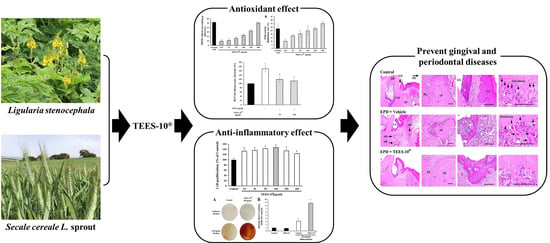
In Vitro and In Vivo Anti-Inflammatory Effects of TEES-10®, a Mixture of Ethanol Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout, on Gingivitis and Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing TEES-10®
2.2. Experiments Using Cells
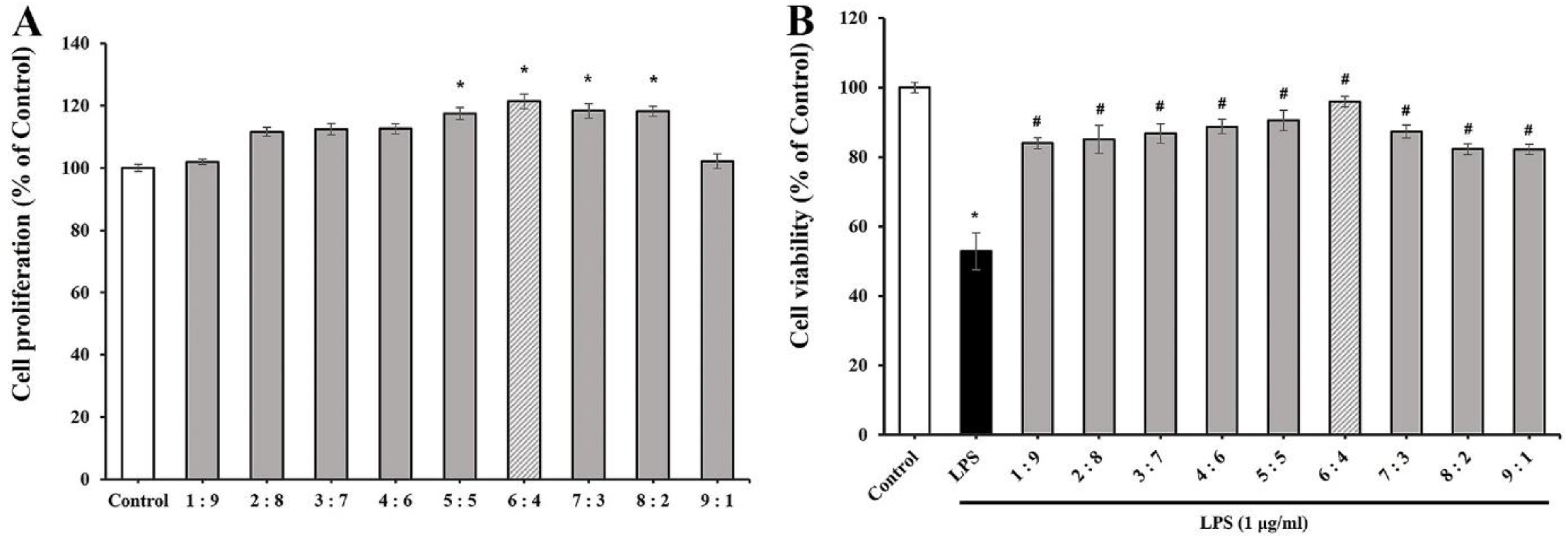
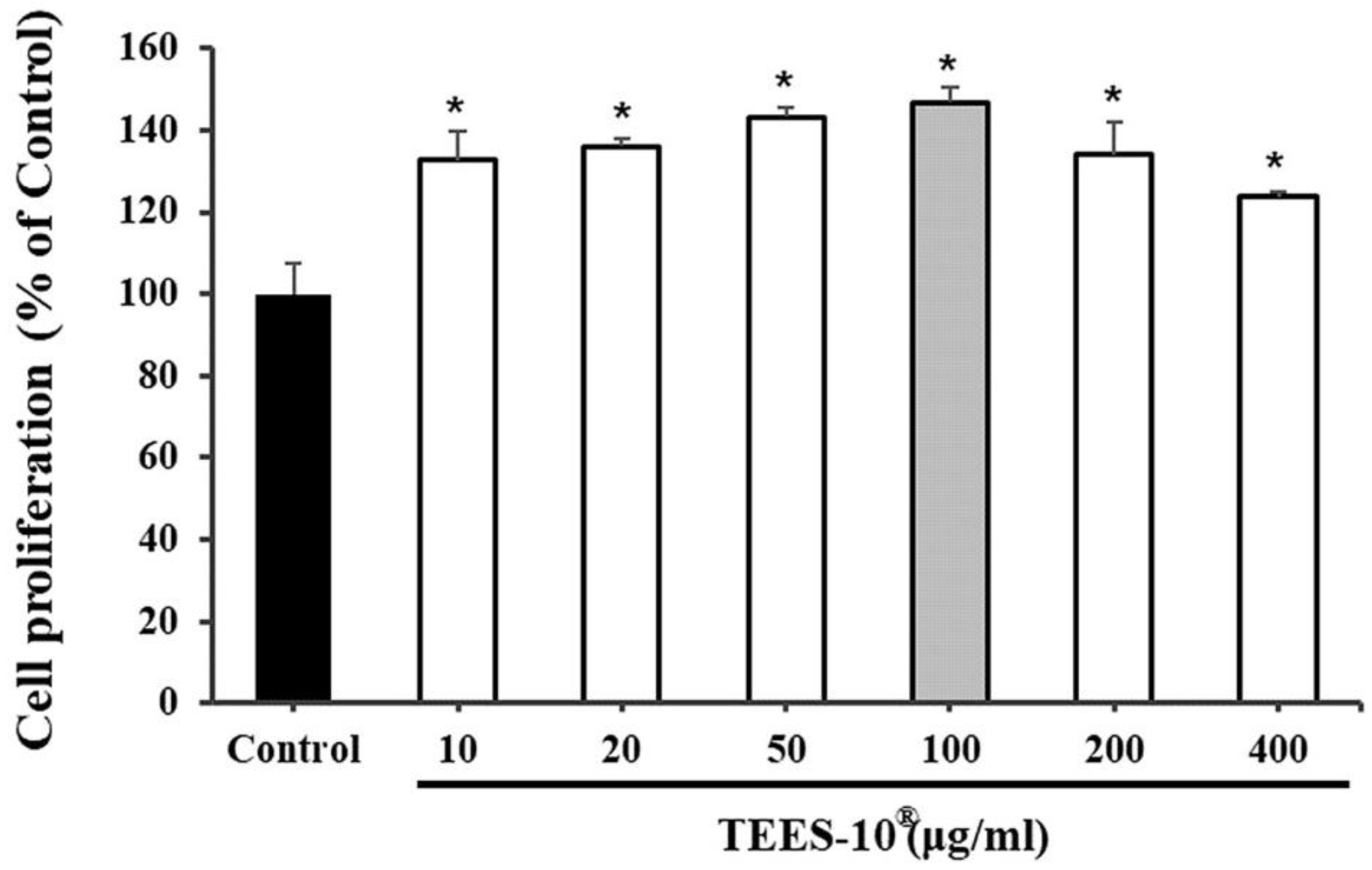
2.2.1. Determining the Optimal TEES-10® Using Periodontal Ligament (PDL) Cells
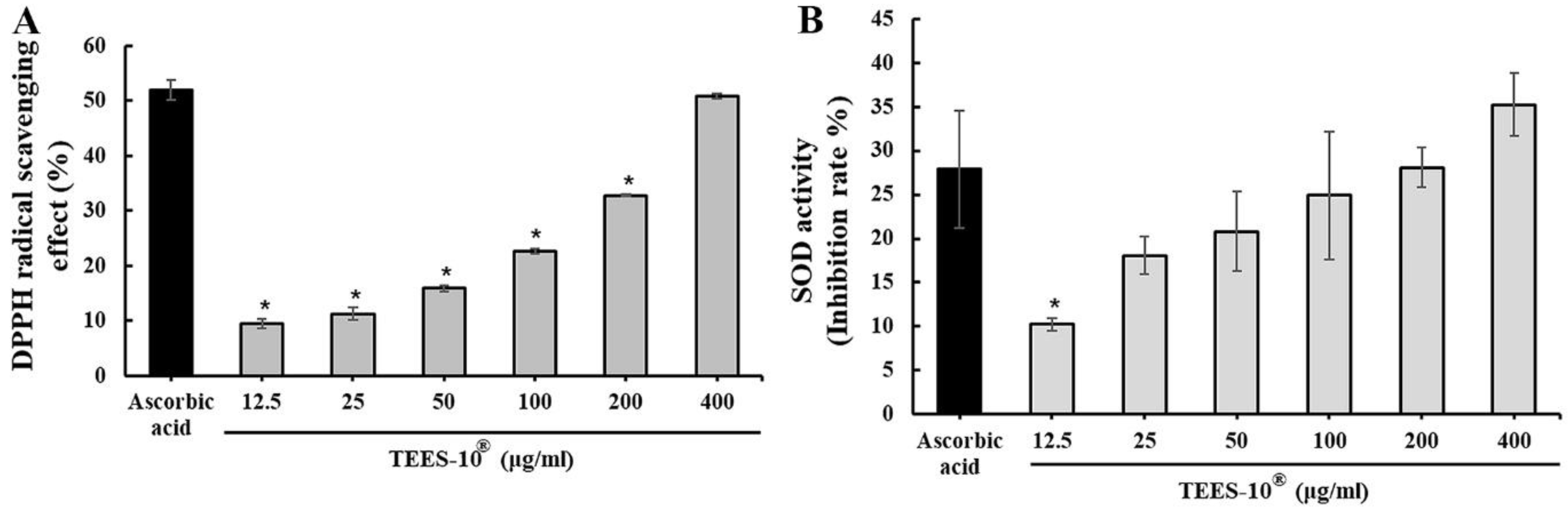
2.2.2. Measuring 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.2.3. Measuring Superoxide Radical Scavenging Activity (SOD-like Activity)
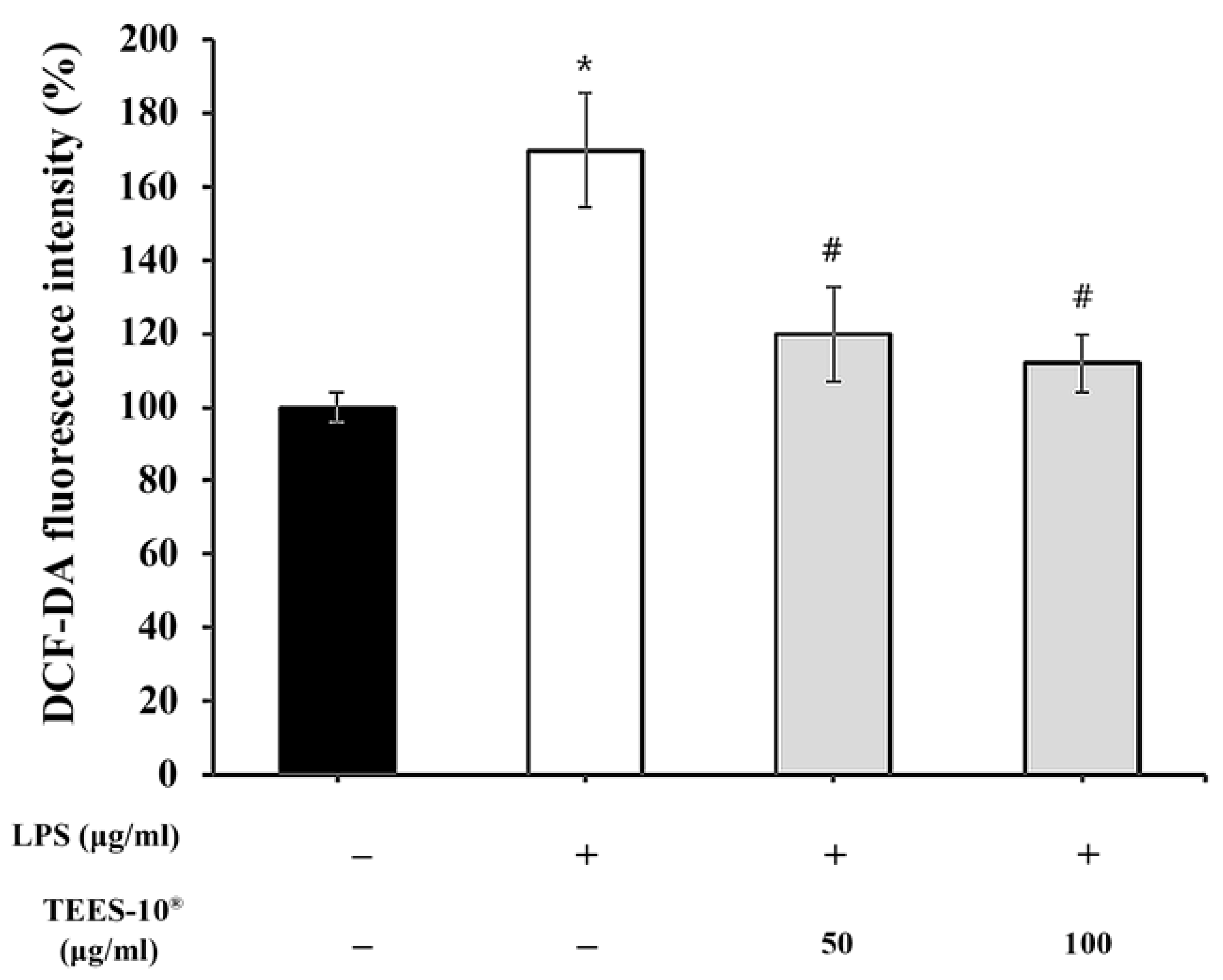
2.2.4. Measuring ROS Production from PDL Cells
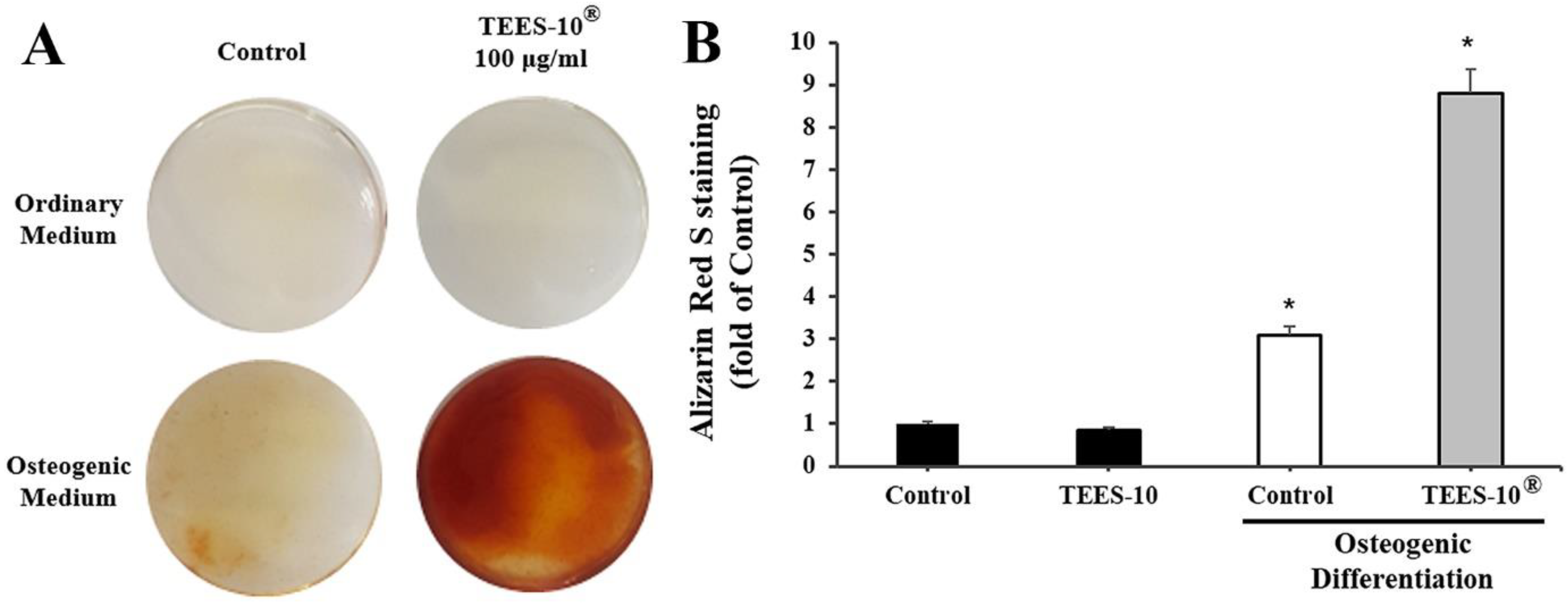
2.2.5. Measuring the Viability of Stem Cells from Human Exfoliated Deciduous Teeth (SHED)
2.2.6. Measuring Osteogenic Differentiation of SHED by Alizarin Red S Staining
2.3. Experiments Using Animals
2.3.1. Inducing Experimental Periodontitis (EPD) in Rats
2.3.2. Histologic Examination of EPD Region
2.3.3. Histomorphometry
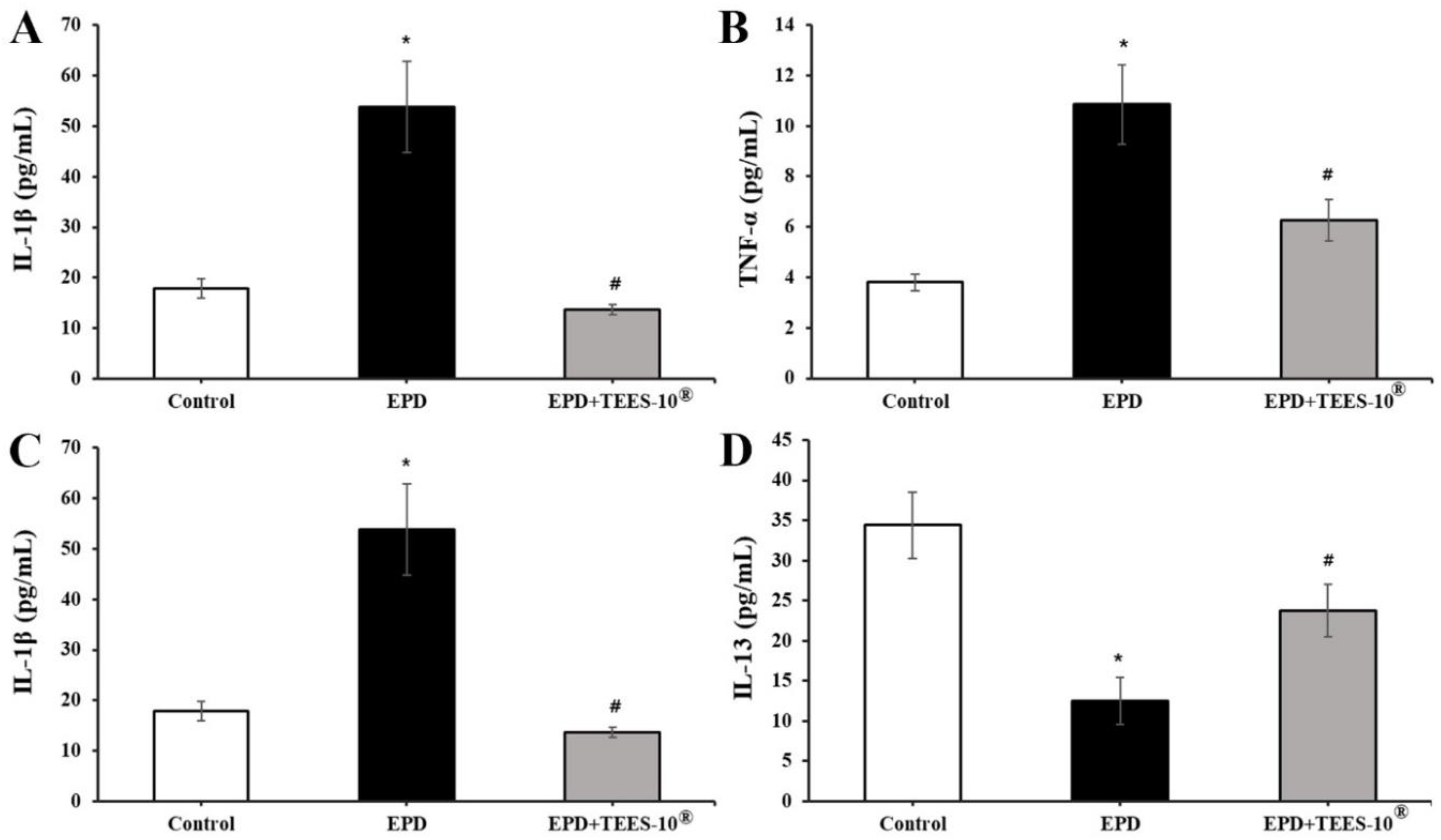
2.3.4. Measuring Blood Levels of Some Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Selecting the TEES-10® with Best Efficacy
3.2. Antioxidant Activities of TEES-10®
3.3. Effects of TEES-10® on Viability and Osteogenic Differentiation of Stem Cells from SHED
3.4. Anti-Inflammatory Effect of TEES-10® on Experimental Periodontitis in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honda, T.; Domon, H.; Okui, T.; Kajita, K.; Amanuma, R.; Yamazaki, K. Balance of inflammatory response in stable gingivitis and progressive periodontitis lesions. Clin. Exp. Immunol. 2006, 144, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Scapoli, L.; Girardi, A.; Palmieri, A.; Martinelli, M.; Cura, F.; Lauritano, D.; Carinci, F. Quantitative analysis of periodontal pathogens in periodontitis and gingivitis. J. Biol. Regul. Homeost Agents 2015, 29, 101–110. [Google Scholar]
