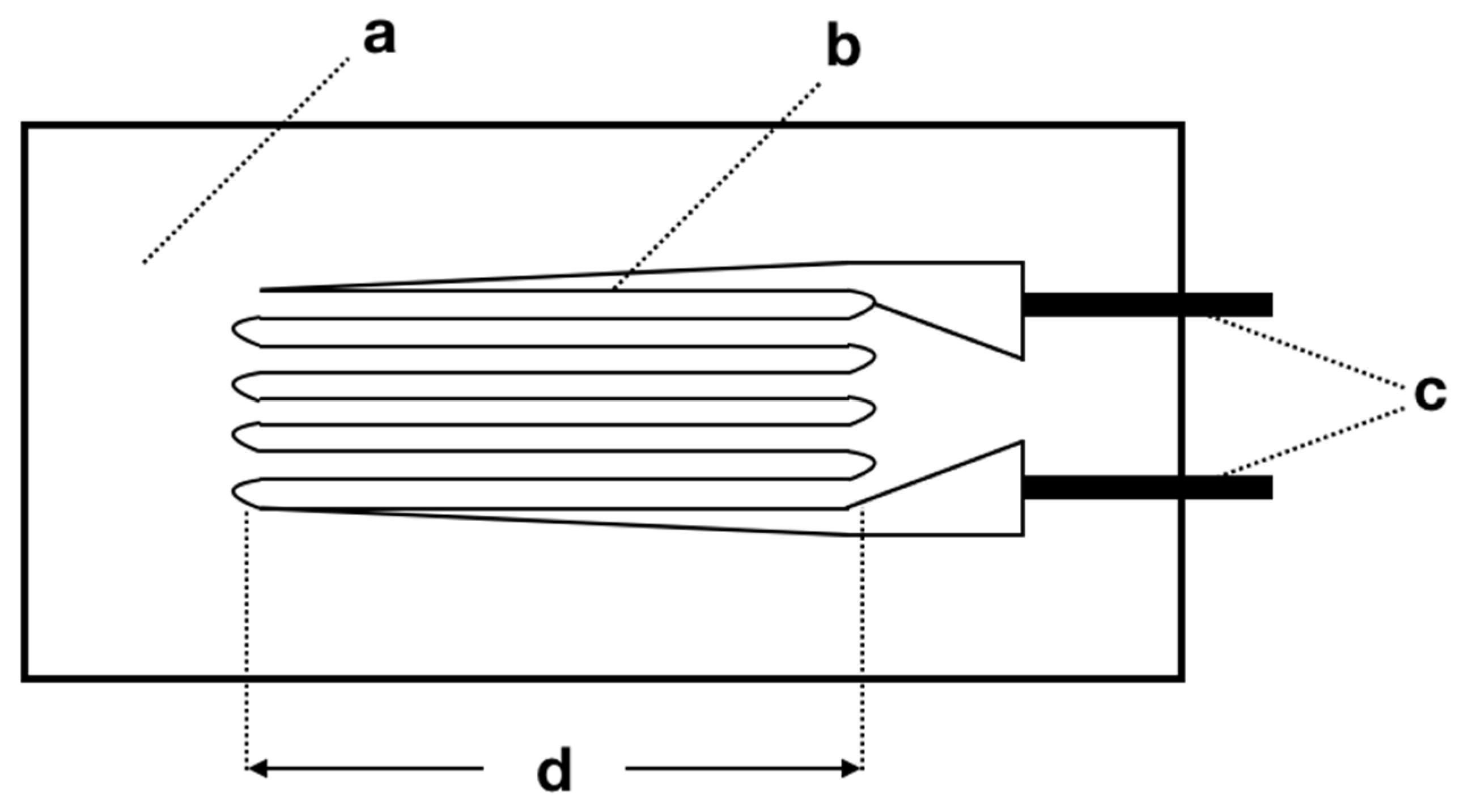
4.3. Strain Gauge
4.4. Photoelastic Analysis
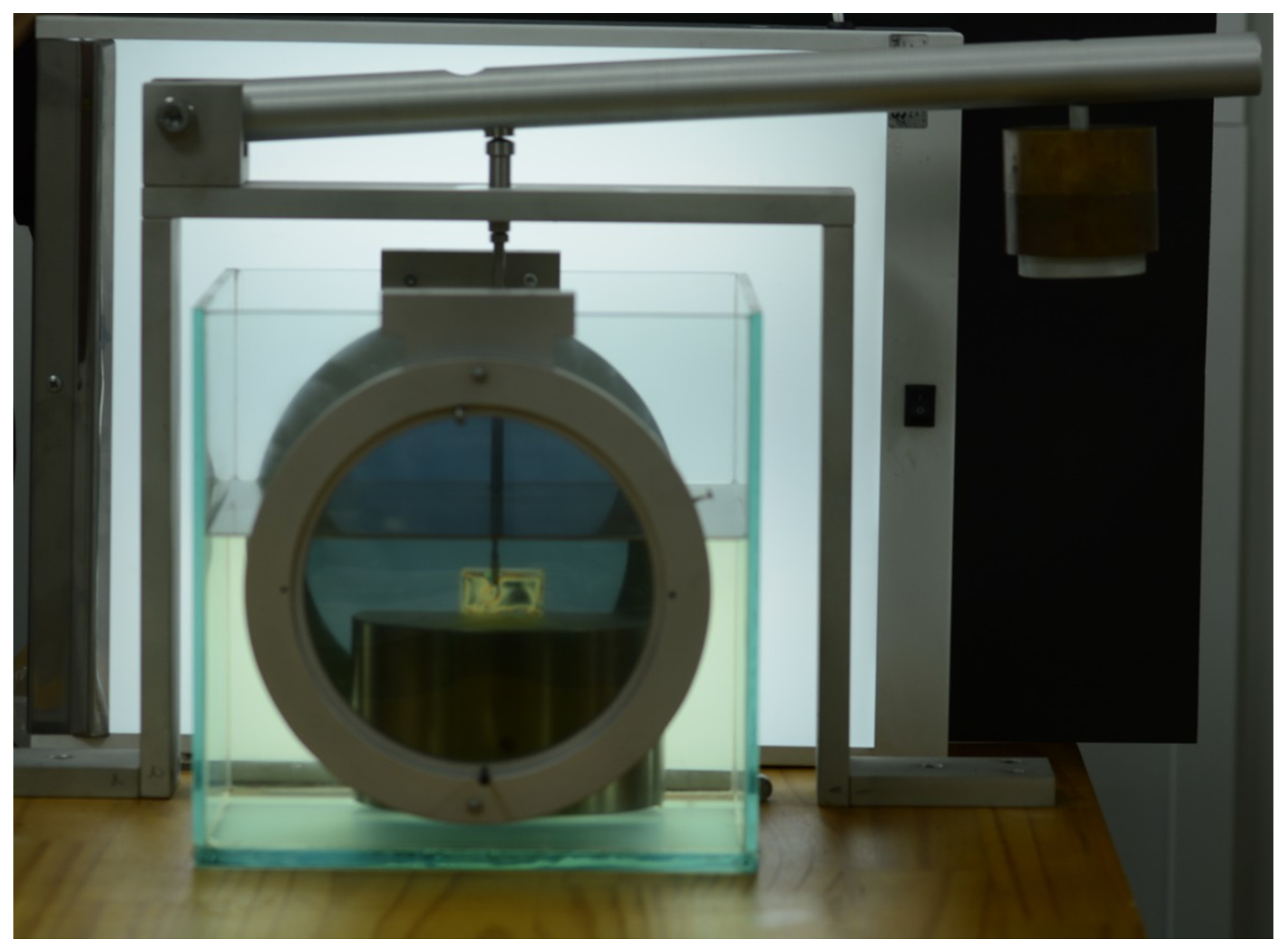
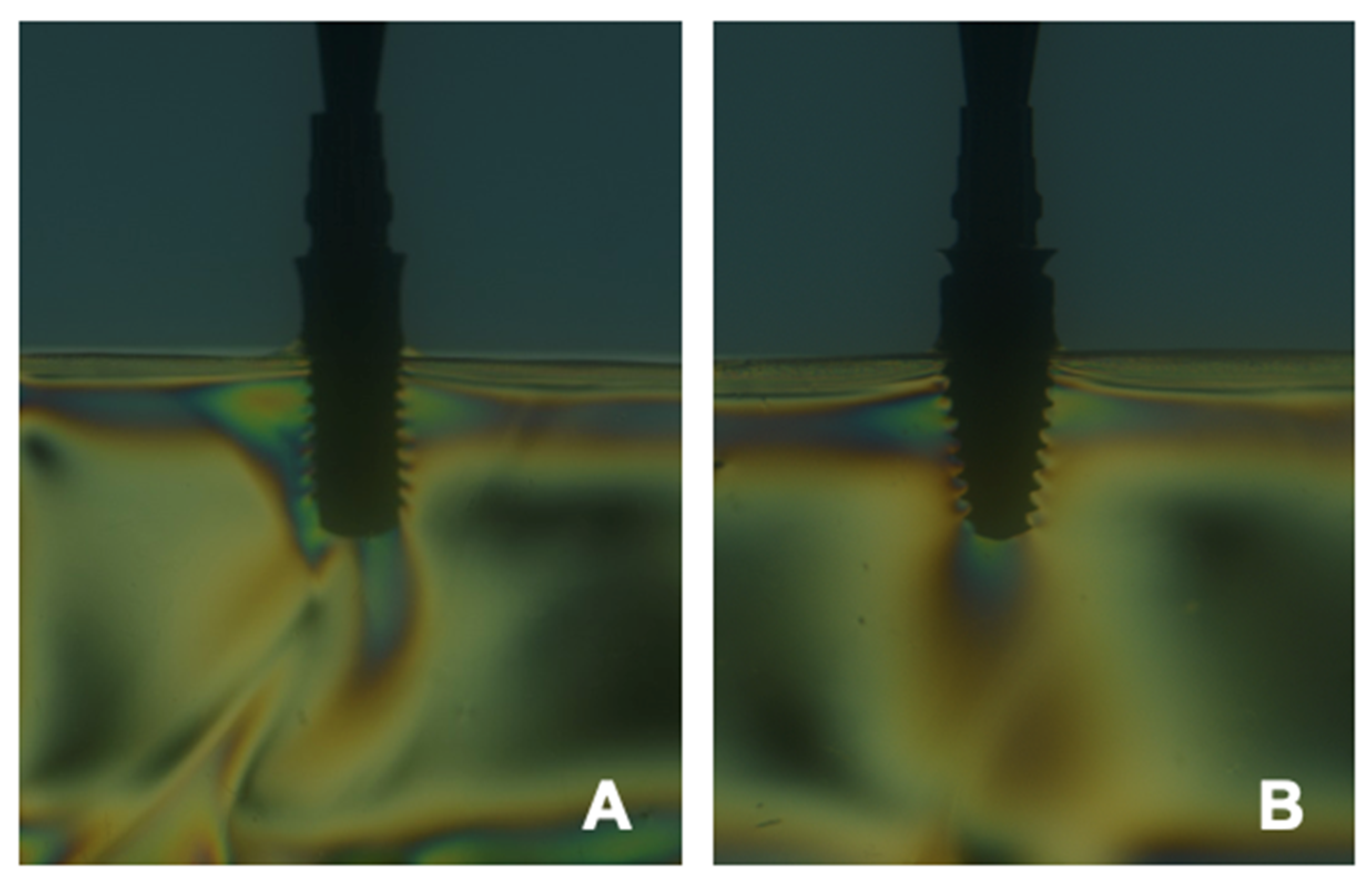
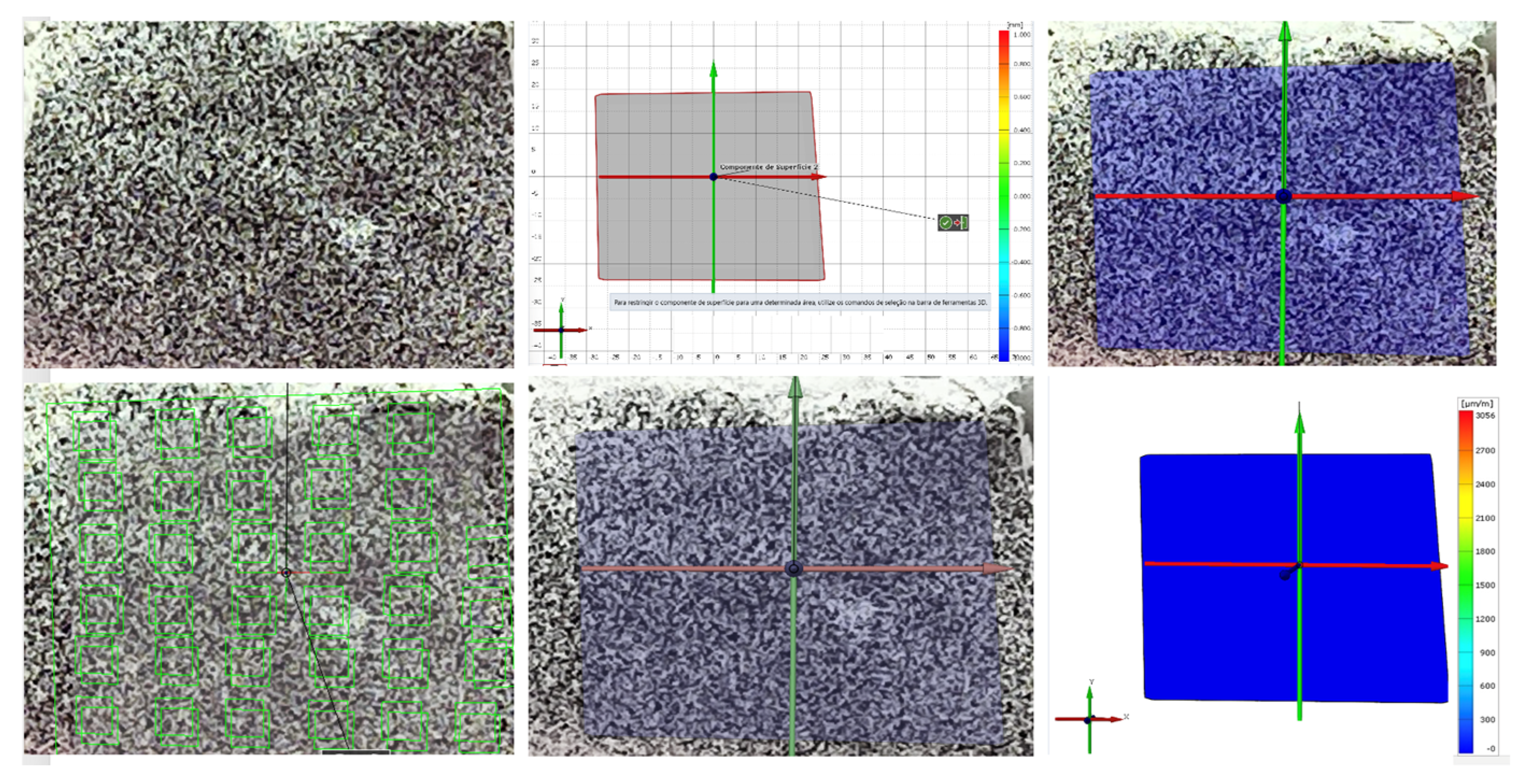
4.5. Digital Image Correlation (DIC)
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, C.J.; Raposo, L.H.; Soares, P.V.; Santos-Filho, P.C.; Menezes, M.S.; Soares, P.B.; Magalhães, D. Effect of different types of cement on the biomechanical behavior of teeth restored with cast dowel-and-cores-in Vitro and FEA analysis. J. Prosthodont. 2010, 19, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Jemt, T.; Carlsson, L.; Boss, A.; Jörneús, L. In vivo load measurements on osseointegrated implants supporting fixed or removable prostheses: A comparative pilot study. Int. J. Oral Maxillofac. Implant. 1991, 6, 413–417. [Google Scholar]
- Glantz, P.O.; Rangert, B.; Svensson, A.; Stafford, G.D.; Arnvidarson, B.; Randow, K.; Lindén, U.; Hultén, J. On clinical loading of osseointegrated implants. A methodological and clinical study. Clin. Oral Implant. Res. 1993, 4, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Brosh, T.; Pilo, R.; Sudai, D. The influence of abutment angulation on strains and stresses along the implant/bone interface: Comparison between two experimental techniques. J. Prosthet. Dent. 1998, 79, 328–334. [Google Scholar] [CrossRef]
- Brito, J.V.C.; Garcia, D.C.; Crispim, S.S.; Matos, J.D.M.; Figueiredo, V.M.G. Application of finite elements in dentistry: A literature review. J. Dent. Public Health. 2017, 8, 77–80. [Google Scholar]
- Lopes, G.R.; Freitas, V.P.; Matos, J.D.; Andrade, V.C.; Nishioka, R.S.; Casas, E.B. Stress distribution in dental roots restored with different post and core materials. J. Int. Oral Health 2019, 11, 127–131. [Google Scholar] [CrossRef]
- Gharechahi, J.; Sharifi, E.; Nosohian, S.; Aghdaee, N.A. Finite element method analysis of stress distribution to supporting tissues in class IV are many removable partial denture (Part II: Bone and mucosal membrane). J. Contemp. Dent. Pract. 2008, 9, 49–56. [Google Scholar] [CrossRef]
- Sandino, C.; McErlain, D.D.; Schipilow, J.; Boyd, S.K. Mechanical stimuli of trabecular bone in osteoporosis: A numerical simulation by finite element analysis of microarchitecture. J. Mech. Behav. Biomed. Mater. 2017, 66, 19–27. [Google Scholar] [CrossRef]
- Pournasrollah, A.; Negahdari, R.; Gharekhani, V.; Torab, A.; Jannati Ataei, S. Investigating the effect of abutment-implant connection type on abutment screw loosening in a dental implant system using finite element methods. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 289–297. [Google Scholar] [CrossRef]
- Ding, X.; Liao, S.H.; Zhu, X.H.; Zhang, X.H.; Zhang, L. Effect of diameter and length on stress distribution of the alveolar crest around immediate loading implants. Clin. Implant Dent. Relat. Res. 2009, 11, 279–287. [Google Scholar] [CrossRef]
