Characterization of SIBLING Proteins in the Mineralized Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Hematoxylin and Eosin (H&E)
2.3. Immunohistochemistry (IHC) Staining
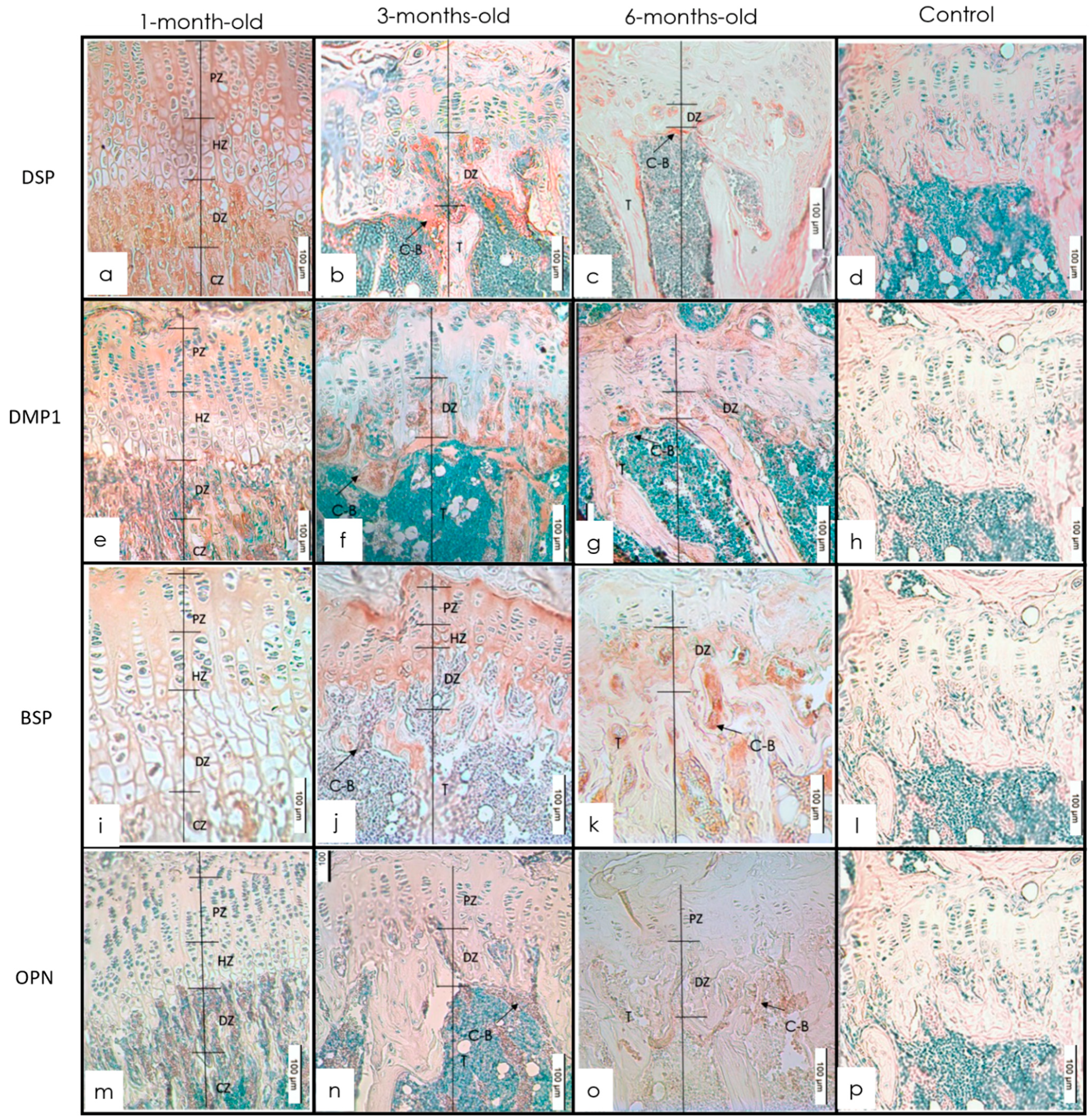
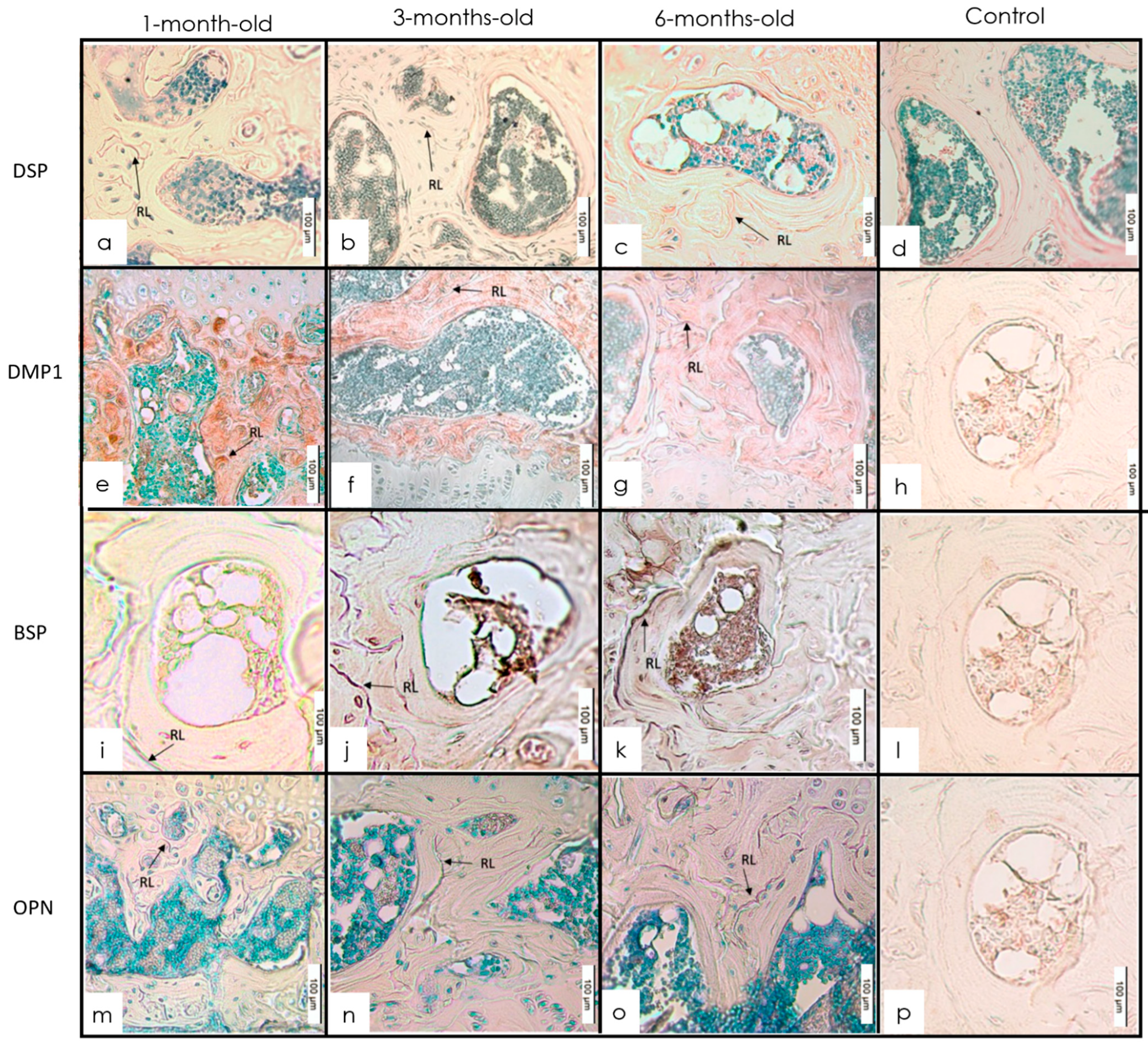
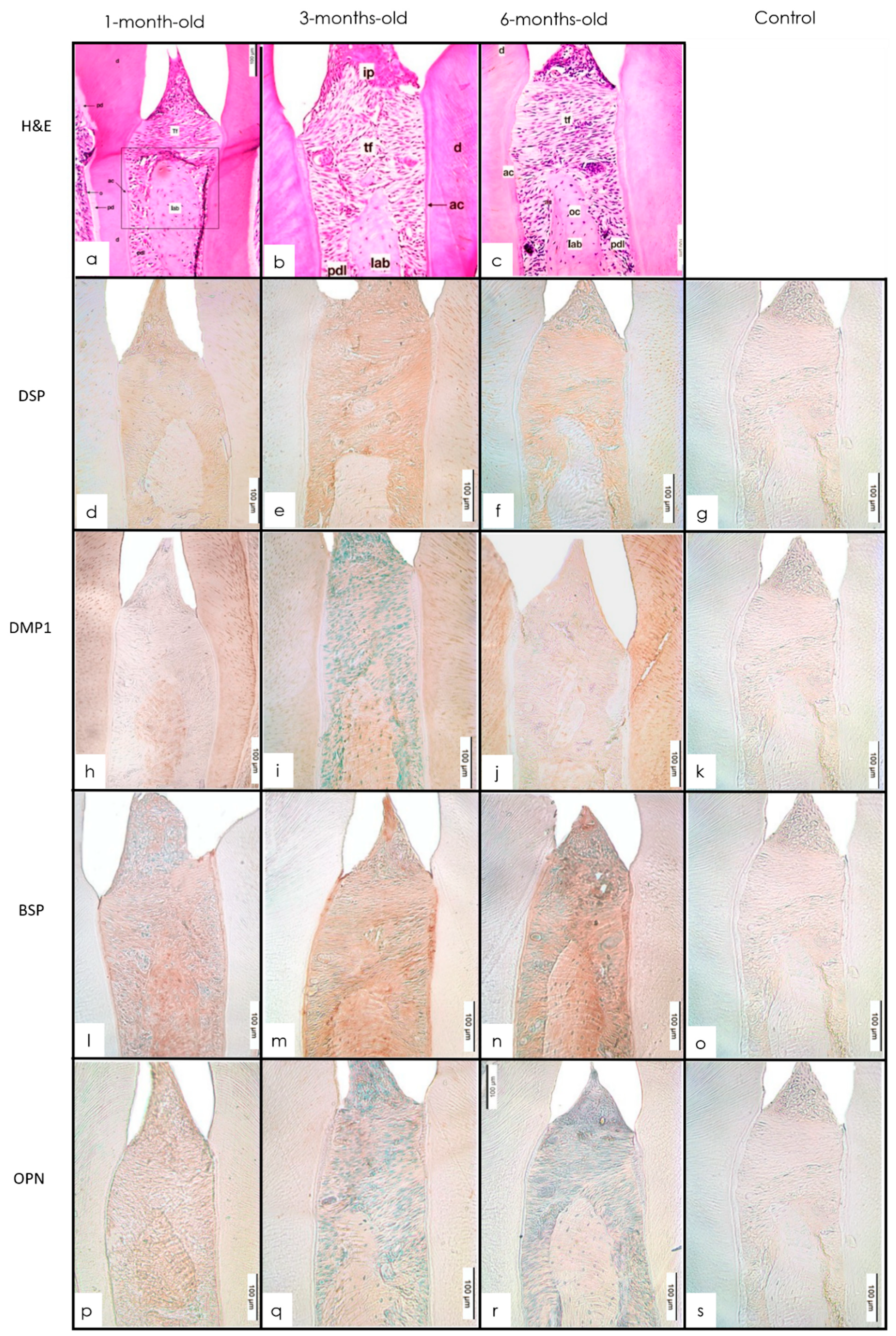
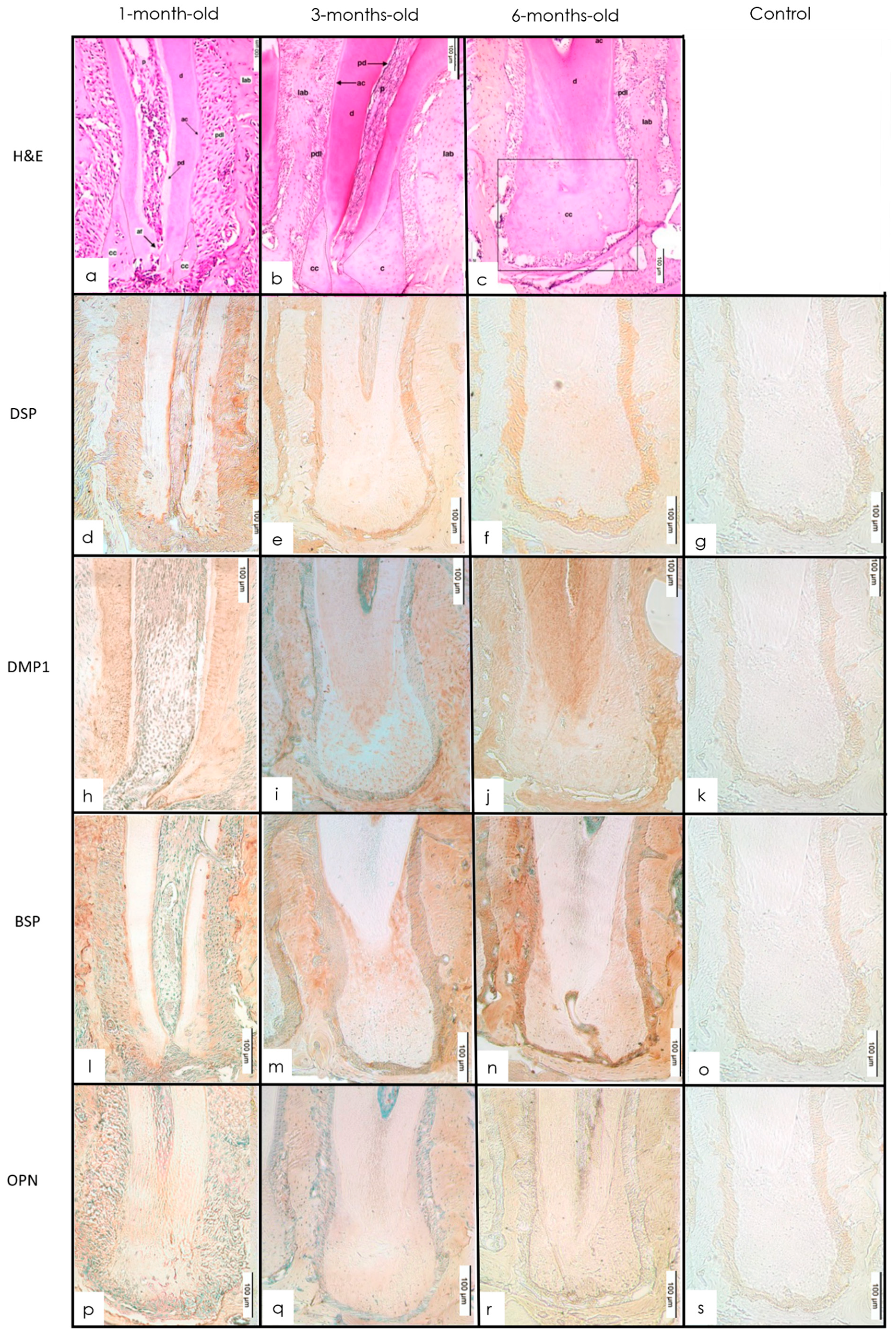
3. Results
3.1. Long Bones
IHC Staining
3.2. Alveolar Bone
3.2.1. H&E Staining
3.2.2. IHC Staining
3.3. Cementum
3.3.1. H&E Staining
3.3.2. IHC Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weatherholt, A.M.; Fuchs, R.K.; Warden, S.J. Specialized connective tissue: Bone, the structural framework of the upper extremity. J. Hand Ther. 2012, 25, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, B.K.; Miyake, T. All for one and one for all: Condensations and the initiation of skeletal development. Bioessays 2000, 22, 138–147. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. Elite 2011, 3, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Silbermann, M.; Kadar, T. Age-related changes in the cellular population of the growth plate of normal mouse. Acta Anat. 1977, 97, 459–468. [Google Scholar] [CrossRef]
- Al-Qtaitat, A.; Aldalaen, S. A review of non-collagenous proteins; their role in bone. Am. J. Life Sci. 2014, 2, 351–355. [Google Scholar] [CrossRef]
- Gibson, M.P.; Liu, Q.; Zhu, Q.; Lu, Y.; Jani, P.; Wang, X.; Liu, Y.; Paine, M.L.; Snead, M.L.; Feng, J.Q.; et al. Role of the NH2-terminal fragment of dentin sialophosphoprotein in dentinogenesis. Eur. J. Oral Sci. 2013, 121, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Butler, W.T.; Brunn, J.C.; Qin, C. Dentin extracellular matrix (ECM) proteins: Comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect. Tissue Res. 2003, 44, 171–178. [Google Scholar] [CrossRef]
- Ye, L.; MacDougall, M.; Zhang, S.; Xie, Y.; Zhang, J.; Li, Z.; Lu, Y.; Mishina, Y.; Feng, J.Q. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J. Biol. Chem. 2004, 279, 19141–19148. [Google Scholar] [CrossRef] [Green Version]
- MacDougall, M. Dental structural diseases mapping to human chromosome 4q21. Connect. Tissue Res. 2003, 44, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.W.; Torchia, D.A.; Fohr, B.; Young, M.F.; Fedarko, N.S. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 2001, 280, 460–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, M.; Septier, D.; Lecolle, S.; Chardin, H.; Quintana, M.A.; Acevedo, A.C.; Gafni, G.; Dillouya, D.; Vermelin, L.; Thonemann, B. Dental mineralization. Int. J. Dev. Biol. 1995, 39, 93–110. [Google Scholar] [PubMed]