- Cardoso, E.M.; Reis, C.; Manzanares-Cespedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef]
- Jaffar, N.; Miyazaki, T.; Maeda, T. Biofilm formation of periodontal pathogens on hydroxyapatite surfaces: Implications for periodontium damage. J. Biomed. Mater. Res. Part A 2016, 104, 2873–2880. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. The role of porphyromonas gingivalis outer membrane vesicles in periodontal disease and related systemic diseases. Front. Cell. Infect. Microbiol. 2020, 10, 585917. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Role of streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S.; Ang, C.S.; Liu, S.W.; Paolini, R.; Veith, P.; Reynolds, E. Inhibition of porphyromonas gingivalis biofilm by oxantel. Antimicrob. Agents Chemother. 2010, 54, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, X.; Lu, Z.; Li, Y.; Lopes-Virella, M.F.; Yu, H.; Haycraft, C.J.; Li, Q.; Kirkwood, K.L.; Huang, Y. Simvastatin inhibits lipopolysaccharide-induced osteoclastogenesis and reduces alveolar bone loss in experimental periodontal disease. J. Periodontal Res. 2014, 49, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Cordero, M.D.; Quiles, J.L.; Morillo, J.M.; del Carmen Ramirez-Tortosa, M.; Battino, M. Mitochondrial dysfunction promoted by porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free. Radic. Biol. Med. 2011, 50, 1336–1343. [Google Scholar] [CrossRef]
- Wang, P.L.; Ohura, K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-cd14 and toll-like receptors. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2002, 13, 132–142. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative stress and antioxidant system in periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Herb, M.; Schramm, M. Functions of ros in macrophages and antimicrobial immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Leto, T.L.; Geiszt, M. Role of nox family nadph oxidases in host defense. Antioxid. Redox Signal. 2006, 8, 1549–1561. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Linhart, K.; Bartsch, H.; Seitz, H.K. The role of reactive oxygen species (ros) and cytochrome p-450 2e1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 2014, 3, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29 (Suppl. S2), 22–32. [Google Scholar] [CrossRef]
- Jones, W.A.; O’Leary, T.J. The effectiveness of in vivo root planing in removing bacterial endotoxin from the roots of periodontally involved teeth. J. Periodontol. 1978, 49, 337–342. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, R.; Eick, S.; Batori-Andronescu, I.; Jepsen, S.; Arweiler, N.B.; Rossler, R.; Conrad, T.; Ramseier, C.A.; Sculean, A. Clinical and microbiological evaluation of local doxycycline and antimicrobial photodynamic therapy during supportive periodontal therapy: A randomized clinical trial. Antibiotics 2021, 10, 277. [Google Scholar] [CrossRef]
- Minic, I.; Pejcic, A.; Bradic-Vasic, M. Effect of the local probiotics in the therapy of periodontitis a randomized prospective study. Int. J. Dent. Hyg. 2022, 20, 401–407. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Soleo, R.; Di Fonso, F.; Politi, L.; Venugopal, A.; Marya, A.; Butera, A. Management of periodontal disease with adjunctive therapy with ozone and photobiomodulation (pbm): A randomized clinical trial. Photonics 2022, 9, 138. [Google Scholar] [CrossRef]
- Lodi, G.; Azzi, L.; Varoni, E.M.; Pentenero, M.; Del Fabbro, M.; Carrassi, A.; Sardella, A.; Manfredi, M. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst. Rev. 2021, 2, CD003811. [Google Scholar] [PubMed]