- Tiossi, R.; de Torres, E.M.; Rodrigues, R.C.; Conrad, H.J.; de Mattos, M.d.G.C.; Fok, A.S.; Ribeiro, R.F. Comparison of the correlation of photoelasticity and digital imaging to characterize the load transfer of implant-supported restorations. J. Prosthet. Dent. 2014, 112, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Sotto-Maior, B.S.; Lima, C.A.; Senna, P.M.; Camargos, G.V.; Del Bel Cury, A.A. Biomechanical evaluation of subcrestal dental implants with different bone anchorages. Braz. Oral Res. 2014, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, R.S.; Vasconcellos, L.G.O.; Nishioka, G.N. Comparative strain gauge analysis of external and internal hexagon, Morse taper, and influence of straight and offset implant configuration. Implant Dent. 2011, 20, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Pesqueira, A.A.; Goiato, M.C.; Filho, H.G.; Monteiro, D.R.; Santos, D.M.; Haddad, M.F.; Pellizzer, E.P. Use of stress analysis methods to evaluate the biomechanics of oral rehabilitation with implants. J. Oral Implantol. 2014, 40, 217–228. [Google Scholar] [CrossRef]
- Marsico, V.S.; Lehmann, R.B.; Assis Claro, C.A.; Amaral, M.; Vitti, R.P.; Neves, A.C.C.; Silva Concilio, L.R. Three-dimensional finite element analysis of occlusal splint and implant connection on stress distribution in implant–supported fixed dental prosthesis and peri-implantal bone. Mater. Sci. Eng. C 2017, 80, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.J.R.; Rocha, E.P.; Santos, P.H.; Ko, C.C.; Martin, M., Jr.; de Almeida, E.O. Mechanics of the maxillary central incisor. Influence of the periodontal ligament represented by beam elements. Comput. Methods Biomech. Biomed. Eng. 2010, 13, 515–521. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; Dal Piva, A.M.O.; Borges, A.L.S.; Bottino, M.A. Influence of Socket-shield technique on the biomechanical response of dental implant: Three-dimensional finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 224–231. [Google Scholar] [CrossRef]
- Nishioka, R.S.; Vasconcellos, L.G.O.; Abreu, C.W. A comparative study of machined copings and plastic copings in three-element prostheses with different types of implant-abutment-joint, strain gauge analysis. J. Appl. Oral. Sci. 2010, 18, 225–230. [Google Scholar] [CrossRef]
- Morrel, R. Measuring Elastic Properties of Advanced Technical Ceramics—A review. UK Natl. Phys. Lab. Rep. 1996, 42, 41. [Google Scholar]
- Wakabayashi, N.; Ona, M.; Suzuki, T.; Igarashi, Y. Nonlinear finite element analyses: Advances and challenges in dental applications. J. Dent. 2008, 36, 463–471. [Google Scholar] [CrossRef]
- Miyashiro, M.; Suedam, V.; Moretti Neto, R.T.; Ferreira, P.M.; Rubo, J.H. Validation of an experimental polyurethane model for biomechanical studies on implant supported prosthesis—Tension tests. J. Appl. Oral Sci. 2011, 19, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Madfa, A.A.; Kadir, M.R.; Kashani, J.; Saidin, S.; Sulaiman, E.; Marhazlinda, J.; Rahbari, R.; Abdullah, B.J.; Abdullah, H.; Abu Kasim, N.H. Stress distributions in maxillary central incisors restored with various types of post materials and designs. Med. Eng. Phys. 2014, 36, 962–967. [Google Scholar] [CrossRef]
- Tribst, J.P.; Rodrigues, V.A.; Dal Piva, A.O.; Borges, A.L.; Nishioka, R.S. The importance of correct implants positioning and masticatory load direction on a fixed prosthesis. J. Clin. Exp. Dent. 2018, 10, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Toniollo, M.B.; Macedo, A.P.; Pupim, D.; Zaparolli, D.; Mattos, M.G.C. Finite element analysis of bone stress in the posterior mandible using regular and short implants, in the same context, with splinted and non-splinted pros-theses. Int. J. Oral Maxillofac. Implants 2017, 32, 