- Embery, G.; Hall, R.; Waddington, R.; Septier, D.; Goldberg, M. Proteoglycans in dentinogenesis. Crit. Rev. Oral Biol. Med. 2001, 12, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Brunn, J.C.; Cadena, E.; Ridall, A.; Tsujigiwa, H.; Nagatsuka, H.; Nagai, N.; Butler, W.T. The expression of dentin sialophosphoprotein gene in bone. J. Dent. Res. 2002, 81, 392–394. [Google Scholar] [CrossRef]
- D’Souza, R.N.; Cavender, A.; Sunavala, G.; Alvarez, J.; Ohshima, T.; Kulkarni, A.B.; MacDougall, M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J. Bone Miner. Res. 1997, 12, 2040–2049. [Google Scholar] [CrossRef]
- Huang, B.; Sun, Y.; Maciejewska, I.; Qin, D.; Peng, T.; McIntyre, B.; Wygant, J.; Butler, W.T.; Qin, C. Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur. J. Oral Sci. 2008, 116, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Baba, O.; Butler, W.T. Post-translational modifications of SIBLING proteins and their roles in osteogenesis and dentinogenesis. Crit. Rev. Oral Biol. Med. 2004, 15, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.Q.; Luan, X.; Wallace, J.; Jing, D.; Ohshima, T.; Kulkarni, A.B.; D’Souza, R.N.; Kozak, C.A.; MacDougall, M. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J. Biol. Chem. 1998, 273, 9457–9464. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.Q.; Huang, H.; Lu, Y.; Ye, L.; Xie, Y.; Tsutsui, T.W.; Kunieda, T.; Castranio, T.; Scott, G.; Bonewald, L.B.; et al. The dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J. Dent. Res. 2003, 82, 776–780. [Google Scholar] [CrossRef]
- Zhu, Q.; Gibson, M.P.; Liu, Q.; Liu, Y.; Lu, Y.; Wang, X.; Feng, J.Q.; Qin, C. Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis. J. Biol. Chem. 2012, 287, 30426–30435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigma-Aldrich Protocol for H&E Staining. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/General_Information/1/hhs.pdf (accessed on 8 March 2021).
