The Interaction of Craniofacial Morphology and Body Mass Index in Obstructive Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
- -
- the SNA angle, SNB angle, ANB angle: sagittal facial projection
- -
- the FMA angle: between the mandibular plane (Go-Me) and the Frankfurt plane
- -
- the occlusal plane angle: formed between the Frankfurt plane and the occlusal plane (OCLP-OCLA)
- -
- AO-BO: the millimeter distance in the occlusal plane between the orthogonal projections of points A and B.
- -
- PFH (posterior facial height)
- -
- AFH (anterior facial height).
- -
- SPAS: the thickness of the airway behind the soft palate along a line parallel to the Go-point B plane.
- -
- PAS: (oropharyngeal space): linear distance between a point at the base of the tongue and another point on the posterior wall of the pharynx, both measured by the extension of a line from point B to point Go.
- -
- MP-H: linear distance between H, the most anterosuperior point of the hyoid bone, and the mandibular plane measured perpendicularly to the latter.
- -
- PNS-P: soft palate length.
- -
- C3-H: linear distance between points C and H, where C3 is the most anteroinferior point of the third cervical vertebra.
3. Results
3.1. Normal-Weight Group
3.2. Overweight Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 15, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 23, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armalaite, J.; Lopatiene, K. Lateral teleradiography of the head as a diagnostic tool used to predict obstructive sleep apnea. Dentomaxillofac. Radiol. 2016, 45, 20150085. [Google Scholar] [CrossRef] [PubMed]