199–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Datte, C.E.; Tribst, J.P.; Dal Piva, A.O.; Nishioka, R.S.; Bottino, M.A.; Evangelhista, A.M.; Monteiro, F.M.M.; Borges, A.L. Influence of different restorative materials on the stress distribution in dental implants. J. Clin. Exp. Dent. 2018, 10, 439–444. [Google Scholar] [CrossRef]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Saab, X.E.; Griggs, J.A.; Powers, J.M.; Engelmeier, R.L. Effect of abutment angulation on the strain on the bone around an implant in the anterior maxilla: A finite element study. J. Prosthet. Dent. 2007, 97, 85–92. [Google Scholar] [CrossRef]
- Tesk, J.A.; Widera, O. Stress distribution in bone arising from loading on endosteal dental implants. J. Biomed. Mater. Res. 1973, 7, 251–261. [Google Scholar] [CrossRef]
- Ye, C.; Wang, Y.Q. On the use of Chebyshev polynomials in the Rayleigh-Ritz method for vibration and buckling analyses of circular cylindrical three-dimensional graphene foam shells. Mech. Based Des. Struct. Mach. 2019, 49, 932–946. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, L.; Zhang, W.; Lei, J. Nonlinear oscillations, bifurcations and chaos of functionally graded materials plate. J. Sound Vib. 2008, 312, 862–892. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, W.; Yang, J. Nonlinear oscillation of a cantilever FGM rectangular plate based on thirdorder plate theory and asymptotic perturbation method. Compos. B Eng. 2011, 42, 402–413. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zu, J.W. Vibration behaviors of functionally graded rectangular plates with porosities and moving in thermal environment. Aerosp. Sci. Technol. 2017, 69, 550–562. [Google Scholar] [CrossRef]
- Hu, C.; Xue, J.; Dong, L.; Jiang, Y.; Wang, X.; Qu, L.; Dai, L. Scalable preparation of multifunctional fireretardant ultralight graphene foams. ACS Nano 2016, 10, 1325–1332. [Google Scholar] [CrossRef]
- Silva, L.S.; Jesus, M.A.; Silva, C.R.; Toffoli, D.J.; Camargo, U.N. Análise quantitativa de tensões em amostras fotoelásticas por meio de fotoelasticidade. Rev. Bras. Fís. Tecnol. Apl. 2017, 4, 34–51. [Google Scholar] [CrossRef]
- Wang, Y.Q. Electro-mechanical vibration analysis of functionally graded piezoelectric porous plates in the translation state. Acta Astronaut. 2018, 143, 263–271. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Huang, X.B.; Li, J. Hydroelastic dynamic analysis of axially moving plates in continuous hot-dip galvanizing process. Int. J. Mech. Sci. 2016, 110, 201–216. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ye, C.; Zu, J.W. Nonlinear vibration of metal foam cylindrical shells reinforced with graphene platelets. Aerosp. Sci. Technol. 2019, 85, 359–370. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zu, J.W. Nonlinear steady-state responses of longitudinally traveling functionally graded material plates in contact with liquid. Compos. Struct. 2017, 164, 130–144. [Google Scholar] [CrossRef]