- Van Dyke, T.E. The management of inflammation in periodontal disease. J. Periodontol. 2008, 79, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Milovanova-Palmer, J.; Pendry, B. Is there a role for herbal medicine in the treatment and management of periodontal disease? J. Herb. Med. 2018, 12, 33–48. [Google Scholar] [CrossRef]
- Eid Abdelmagyd, H.A.; Ram Shetty, D.S.; Musa Musleh Al-Ahmari, D.M. Herbal medicine as adjunct in periodontal therapies- a review of clinical trials in past decade. J. Oral Biol. Craniofacial Res. 2019, 9, 212–217. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Kharaeva, Z.F.; Mustafaev, M.S.; Khazhmetov, A.V.; Gazaev, I.H.; Blieva, L.Z.; Steiner, L.; Mayer, W.; Luca, C.; Korkina, L.G. Anti-bacterial and anti-inflammatory effects of toothpaste with swiss medicinal herbs towards patients suffering from gingivitis and initial stage of periodontitis: From clinical efficacy to mechanisms. Dent. J. 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Kim, E.-K.; Deb Nath, N.C.; Lee, K.-G. Therapeutic effects of Ligularia stenocephala against inflammatory bowel disease by regulating antioxidant and inflammatory mediators. Food Agric. Immunol. 2017, 28, 1142–1154. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kim, W.-B.; Nam, J.-H.; Park, H.-J. Anti-diabetic effect of the methanolic extract of Ligularia stenocephala leaves in the streptozotocin-induced rat. Korean J. Plant Resour. 2007, 20, 362–366. [Google Scholar]
- Seo, D.; Cheon, W.; Kim, Y. Antioxidant activity and anti-adipogenic effect of Ligularia stenocephala extract. Korean J. Food Nutr. 2017, 30, 1292–1298. [Google Scholar]
- Gong, Z.; Chen, W.; Bao, G.; Sun, J.; Ding, X.; Fan, C. Physiological response of Secale cereale L. Seedlings under freezing-thawing and alkaline salt stress. Environ. Sci. Pollut. Res. Int. 2020, 27, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.F.; Landbo, A.K.; Christensen, L.P.; Hansen, A.; Meyer, A.S. Antioxidant