- Battagel, J.M.; L’Estrange, P.R. The cephalometric morphology of patients with obstructive sleep apnoea (OSA). Eur. J. Orthod. 1996, 18, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, B.C.; Kharbanda, O.P.; Sardana, H.K.; Balachandran, R.; Sardana, V.; Kapoor, P.; Gupta, A.; Vasamsetti, S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 2017, 31, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Gungor, A.Y.; Turkkahraman, H.; Yilmaz, H.H.; Yariktas, M. Cephalometric comparison of obstructive sleep apnea patients and healthy controls. Eur. J. Dent. 2013, 7, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Iked, N.; Hazime, N.; Dekeister, C.; Folia, M.; Tiberge, M.; Paoli, J.R. Comparison of the cephalometric characteristics of snoring patients and apneic patients as a function of the degree of obesity. Apropos of 162 cases. Rev. Stomatol. Chir. Maxillofac. 2001, 102, 305–311. [Google Scholar]
- Insalaco, G.; Calabro, S. Nuovi Orizzonti. Vol. Il Sonno e le Patologie Respiratorie. AIPO. Available online: http://www.aiponet.it/component/attachments/download/1075.html (accessed on 13 January 2022).
- Hoekema, A.; Stegenga, B.; Wijkstra, P.J.; van der Hoeven, J.H.; Meinesz, A.F.; de Bont, L.G. Obstructive sleep apnea therapy. J. Dent. Res. 2008, 87, 882–887. [Google Scholar] [CrossRef]
- Iftikhar, I.H.; Bittencourt, L.; Youngstedt, S.D.; Ayas, N.; Cistulli, P.; Schwab, R.; Durkin, M.W.; Magalang, U.J. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: A network meta-analysis. Sleep Med. 2017, 30, 7–14. [Google Scholar] [CrossRef]