- Zhang, W. Global and chaotic dynamics for a parametrically excited thin plate. J. Sound Vib. 2001, 239, 1013–1036. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, Y.; Guo, X.; Chen, L. Complicated nonlinear responses of a simply supported FGM rectangular plate under combined parametric and external excitations. Meccanica 2012, 47, 985–1014. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, Y.; Yang, J. Nonlinear dynamics of FGM circular cylindrical shell with clamped–clamped edges. Compos. Struct. 2012, 94, 1075–1086. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, T.; Xi, A.; Wang, Y.N. Resonant responses and chaotic dynamics of composite laminated circular cylindrical shell with membranes. J. Sound Vib. 2018, 423, 65–99. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, S.W.; Mao, J.J. Nonlinear radial breathing vibrations of CFRP laminated cylindrical shell with non-normal boundary conditions subjected to axial pressure and radial line load at two ends. Compos. Struct. 2018, 190, 52–78. [Google Scholar] [CrossRef]
- Clough, R.W. The finite element method in plane stress analysis. In Proceedings of the 2nd ASCE Conference on Electronic Computation, Pittsburgh, PA, USA, 8–9 September 1960. [Google Scholar]
- Clough, R.W. The finite element method in structural mechanics. In Stress Analysis; Zienkiewicz, O.C., Holister, G.S., Eds.; Wiley: London, UK, 1965; Volume 1, pp. 85–119. [Google Scholar]
- Lopes, G.D.R.S.; Matos, J.D.M.; Barbosa, G.P.S.; Rodrigues, A.G.; Nishioka, R.S.; Andrade, V.C.; Guerra, S.M.G. Etiología de las Pérdidas Dentales en Pacientes Rehabilitados con Prótesis sobre Implantes. Int. J. Odontostomatol. 2018, 12, 280–286. [Google Scholar] [CrossRef]
- Lopes, G.D.R.S.; Feitosa, A.C.R.; Suaid, F.F.; Matos, J.D.M.; Vasconcelos, J.E.L.; Vaz, S.L.A.; Andrade, V.C.; Nishioka, R.S.; Guerra, S.M.G. Evaluation of peri-implant condition in periodontally compromised patients. J. Indian Prosthodont. Soc. 2019, 19, 283–289. [Google Scholar] [CrossRef]
- Clough, R.W.; Penzien, J. Dynamics of Structures, 2nd ed.; MacGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Clough, R.W.; Wilson, E.L. Early finite element research at Berkeley. In Proceedings of the 5th US National Conference on Computational Mechanics, Boulder, CO, USA, 4–6 August 1999. [Google Scholar]
- Clough, R.W. The finite element method—A personal view of its original formulation. In From Finite Elements to the Troll Platform—The Ivar Holand 70th Anniversary Volume; Bell, K., Ed.; Tapir: Lofoten Islands, Norway, 1994; Volume 1, pp. 89–100. [Google Scholar]
- Beleza, S.C.A. Medição de Deformações Através da Técnica de Correlação Digital de Imagem. Master’s Thesis, Universidade Nova de Lisboa, Lisboa, Portugal, 2017. [Google Scholar]
- Rengo, S. Behavior of RTD fiber posts in finite element analysis (FEM) on three dimensional models. In Proceedings of the 3rd International Symposium, Margherita Ligure, Italy, 26–27 March 1999; Volume 1, pp. 20–27. [Google Scholar]
- Yu, W.J.; Kwon, T.Y.; Kyung, H.M.; Kim, K.H. An evaluation of localized debonding between fiber post and root canal wall by finite element simulation. Int. Endod. J. 2006, 39, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Sorrentino, R.; Apicella, D.; Valentino, B.; Ferrari, M.; Aversa, R.; Apicella, A. Evaluation of the biomechanical behavior of maxillary central incisors restored by means of endocrowns compared to a natural tooth: A 3D static linear finite elements analysis. Dent. Mater. 2006, 22, 1035–1044. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; Dal Piva, A.M.O.; Bottino, M.A.; Nishioka, R.S.; Borges, A.L.S.; Özcan, M. Digital Image Correlation and Finite Element Analysis of Bone Strain Generated by Implant-Retained Cantilever Fixed Prosthesis. Eur. J. Prosthodont. Restor. Dent. 2020, 28, 10–17. [Google Scholar]