effects of phenolic rye (Secale cereale L.) extracts, monomeric hydroxycinnamates, and ferulic acid dehydrodimers on human low-density lipoproteins. J. Agric. Food Chem. 2001, 49, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Branning, C.; Adawi, D.; Molin, G.; Nyman, M.; Jeppsson, B.; Ahrne, S. Blueberry husks, rye bran and multi-strain probiotics affect the severity of colitis induced by dextran sulphate sodium. Scand. J. Gastroenterol. 2009, 44, 1213–1225. [Google Scholar] [CrossRef]
- Hong, I.; Park, J.Y.; Noh, Y.H.; Jeon, S.H.; Paik, J.W.; Lee, J.S.; Choi, S.H.; Cha, J.K. Anti-inflammatory potential of complex extracts of Ligularia stenocephala matsum. & koidz. And Secale cereale L. Sprout in chronic gingivitis: In vitro investigation and randomized clinical trial. Antioxidants 2021, 10, 1586. [Google Scholar]
- Kukic, J.; Petrovic, S.; Niketic, M. Antioxidant activity of four endemic stachys taxa. Biol. Pharm. Bull. 2006, 29, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Gamisonia, A.M.; Yushina, M.N.; Fedorova-Gogolina, I.A.; Akimov, M.G.; Eldarov, C.M.; Pavlovich, S.V.; Bezuglov, V.V.; Gretskaya, N.M.; Sukhikh, G.T.; Bobrov, M.Y. N-acyl dopamines induce apoptosis in endometrial stromal cells from patients with endometriosis. Int. J. Mol. Sci. 2021, 22, 10648. [Google Scholar] [CrossRef] [PubMed]
- Boonanantanasarn, K.; Janebodin, K.; Suppakpatana, P.; Arayapisit, T.; Rodsutthi, J.A.; Chunhabundit, P.; Boonanuntanasarn, S.; Sripairojthikoon, W. Morinda citrifolia leaves enhance osteogenic differentiation and mineralization of human periodontal ligament cells. Dent. Mater. J. 2014, 33, 157–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, S.I.; Kang, S.J.; Han, C.H.; Kim, J.W.; Song, C.H.; Lee, S.N.; Ku, S.K.; Lee, Y.J. The effects of topical application of polycal (a 2:98 (g/g) mixture of polycan and calcium gluconate) on experimental periodontitis and alveolar bone loss in rats. Molecules 2016, 21, 527. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Patil, O.N.; Karasik, D.; Razdan, R. Development and evaluation of novel biodegradable chitosan based metformin intrapocket dental film for the management of periodontitis and alveolar bone loss in a rat model. Arch. Oral Biol. 2018, 85, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.M.; Rocha, F.A.; Chaves, H.V.; Carvalho, C.B.; Ribeiro, R.A.; Brito, G.A. Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. J. Periodontol. 2005, 76, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Cho, H.R.; Sung, Y.S.; Kang, S.J.; Lee, Y.J. Effects of calcium gluconate on experimental periodontitis and alveolar bone loss in rats. Basic Clin. Pharmacol. Toxicol. 2011, 108, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kang, S.J.; Kim, J.W.; Cho, H.R.; Moon, S.B.; Kim, K.Y.; Lee, H.S.; Han, C.H.; Ku, S.K.; Lee, Y.J. Effects of polycan, a beta-glucan, on experimental periodontitis and alveolar bone loss in sprague-dawley rats. J. Periodontal Res. 2012, 47, 800–810. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, C.A.; Lekic, P.; McKee, M.D. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontology 2000 2000, 24, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Merkel, A.; Chen, Y.; George, A. Endocytic trafficking of dmp1 and grp78 complex facilitates osteogenic differentiation of human periodontal ligament stem cells. Front. Physiol. 2019, 10, 1175. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef] [PubMed]
- Lymperi, S.; Ligoudistianou, C.; Taraslia, V.; Kontakiotis, E.; Anastasiadou, E. Dental stem cells and their applications in dental tissue engineering. Open Dent. J. 2013, 7, 76–81. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. Shed: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Chadipiralla, K.; Yochim, J.M.; Bahuleyan, B.; Huang, C.Y.; Garcia-Godoy, F.; Murray, P.E.; Stelnicki, E.J. Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res. 2010, 340, 323–333. [Google Scholar] [CrossRef]