- Hou, C.; Liu, Z.X.; Tang, K.L.; Wang, M.G.; Sun, J.; Wang, J.; Li, S. Developmental changes and regional localization of Dspp, Mepe, Mimecan and Versican in postnatal developing mouse teeth. J. Mol. Histol. 2012, 43, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Huang, S.; Miclau, T.; Helms, J.A.; Colnot, C. Mepe is expressed during skeletal development and regeneration. Histochem. Cell Biol. 2004, 121, 493–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, O.; Qin, C.; Brunn, J.C.; Jones, J.E.; Wygant, J.N.; McIntyre, B.W.; Butler, W.T. Detection of dentin sialoprotein in rat periodontium. Eur. J. Oral Sci. 2004, 112, 163–170. [Google Scholar] [CrossRef]
- Baba, O.; Qin, C.; Brunn, J.C.; Wygant, J.N.; McIntyre, B.W.; Butler, W.T. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004, 23, 371–379. [Google Scholar] [CrossRef]
- Gibson, M.P.; Zhu, Q.; Wang, S.; Liu, Q.; Liu, Y.; Wang, X.; Yuan, B.; Ruest, L.B.; Feng, J.Q.; D’Souza, R.N.; et al. The rescue of dentin matrix protein 1 (DMP1)-deficient tooth defects by the transgenic expression of dentin sialophosphoprotein (DSPP) indicates that DSPP is a downstream effector molecule of DMP1 in dentinogenesis. J. Biol. Chem. 2013, 288, 7204–7214. [Google Scholar] [CrossRef] [Green Version]
- Jani, P.H.; Gibson, M.P.; Liu, C.; Zhang, H.; Wang, X.; Lu, Y.; Qin, C. Transgenic expression of Dspp partially rescued the long bone defects of Dmp1-null mice. Matrix Biol. 2016, 52–54, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peça, J.; Santos, J.M. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: In vivo interaction with two bioactive materials. Clin. Oral Investig. 2021, 25, 5317–5329. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, V.L.; Ayers, R.A.; Bateman, T.A.; Simske, S.J. Bone development and age-related bone loss in male C57BL/6J mice. Bone 2003, 33, 387–398. [Google Scholar] [CrossRef]
- Glatt, V.; Canalis, E.; Stadmeyer, L.; Bouxsein, M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007, 22, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Verdelis, K.; Ling, Y.; Sreenath, T.; Haruyama, N.; MacDougall, M.; van der Meulen, M.C.; Lukashova, L.; Spevak, L.; Kulkarni, A.B.; Boskey, A.L. DSPP effects on in vivo bone mineralization. Bone 2008, 43, 983–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Sreenath, T.; Haruyama, N.; Honeycutt, C.; Terse, A.; Cho, A.; Kohler, T.; Müller, R.; Goldberg, M.; Kulkarni, A.B. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009, 28, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, M.; Butler, W.T.; Qin, C. Dentin sialophosphoprotein in biomineralization. Connect. Tissue Res. 2010, 51, 404–417. [Google Scholar] [CrossRef] [Green Version]
- Tartaix, P.H.; Doulaverakis, M.; George, A.; Fisher, L.W.; Butler, W.T.; Qin, C.; Salih, E.; Tan, M.; Fujimoto, Y.; Spevak, L.; et al. In Vitro Effects of Dentin Matrix Protein-1 on Hydroxyapatite Formation Provide Insights into in Vivo Functions. J. Biol. Chem. 2004, 279, 18115–18120. [Google Scholar] [CrossRef] [Green Version]