- Zhang, S.J.; Zeng, J.Y.; Li, J.; Zhang, R.; Yin, J.Y.; Wang, H. Photoelastic analysis of the influence of prosthetic material on the stress distribution in bone around implant. Zhonghua Kou Qiang Yi Xue Za Zhi. 2018, 53, 30–35. [Google Scholar]
- Li, L.L.; Wang, Z.Y.; Bai, Z.C.; Mao, Y.; Gao, B.; Xin, H.T.; Zhou, B.; Zhang, Y.; Liu, B. Three-dimensional finite element analysis of weakened roots restored with different cements in combination with titanium alloy posts. Chin. Med. J. 2006, 119, 305–311. [Google Scholar] [CrossRef]
- Palamara, J.E.; Wilson, P.R.; Thomas, C.D.; Messer, H.H. A new imaging technique for measuring the surface strains applied to dentine. J. Dent. 2000, 28, 141–146. [Google Scholar] [CrossRef]
- Pignataro, R.R.D.; Matos, J.D.M.; Ribeiro Pinto, I.L.R.; Samico, R.P.; Bottino, M.A.; Ramos, N.C.; Marinho, R.M.M. Survival Analysis and Torque Loosening of Experimental Screw After Mechanical Performance for Micro Conical Abutment. Int. J. Odontostomatol. 2022, 16, 27–32. [Google Scholar] [CrossRef]
- Clough, R.W. The finite element method after twenty-five years: A personal view. Comput. Struct. 1980, 12, 361–370. [Google Scholar] [CrossRef]
- Carr, A.J. A Refined Finite Element Analysis of Thin Shell Structures Including Dynamic Loadings. Ph.D. Thesis, University of California at Berkeley, Berkeley, CA, USA, 1968. [Google Scholar]
- Demircan, S.; Uretürk, E.U.; Apaydın, A.; Şen, S. Fixation Methods for Mandibular Advancement and Their Effects on Temporomandibular Joint: A Finite Element Analysis Study. Biomed. Res. Int. 2020, 2020, 2810763. [Google Scholar] [CrossRef]
- Cook, R.D.; Malkus, D.S.; Plesha, M.E. Concepts and Application of Finite Element Methods, 3rd ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Cools, R. Constructing cubature formulas—The science behind the art. Acta Numer. 1999, 6, 1–54. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.; Kwon, K.R.; Noh, G. Effects of cementless fixation of implant prosthesis: A finite element study. J. Adv. Prosthodont. 2019, 11, 341–349. [Google Scholar] [CrossRef]
- Bakitian, F.; Papia, E.; Larsson, C.; von Steyern, P.V. Evaluation of Stress Distribution in Tooth-Supported Fixed Dental Prostheses Made of Translucent Zirconia with Variations in Framework Designs: A Three-Dimensional Finite Element Analysis. J. Prosthodont. 2020, 29, 315–322. [Google Scholar] [CrossRef]
- Rezende, C.E.E.; Griggs, J.A.; Duan, Y.; Mushashe, A.M.; Nolasco, G.M.C.; Borges, A.F.S.; Rubo, J.H. An indirect method to measure abutment screw preload: A pilot study based on micro-CT scanning. Braz. Dent. J. 2015, 26, 596–601. [Google Scholar] [CrossRef]
- Wain, E.A. A method of measuring stress in dentures. Nature 1955, 175, 1082–1083. [Google Scholar] [CrossRef]
- Asvanund, P.A. Strain gauge analysis comparing external and internal implant-abutment connections. Implant Dent. 2014, 23, 206–211. [Google Scholar] [CrossRef]