- Segù, M.; Cosi, A.; Santagostini, A.; Scribante, A. Efficacy of a trial oral appliance in OSAS management: A new protocol to recognize responder/nonresponder patients. Int. J. Dent. 2021, 2021, 8811700. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Isono, S.; Tanaka, A.; Tanzawa, H.; Nishino, T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2002, 165, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Lee, R.W.W.; Cistulli, P.A. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: Impact of ethnicity. Respirology 2012, 17, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Pillar, G.; Peretz, L. Obstructive sleep apnea: Diagnosis, risk factors and pathophysiology. In Handbook of Clinical Neurology, 3rd ed.; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Sleep Disorders, Part I.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 98. [Google Scholar]
- Carberry, J.C.; Amatoury, J.; Eckert, D.J. Personalized management approach for OSA. Chest 2018, 153, 744–755. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Bhattacharya, D.; Thukral, B.B.; Suri, J.C. Craniofacial and upper airway profile assessment in North Indian patients with obstructive sleep apnea. Lung India 2019, 36, 94. [Google Scholar]
- Ryu, H.-H.; Kim, C.-H.; Cheon, S.-M.; Bae, W.-Y.; Kim, S.-H.; Koo, S.-K.; Kim, M.S.; Kim, B.J. The usefulness of cephalometric measurement as a diagnostic tool for obstructive sleep apnea syndrome: A retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 20–31. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and management of obstructive sleep apnea: A review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Stipa, C.; Cameli, M.; Sorrenti, G.; Ippolito, D.R.; Pelligra, I.; Alessandri-Bonetti, G. Relationship between cephalometric parameters and the apnoea-hypopnoea index in OSA patients: A retrospective cohort study. Eur. J. Orthod. 2020, 42, 101–106. [Google Scholar] [CrossRef]
- Borges, P.d.T.M.; Filho, E.S.F.; Araujo TME de Neto, J.M.M.; Borges, N.E.d.S.; Neto, B.M.; Campleo, V.; Paschoal, J.R.; Li, L.M. Correlation of cephalometric and anthropometric measures with obstructive sleep apnea severity. Int. Arch. Otorhinolaryngol. 2013, 17, 321–328. [Google Scholar]
- Silva, V.G.; Pinheiro, L.A.M.; Silveira PL da Duarte, A.S.M.; Faria, A.C.; Carvalho EGBZancarella, E.; Crespo, A.N. Correlation between cephalometric data and severity of sleep apnea. Braz. J. Otorhinolaryngol. 2014, 80, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Bertuzzi, F.; Santagostini, A.; Pollis, M.; Segù, M. Analisi cefalometrica craniofacciale e delle vie aeree in pazienti OSAS. Revisione della letteratura. Dent. Cadmos. 2022. [Google Scholar] [CrossRef]
| Sella | S | Midpoint of the sella turcica |
| Nasion | N | Most anterior point of the frontonasal suture |
| Point A | A | Deepest anterior point on the maxilla anterior concavity |
| Point B | B | Deepest anterior point on the mandibular symphysis |
| Porion | Po | Most superior point on the external auditory meatus |
| Pogonion | Pg | Most anterior point on the mandibular symphysis |
| Pterion | Pt | Most posterior superior point on the pterygomaxillary fissure |