- Akalin, F.A.; Baltacioglu, E.; Alver, A.; Karabulut, E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J. Clin. Periodontol. 2007, 34, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Brock, G.R.; Milward, M.R.; Ling, N.; Matthews, J.B. Compromised gcf total antioxidant capacity in periodontitis: Cause or effect? J. Clin. Periodontol. 2007, 34, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Jeong, K.; Rodriguez, Y.A.R.; Kim, J.H.; Ahn, E.E.; Lim, S.S. Fak and pyk2 activity promote tnf-alpha and il-1beta-mediated pro-inflammatory gene expression and vascular inflammation. Sci. Rep. 2019, 9, 7617. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
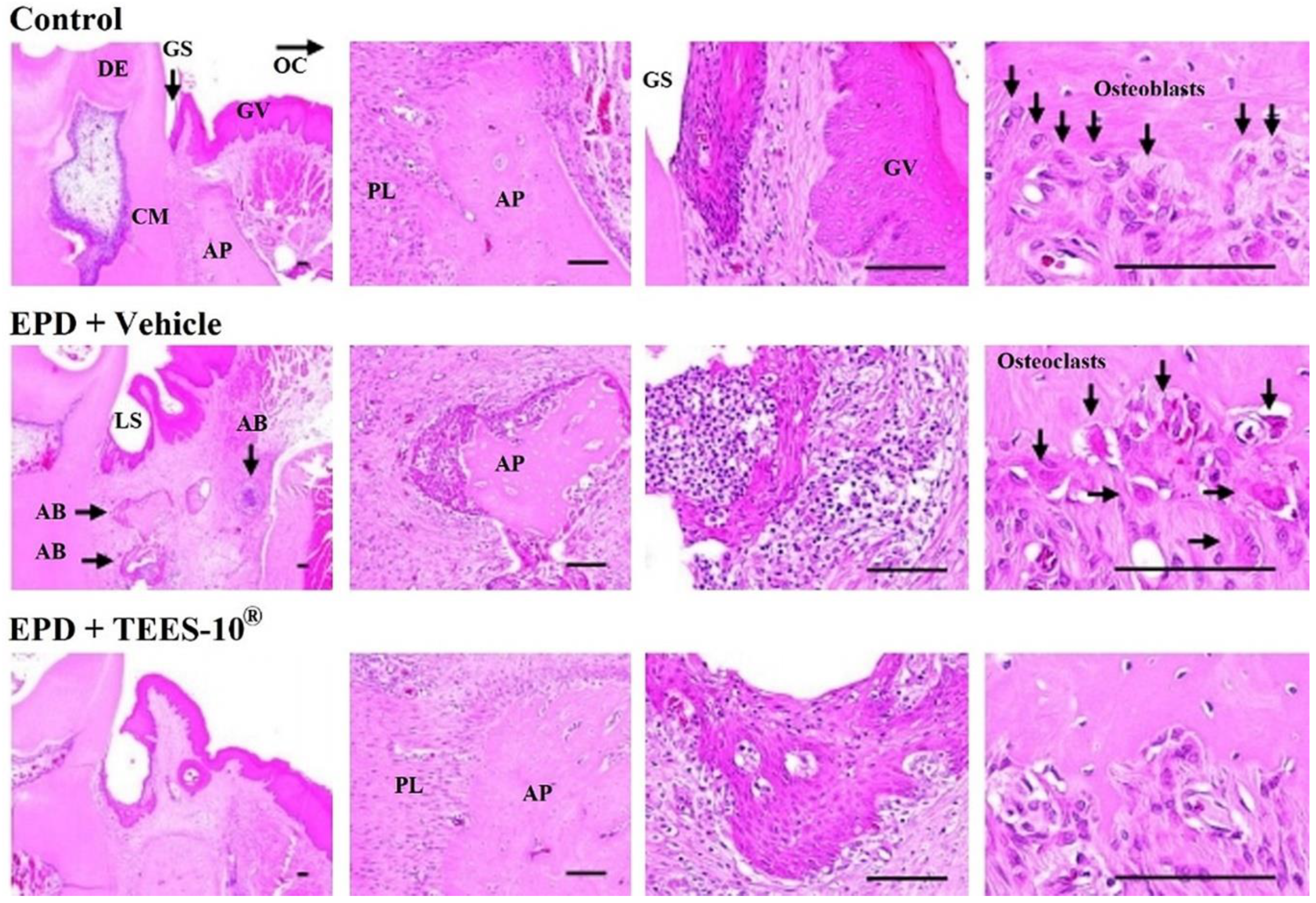
| Scores | Remarks |
|---|---|
| 0 | Absence or only a discrete cellular infiltration (inflammatory cell infiltration is sparse and restricted to the region of the marginal gingival), preserved alveolar process and cementum |
| 1 | Moderate cellular infiltration (inflammatory cellular infiltration present all over the insert gingival), some but minor alveolar process resorption and intact cementum |
| 2 | Accentuated cellular infiltration (inflammatory cellular infiltration present in both gingival and periodontal ligament), accentuated degradation of the alveolar process and partial destruction of cementum |
| 3 | Accentuated cellular infiltrate, complete resorption of the alveolar process and severe destruction of cementum |
| Max = 3 | |
| Groups | Histological Scores (Max = 3) | Inflammatory Cell Numbers (cells/mm2 of Gingival Tissues) | Alveolar Process Volumes (%) | Osteoclast Cell Numbers (cells/mm2 of Alveolar Gingival Tissues) | Osteoblast Cell Numbers (cells/mm2 of Alveolar Gingival Tissues) |