- Rodrigues, V.A.; Tribst, J.P.M.; Santis, L.R.; Lima, D.R.; Nishioka, R.S. Influence of angulation and vertical misfit in the evaluation of micro-deformations around implants. Braz. Dent. Sci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Epprecht, A.; Zeltner, M.; Benic, G.; Özcan, M. A strain gauge analysis comparing 4-unit veneered zirconium dioxide implant-borne fixed dental prosthesis on engaging and non-engaging abutments before and after torque application. Clin. Exp. Dent. Res. 2018, 4, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Bhering, C.L.B.; Marques, I.D.S.V.; Takahashi, J.M.F.K.; Barão, V.A.R.; Consani, R.L.X.; Mesquita, M.F. The effect of casting and masticatory simulation on strain and misfit of implant-supported metal frameworks. Mater. Sci. Eng. C 2016, 62, 746–751. [Google Scholar] [CrossRef]
- Torres, E.M. Estudo da Correlação Entre Adaptação Marginal e Tensões Transmitidas aos Implantes por Estruturas Metálicas Fundidas em Monobloco: Análise Fotoelástica. Master’s Thesis, Faculdade de Odontologia de Ribeirão Preto, Ribeirão Preto, Brazil, 2005. [Google Scholar]
- Zaparolli, D.; Peixoto, R.F.; Pupim, D.; Macedo, A.P.; Toniollo, M.B.; Mattos, M.D.G.C. Photoelastic analysis of mandibular full-arch implant-supported fixed dentures made with different bar materials and manufacturing techniques. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 144–147. [Google Scholar] [CrossRef]
- Ozyilmaz, O.Y.; Aykent, F.; Sayin Ozel, G. Effect of mucosa thicknesses on stress distribution of implant-supported overdentures under unilateral loading: Photoelastic analysis. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019882645. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; de Medeiros, R.A.; da Silva, E.V.; Sônego, M.V.; Dos Santos, D.M. Biomechanical evaluation of spring system for implant-supported prosthesis: Analysis by photoelasticity and extensometry. J. Med. Eng. Technol. 2017, 41, 309–313. [Google Scholar] [CrossRef][Green Version]
- Valente, M.L.D.C.; de Castro, D.T.; Macedo, A.P.; Shimano, A.C.; Dos Reis, A.C. Comparative analysis of stress in a new proposal of dental implants. Mater. Sci. Eng. C Mater. Biol Appl. 2017, 77, 360–365. [Google Scholar] [CrossRef]
- Tiossi, R.; Lin, L.; Rodrigues, R.C.; Heo, Y.C.; Conrad, H.J.; de Mattos, M.d.G.C.; Ribeiro, R.F.; Fok, A.S. Digital image correlation analysis of the load transfer by implant-supported restorations. J. Biomech. 2011, 44, 1008–1013. [Google Scholar] [CrossRef]
- Tiossi, R.; Vasco, M.A.; Lin, L.; Conrad, H.J.; Bezzon, O.L.; Ribeiro, R.F.; Fok, A.S. Validation of finite element models for strain analysis of implant-supported prostheses using digital image correlation. Dent. Mater. 2013, 29, 788–796. [Google Scholar] [CrossRef]
- Valente, M.L.C.; Bolfarini, C.; de Oliveira, D.P.; Dos Reis, A.C. Dental mini-implant designs to support overdentures: Development, biomechanical evaluation, and 3D digital image correlation. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Peixoto, R.F.; Macedo, A.P.; Martinelli, J.; Faria, A.C.L.; Tiossi, R.; Ribeiro, R.F.; de Mattos, M.D.G.C. A Digital Image Correlation Analysis of Strain Generated by 3-Unit Implant-Supported Fixed Dental Prosthesis: An In Vitro Study. Implant Dent. 2017, 26, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Cantó-Navés, O.; Marimon, X.; Ferrer, M.; Cabratosa-Termes, J. Comparison between experimental digital image processing and numerical methods for stress analysis in dental implants with different restorative materials. J. Mech. Behav. Biomed. Mater. 2021, 113, 104092. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.Q.; Xiao, Y.; Ma, R.Y.; Huang, C.P.; Wu, Y.; Yang, B.C.; Yang, Q.; Bao, C.Y.; Yu, H.Y. Expert consensus on biomechanical research of dental implant. Hua Xi Kou Qiang Yi Xue Za Zhi 2019, 37, 115–123. [Google Scholar] [PubMed]