| Orbitale | Or | Most inferior point on the lower border of the bony orbit |
| Basion | Ba | Most anterior-inferior point on the foramen magnum |
| Articulare | Ar | Most posterior point on the condylar neck |
| Gonion | Go | Point of intersection between the mandibular plane and the tangent line to the posterior mandibular border |
| Menton | Me | Most inferior point on the mandibular symphysis |
| Anterior nasal spine | ANS | Most anterior point of the hard palate |
| Posterior nasal spine | PNS | Most posterior point of the hard palate |
| Condilo | Co | Most superior point on the condylar head |
| Sigmoid incision | Sg | Deepest point on the sigmoid incision |
| Anterior occlusal point | OCLA | Midpoint of the segment joining the upper incisal point to the lower one |
| Posterior occlusal point | OCLP | Midpoint of the occlusal surface of the first permanent molars |
| Hyoid | H | Most anterior-superior point on the hyoid bone |
| Upper posterior airway space | SPAS | Thickness of the airway behind the soft palate along a line parallel to the Go-point B plane |
| Posterior airway space | PAS: | Linear distance between a point at the base of the tongue and another point on the posterior wall of the pharynx, both measured by the extension of a line from point B to point Go |
| Mandibular plane | MP | Plane tangent to the lower edge of the mandible passing through Go and Me |
| Cervical vertebra | C3 | Most anteroinferior point of the third cervical vertebra |
| Uvula apex | P | Inferior tip of the uvula |