- Gajjeraman, S.; Narayanan, K.; Hao, J.; Qin, C.; George, A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J. Biol. Chem. 2007, 282, 1193–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, K.; Srinivas, R.; Ramachandran, A.; Hao, J.; Quinn, B.; George, A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc. Natl. Acad. Sci. USA 2001, 98, 4516–4521. [Google Scholar] [CrossRef] [Green Version]
- Gluhak-Heinrich, J.; Ye, L.; Bonewald, L.F.; Feng, J.Q.; MacDougall, M.; Harris, S.E.; Pavlin, D. Mechanical Loading Stimulates Dentin Matrix Protein 1 (DMP1) Expression in Osteocytes In Vivo. J. Bone Miner. Res. 2003, 18, 807–817. [Google Scholar] [CrossRef]
- Narayanan, K.; Gajjeraman, S.; Ramachandran, A.; Hao, J.; George, A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J. Biol. Chem. 2006, 281, 19064–19071. [Google Scholar] [CrossRef] [Green Version]
- Moses, K.D.; Butler, W.T.; Qin, C. Immunohistochemical study of small integrin-binding ligand, N-linked glycoproteins in reactionary dentin of rat molars at different ages. Eur. J. Oral Sci. 2006, 114, 216–222. [Google Scholar] [CrossRef]
- Baht, G.S.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008, 27, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.L.; Rattray, K.R.; Tye, C.E.; Underhill, T.M.; Somerman, M.J.; D’errico, J.A.; Chambers, A.F.; Hunter, G.K.; Goldberg, H.A. Functional analysis of bone sialoprotein: Identification of the hydroxyapatite-nucleating and cell-binding domains by recombinant peptide expression and site-directed mutagenesis. Bone 2000, 27, 795–802. [Google Scholar] [CrossRef]
- Foster, B.L.; Ao, M.; Willoughby, C.; Soenjaya, Y.; Holm, E.; Lukashova, L.; Tran, A.B.; Wimer, H.F.; Zerfas, P.M.; Nociti, F.H., Jr.; et al. Mineralization defects in cementum and craniofacial bone from loss of bone sialoprotein. Bone 2015, 78, 150–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Sun, Y.; Chen, L.; Guan, C.; Guo, L.; Qin, C. Expression and distribution of SIBLING proteins in the predentin/dentin and mandible of hyp mice. Oral Dis. 2010, 16, 453–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskey, A.L.; Spevak, L.; Paschalis, E.; Doty, S.B.; McKee, M.D. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif. Tissue Int. 2002, 71, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.K.; Kyle, C.L.; Goldberg, H.A. Modulation of crystal formation by bone phosphoproteins: Structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994, 300, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dab, S.; Abdelhay, N.; Figueredo, C.A.; Ganatra, S.; Gibson, M.P. Characterization of SIBLING Proteins in the Mineralized Tissues. Dent. J. 2022, 10, 144. https://doi.org/10.3390/dj10080144
Dab S, Abdelhay N, Figueredo CA, Ganatra S, Gibson MP. Characterization of SIBLING Proteins in the Mineralized Tissues. Dentistry Journal. 2022; 10(8):144. https://doi.org/10.3390/dj10080144
Chicago/Turabian StyleDab, Sandeep, Nancy Abdelhay, Carlos Alberto Figueredo, Seema Ganatra, and Monica Prasad Gibson. 2022. "Characterization of SIBLING Proteins in the Mineralized Tissues" Dentistry Journal 10, no. 8: 144. https://doi.org/10.3390/dj10080144
APA StyleDab, S., Abdelhay, N., Figueredo, C. A., Ganatra, S., & Gibson, M. P. (2022). Characterization of SIBLING Proteins in the Mineralized Tissues. Dentistry Journal, 10(8), 144. https://doi.org/10.3390/dj10080144