- Silva, J.D.; Ferreira, J.A. O Princípio de Saint-Venant em Elasticidade Não Linear. TEMA—Tend. Mat. Apl. Comput. 2004, 5, 13. [Google Scholar] [CrossRef]
- Yoon, S.; Jung, H.J.; Knowles, J.C.; Lee, H.H. Digital image correlation in dental materials and related research: A review. Dent. Mater. 2021, 37, 758–771. [Google Scholar] [CrossRef]
- Niroomand, M.R.; Arabbeiki, M. Effect of the dimensions of implant body and thread on bone resorption and stability in trapezoidal threaded dental implants: A sensitivity analysis and optimization. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Robau-Porrua, A.; Pérez-Rodríguez, Y.; Soris-Rodríguez, L.M.; Pérez-Acosta, O.; González, J.E. The effect of diameter, length and elastic modulus of a dental implant on stress and strain levels in peri-implant bone: A 3D finite element analysis. Biomed. Mater. Eng. 2020, 30, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.V.; Pérez, R.A.; Manero, J.M.; Gil Mur, J. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef]
- Tsitsiashvili, A.M.; Silant’ev, A.S.; Panin, A.M.; Arutyunov, S.D. Biomekhanika korotkogo dental’nogo implantata v kostnoi tkani nizhnei chelyusti [Short dental implant biomechanics in the mandible bone tissue]. Stomatologiia 2019, 98, 33–36. [Google Scholar] [CrossRef]
- Setz, J.; Krämer, A.; Benzing, U.; Weber, H. Complete dentures fixed on dental implants: Chewing patterns and implant stress. Int. J. Oral Maxillofac. Implant. 1989, 4, 107–111. [Google Scholar]
- Falk, H.; Laurell, L.; Lundgren, D. Occlusal force pattern in dentitions with mandibular implant-supported fixed cantilever prostheses occluded with complete dentures. Int. J. Oral Maxillofac. Implant. 1989, 4, 55–62. [Google Scholar]
- Akça, K.; Cehreli, M.C. A photoelastic and strain-gauge analysis of interface force transmission of internal-cone implants. Int. J. Periodontics Restor. Dent. 2008, 28, 391–399. [Google Scholar]
- Clelland, N.L.; Gilat, A.; McGlumphy, E.A.; Brantley, W.A. A photoelastic and strain gauge analysis of angled abutments for an implant system. Int. J. Oral Maxillofac. Implant. 1993, 8, 541–548. [Google Scholar]
- Anami, L.C.; da Costa Lima, J.M.; Takahashi, F.E.; Neisser, M.P.; Noritomi, P.Y.; Bottino, M.A. Stress distribution around osseointegrated implants with different internal-cone connections: Photoelastic and finite element analysis. J. Oral Implantol. 2015, 41, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Tanimoto, Y.; Iida, T.; Murakami, H. Effects of material and coefficient of friction on taper joint dental implants. J. Prosthodont. Res. 2020, 64, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Su, K.; Zhou, Y.; Hossaini-Zadeh, M.; Lewis, G.S.; Du, J. Voxel-based micro-finite element analysis of dental implants in a human cadaveric mandible: Tissue modulus assignment and sensitivity analyses. J. Mech. Behav. Biomed. Mater. 2019, 94, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.L.; Rodriguez, M.S. Tensile strength and modulus of elasticity of tooth structure and several restorative materials. J. Am. Dent. Assoc. 1962, 64, 378–387. [Google Scholar] [CrossRef]