| AHI | AHI Supine | Mean SaO2% | ODI | ||
|---|---|---|---|---|---|
| SNA angle | Pearson correlation | −0.004 | −0.342 | 0.044 | −0.068 |
| Sig. (2-tailed) | 0.982 | 0.120 | 0.826 | 0.729 | |
| N | 30 | 22 | 28 | 28 | |
| SNB angle | Pearson correlation | 0.008 | −0.160 | −0.103 | 0.038 |
| Sig. (2-tailed) | 0.968 | 0.476 | 0.602 | 0.848 | |
| N | 30 | 22 | 28 | 28 | |
| ANB angle | Pearson correlation | −0.022 | −0.171 | 0.180 | −0.120 |
| Sig. (2-tailed) | 0.907 | 0.447 | 0.359 | 0.543 | |
| N | 30 | 22 | 28 | 28 | |
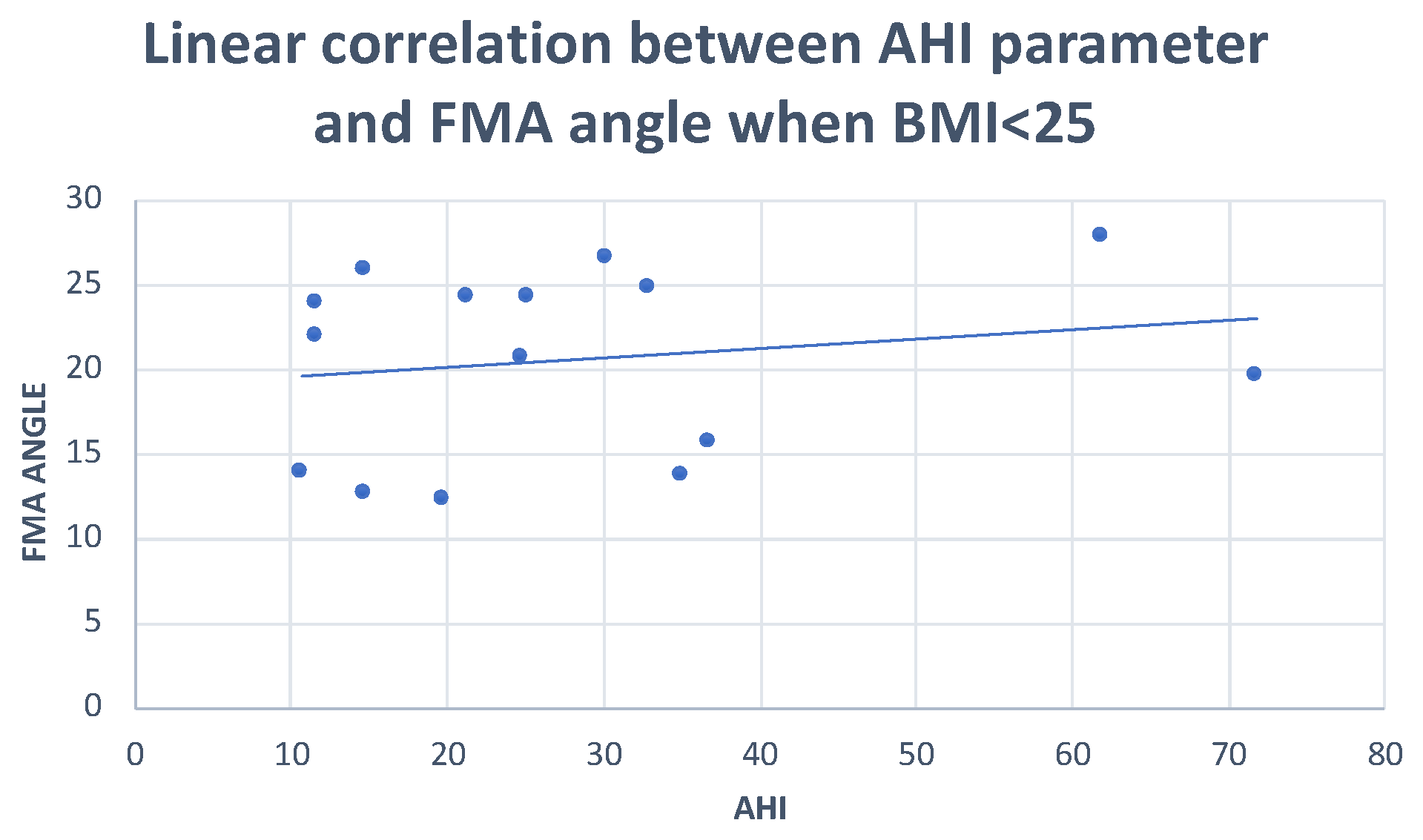
| FMA angle | Pearson correlation | −0.018 | 0.043 | 0.161 | −0.175 |
| Sig. (2-tailed) | 0.925 | 0.849 | 0.412 | 0.372 | |
| N | 30 | 22 | 28 | 28 | |
| AO-BO | Pearson correlation | −0.080 | −0.155 | 0.177 | −0.166 |
| Sig. (2-tailed) | 0.675 | 0.491 | 0.367 | 0.399 | |
| N | 30 | 22 | 28 | 28 | |
| Occlusal plane angle | Pearson correlation | 0.032 | 0.091 | 0.076 | 0.003 |
| Sig. (2-tailed) | 0.867 | 0.686 | 0.699 | 0.988 | |
| N | 30 | 22 | 28 | 28 | |
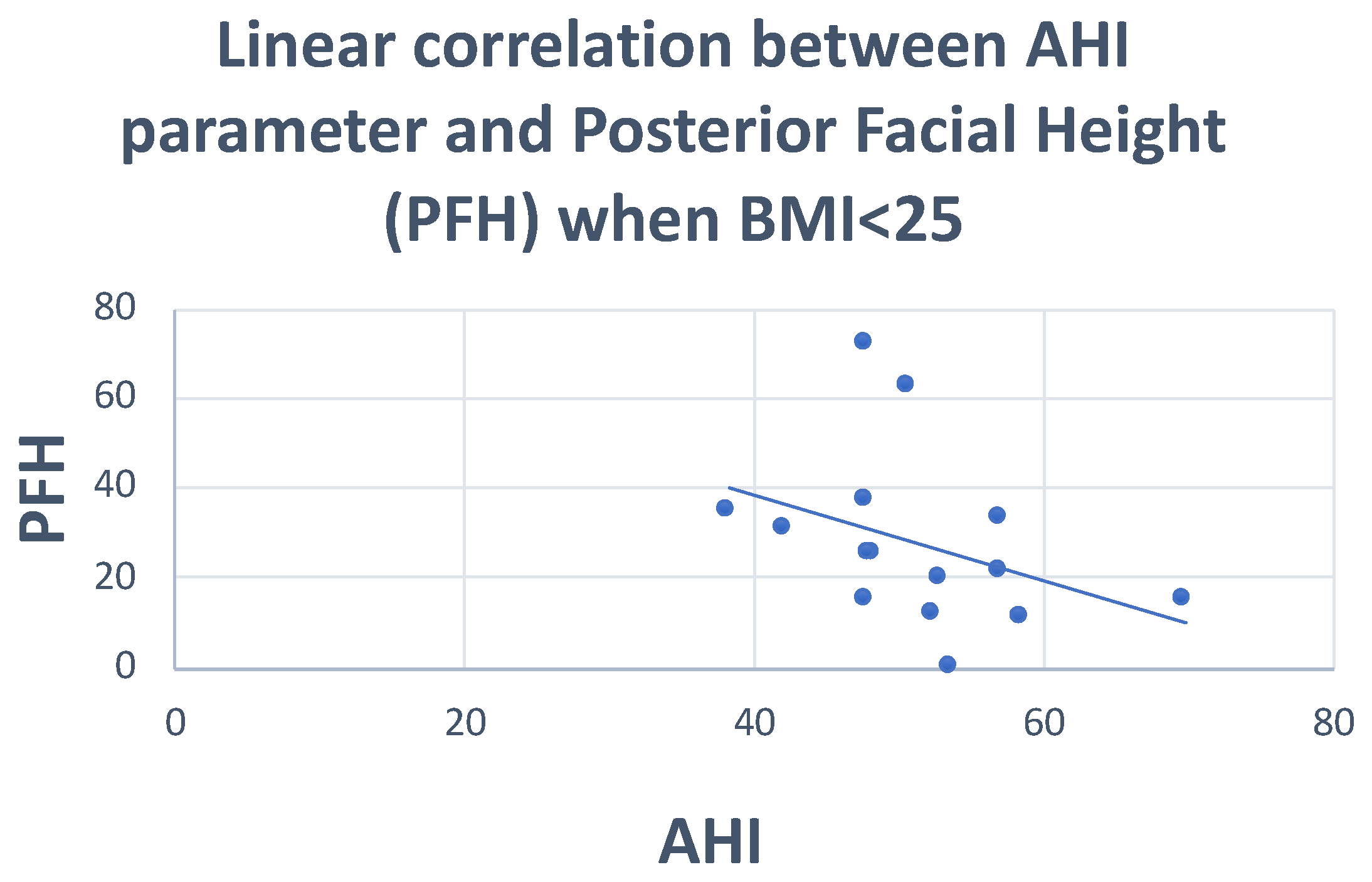
| PFH | Pearson correlation | −0.110 | −0.096 | −0.099 | 0.103 |
| Sig. (2-tailed) | 0.564 | 0.671 | 0.616 | 0.601 | |
| N | 30 | 22 | 28 | 28 | |
| AFH | Pearson correlation | 0.228 | 0.191 | −0.235 | 0.397 |
| Sig. (2-tailed) | 0.225 | 0.394 | 0.229 | 0.036 | |
| N | 30 | 22 | 28 | 28 | |
| SPAS | Pearson correlation | −0.148 | 0.142 | 0.092 | −0.159 |
| Sig. (2-tailed) | 0.436 | 0.529 | 0.640 | 0.420 | |
| N | 30 | 22 | 28 | 28 | |
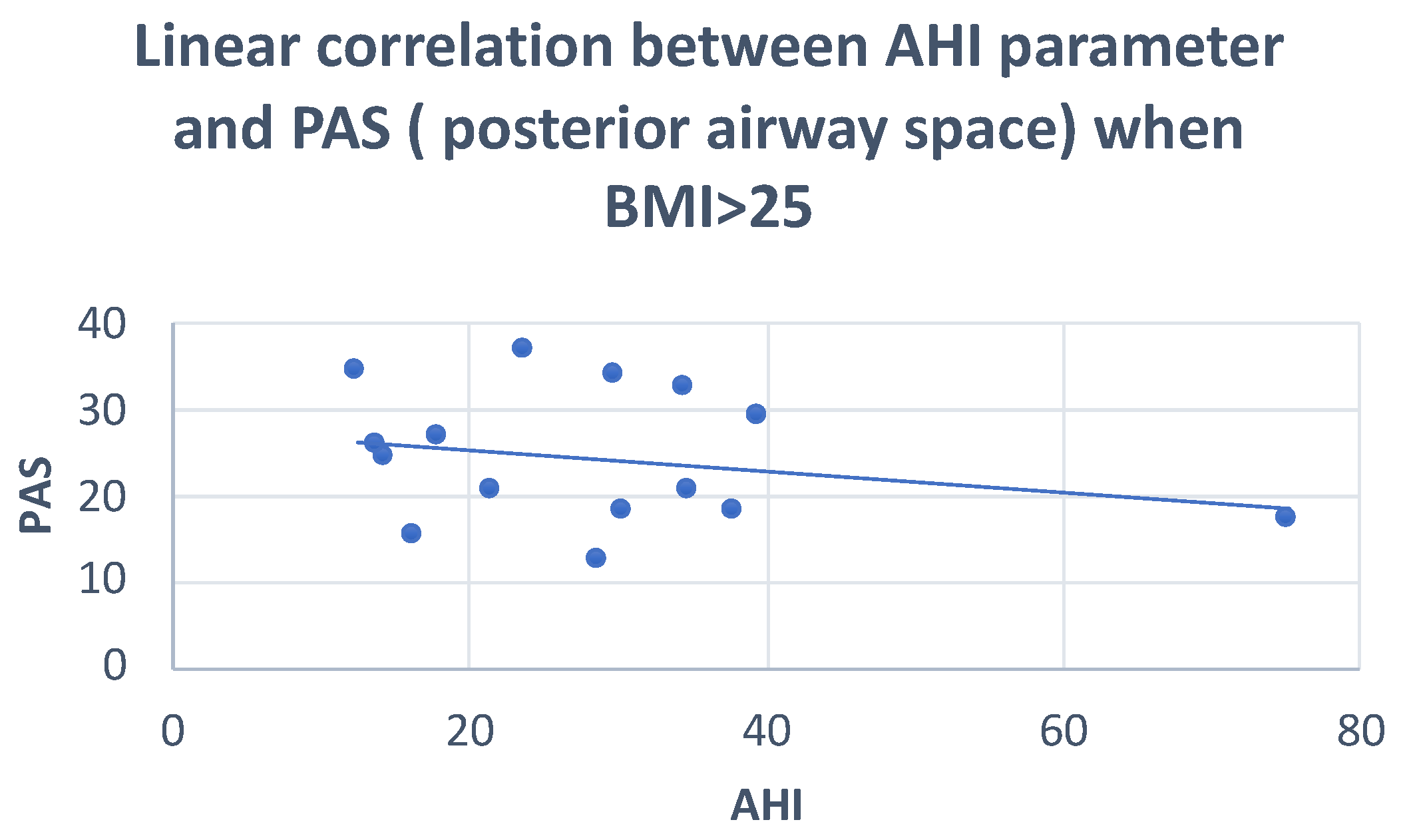
| PAS | Pearson correlation | −0.068 | 0.064 | −0.070 | 0.013 |