|---|---|---|---|---|---|
| Control | 0.20 ± 0.45 | 27.20 ± 8.20 | 70.93 ± 6.50 | 9.60 ± 4.56 | 91.60 ± 15.65 |
| EPD + Vehicle | 2.44 ± 0.73 a | 414.89 ± 280.59 c | 36.17 ± 12.20 c | 38.00 ± 9.27 a | 18.33 ± 7.14 a |
| EPD + TEES-10® | 0.89 ± 0.33 a, b | 133.78 ± 50.35 c, d | 61.77 ± 5.39 c, d | 2.89 ± 3.89 b | 56.67 ± 12.49 a, b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kim, I.H.; Hong, J.; Jeon, B.-J.; Kim, S.-S.; Lee, J.-W.; Park, J.-Y.; Choi, S.-H.; Lee, T.-K.; Cha, J.-K.; et al. In Vitro and In Vivo Anti-Inflammatory Effects of TEES-10®, a Mixture of Ethanol Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout, on Gingivitis and Periodontitis. Dent. J. 2022, 10, 143. https://doi.org/10.3390/dj10080143
Lee S, Kim IH, Hong J, Jeon B-J, Kim S-S, Lee J-W, Park J-Y, Choi S-H, Lee T-K, Cha J-K, et al. In Vitro and In Vivo Anti-Inflammatory Effects of TEES-10®, a Mixture of Ethanol Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout, on Gingivitis and Periodontitis. Dentistry Journal. 2022; 10(8):143. https://doi.org/10.3390/dj10080143
Chicago/Turabian StyleLee, Seungah, In Hye Kim, Junkee Hong, Byung-Ju Jeon, Sung-Su Kim, Ji-Won Lee, Jin-Young Park, Seong-Ho Choi, Tae-Kyeong Lee, Jae-Kook Cha, and et al. 2022. "In Vitro and In Vivo Anti-Inflammatory Effects of TEES-10®, a Mixture of Ethanol Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout, on Gingivitis and Periodontitis" Dentistry Journal 10, no. 8: 143. https://doi.org/10.3390/dj10080143
APA StyleLee, S., Kim, I. H., Hong, J., Jeon, B.-J., Kim, S.-S., Lee, J.-W., Park, J.-Y., Choi, S.-H., Lee, T.-K., Cha, J.-K., & Won, M.-H. (2022). In Vitro and In Vivo Anti-Inflammatory Effects of TEES-10®, a Mixture of Ethanol Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout, on Gingivitis and Periodontitis. Dentistry Journal, 10(8), 143. https://doi.org/10.3390/dj10080143