- Tiossi, R.; Lin, L.; Conrad, H.J.; Rodrigues, R.C.; Heo, Y.C.; de Mattos, M.d.G.C.; Fok, A.S.; Ribeiro, R.F. Digital image correlation analysis on the influence of crown material in implant-supported prostheses on bone strain distribution. J. Prosthodont. Res. 2012, 56, 25–31. [Google Scholar] [CrossRef]
- Sojic, L.T.; Milic Lemic, A.; Tanasic, I.; Mitrovic, N.; Milosevic, M.; Petrovic, A. Compressive strains and displacement in a partially dentate lower jaw rehabilitated with two different treatment modalities. Gerodontology 2012, 29, 851–857. [Google Scholar] [CrossRef]
- Lopez, C.A.V.; Vasco, M.A.A.; Ruales, E.; Bedoya, K.A.; Benfatti, C.M.; Bezzon, O.L.; Deliberador, T.M. Three-Dimensional Finite Element Analysis of Stress Distribution in Zirconia and Titanium Dental Implants. J. Oral Implantol. 2018, 44, 409–415. [Google Scholar] [CrossRef]
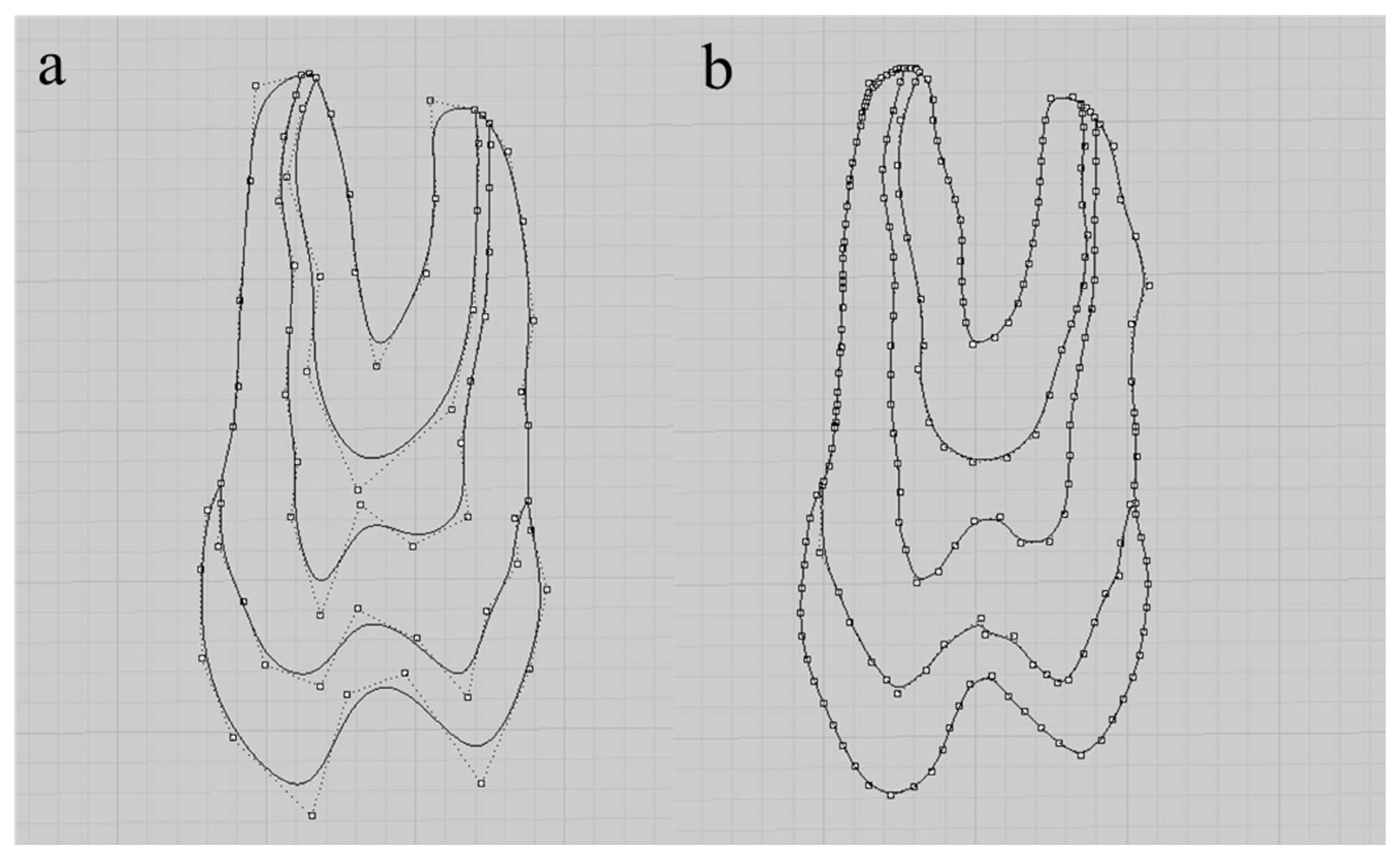
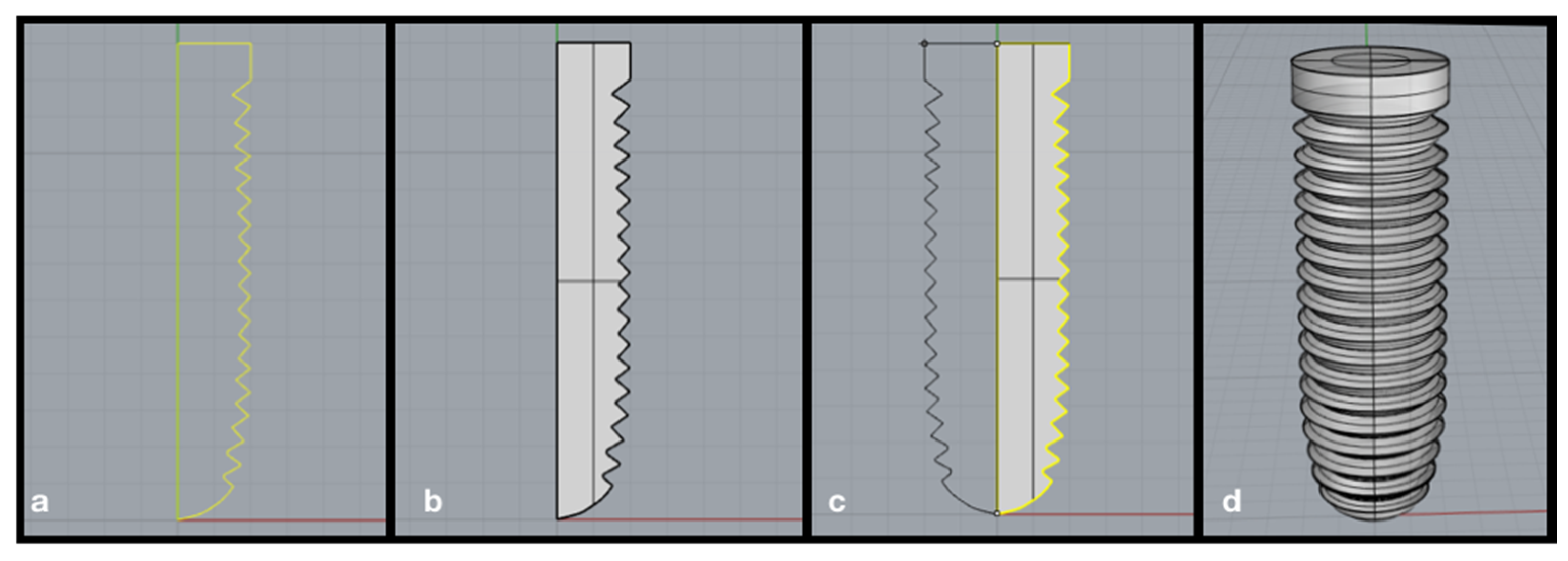
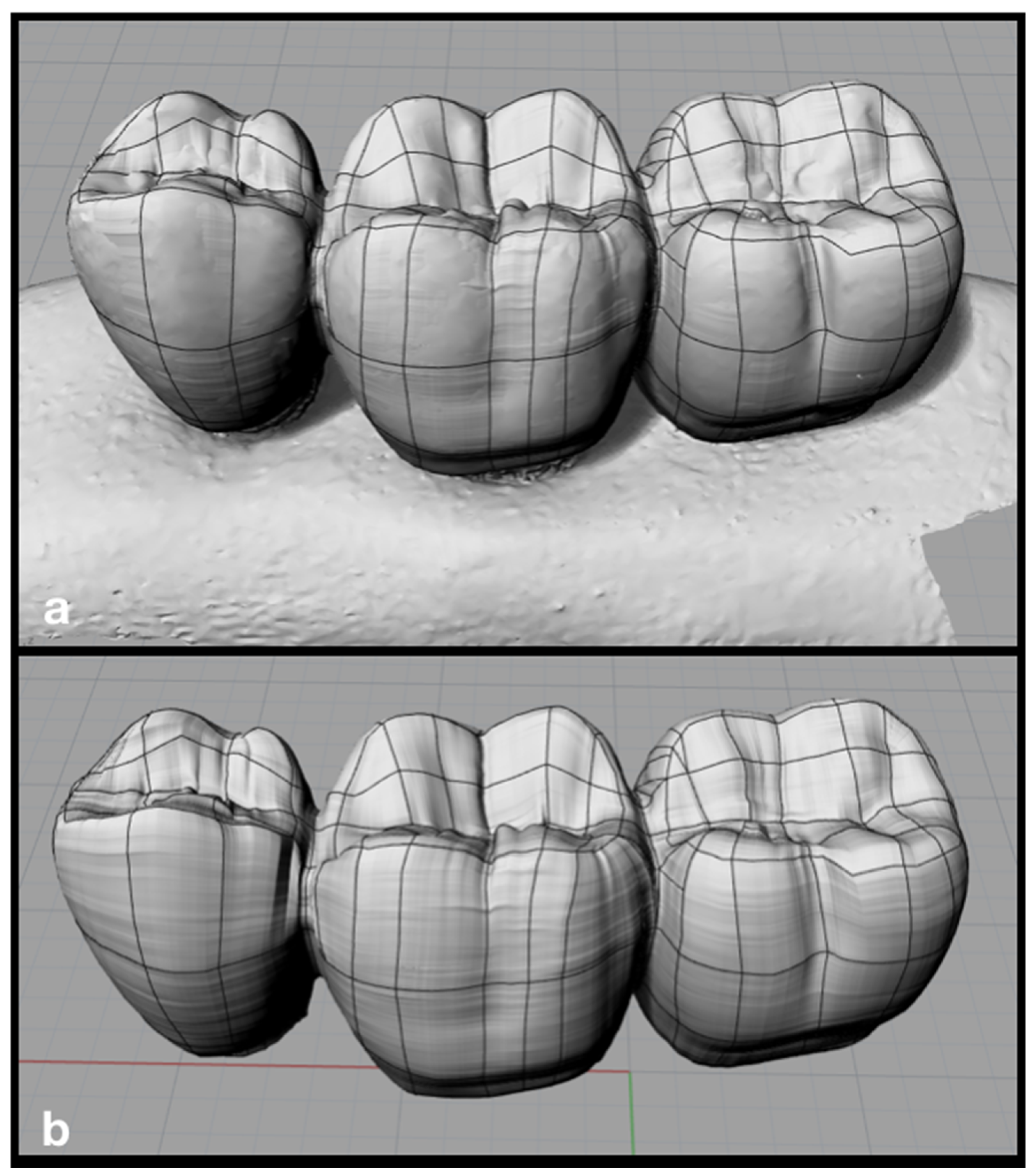
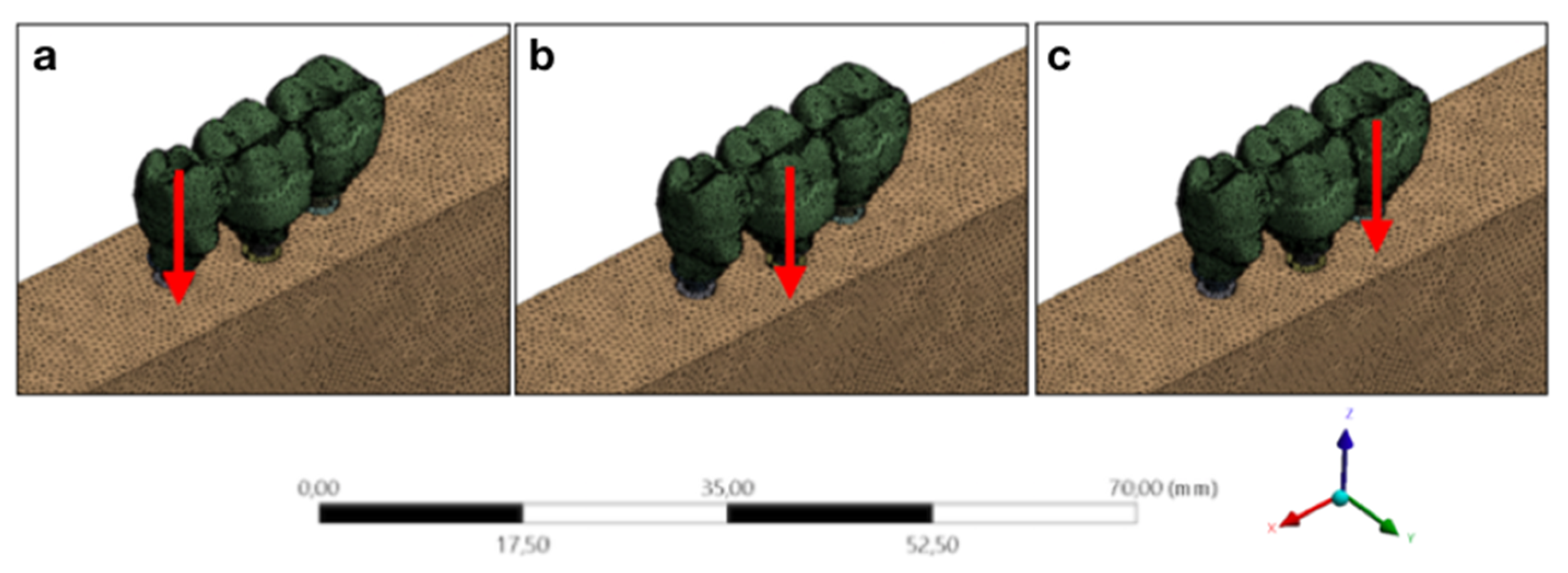



| Database | Mean Value ± Standard Deviation | CI 95% |
|---|---|---|
| Pubmed | 2.03 ± 1.89 a | (0.19–3.92) |
| Google Scholar | 0.78 ± 0.90 b | (0.12–1.68) |
| Raleigh-Ritz Method | Finite Element Method |
|---|---|
| The structure is treated as a single entity; therefore, it consists of a single element [29,30,31,35]. | The structure consists of multiple elements connected by nodes [6,44,45,46,47,48,49,50]. |
| The variables to be optimized are the coefficients A, B, C, etc., of the equations describing the problem [29,32,35,36,37,38]. | Offsets and rotations are the variables to be optimized [5,6,44,46,47,51]. |
| Less intuitive. You need to specify boundary conditions and restrictions regarding the amplitude of sine waves [29,39,40,41,42,43]. | More intuitive, as the boundary conditions and restrictions refer to displacements and rotations [5,6,44,45,46,47,48,49,50,51,52,53,54,55,56]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, J.D.M.d.; Queiroz, D.A.; Nakano, L.J.N.; Andrade, V.C.; Ribeiro, N.d.C.R.; Borges, A.L.S.; Bottino, M.A.; Lopes, G.d.R.S. Bioengineering Tools Applied to Dentistry: Validation Methods for In Vitro and In Silico Analysis. Dent. J. 2022, 10, 145. https://doi.org/10.3390/dj10080145
Matos JDMd, Queiroz DA, Nakano LJN, Andrade VC, Ribeiro NdCR, Borges ALS, Bottino MA, Lopes GdRS. Bioengineering Tools Applied to Dentistry: Validation Methods for In Vitro and In Silico Analysis. Dentistry Journal. 2022; 10(8):145. https://doi.org/10.3390/dj10080145
Chicago/Turabian StyleMatos, Jefferson David Melo de, Daher Antonio Queiroz, Leonardo Jiro Nomura Nakano, Valdir Cabral Andrade, Nathália de Carvalho Ramos Ribeiro, Alexandre Luiz Souto Borges, Marco Antonio Bottino, and Guilherme da Rocha Scalzer Lopes. 2022. "Bioengineering Tools Applied to Dentistry: Validation Methods for In Vitro and In Silico Analysis" Dentistry Journal 10, no. 8: 145. https://doi.org/10.3390/dj10080145
APA StyleMatos, J. D. M. d., Queiroz, D. A., Nakano, L. J. N., Andrade, V. C., Ribeiro, N. d. C. R., Borges, A. L. S., Bottino, M. A., & Lopes, G. d. R. S. (2022). Bioengineering Tools Applied to Dentistry: Validation Methods for In Vitro and In Silico Analysis. Dentistry Journal, 10(8), 145. https://doi.org/10.3390/dj10080145