| Sig. (2-tailed) | 0.720 | 0.779 | 0.725 | 0.947 | |
| N | 30 | 22 | 28 | 28 | |
| Pns-P | Pearson correlation | 0.147 | −0.183 | −0.080 | 0.082 |
| Sig. (2-tailed) | 0.439 | 0.414 | 0.687 | 0.679 | |
| N | 30 | 22 | 28 | 28 | |
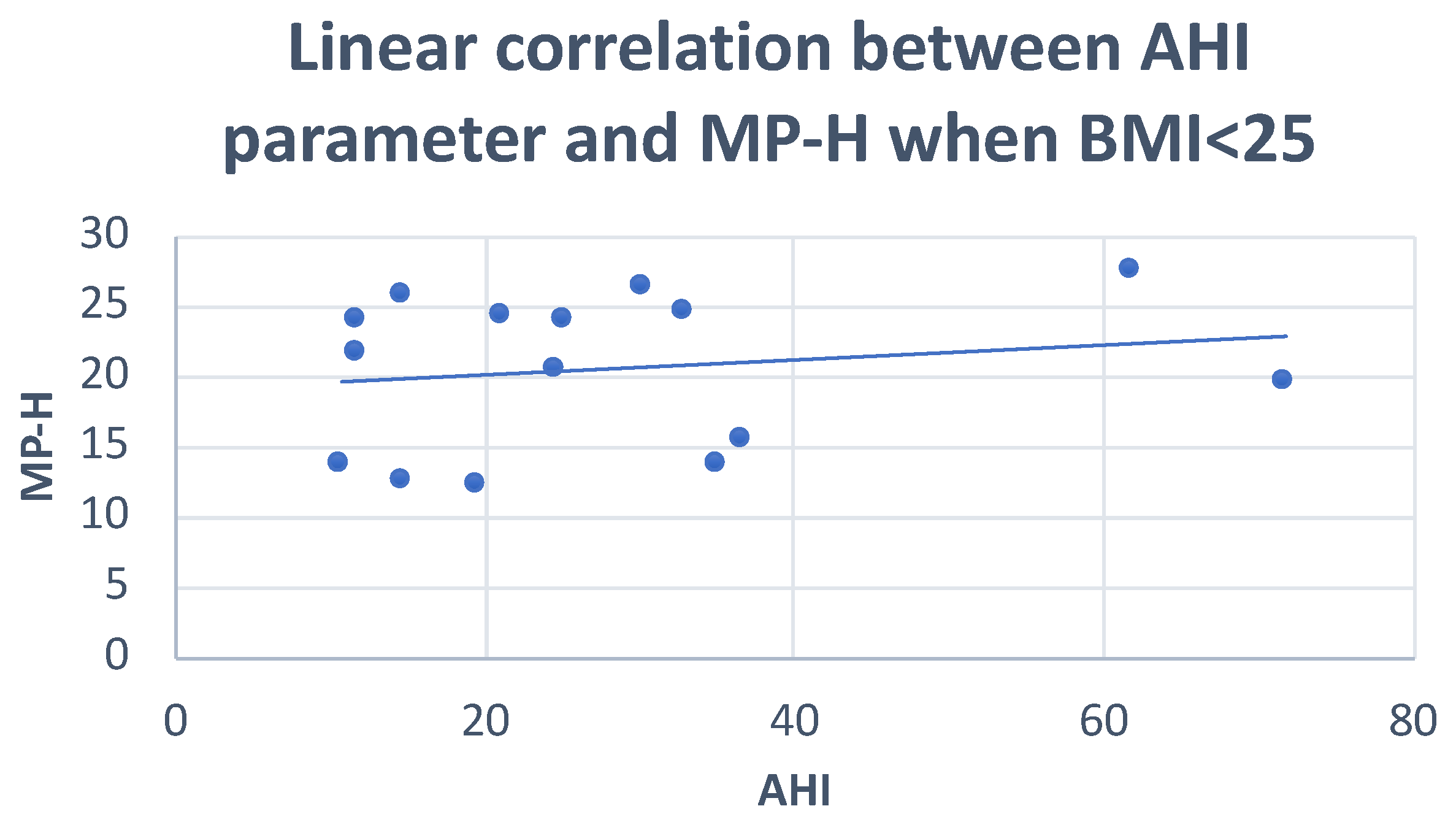
| MP-H | Pearson correlation | 0.013 | 0.287 | −0.136 | 0.053 |
| Sig. (2-tailed) | 0.945 | 0.196 | 0.489 | 0.788 | |
| N | 30 | 22 | 28 | 28 | |
| H-C3 | Pearson correlation | 0.106 | 0.087 | −0.216 | 0.213 |
| Sig. (2-tailed) | 0.578 | 0.700 | 0.269 | 0.276 | |
| N | 30 | 22 | 28 | 28 | |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | Sig. | ||
| SNA angle | Between groups | 17,051 | 2 | 8525 | 0.938 | 0.404 |
| Within groups | 245,381 | 27 | 9088 | |||
| Total | 262,432 | 29 | ||||
| SNB angle | Between groups | 11,414 | 2 | 5707 | 0.433 | 0.653 |
| Within groups | 355,725 | 27 | 13,175 | |||
| Total | 367,139 | 29 | ||||
| ANB angle | Between groups | 853 | 2 | 426 | 0.052 | 0.949 |
| Within groups | 219,421 | 27 | 8127 | |||
| Total | 220,274 | 29 | ||||
| FMA angle | Between groups | 4312 | 2 | 2156 | 0.045 | 0.956 |
| Within groups | 1,302,595 | 27 | 48,244 | |||
| Total | 1,306,907 | 29 | ||||
| AO-BO | Between groups | 7748 | 2 | 3874 | 0.175 | 0.840 |
| Within groups | 597,539 | 27 | 22,131 | |||
| Total | 605,287 | 29 | ||||
| Occlusal plane angle | Between groups | 2350 | 2 | 1175 | 0.046 | 0.955 |
| Within groups | 682,843 | 27 | 25,290 | |||
| Total | 685,194 | 29 | ||||
| PFH | Between groups | 506,211 | 2 | 253,105 | 2.132 | 0.138 |
| Within groups | 3,205,448 | 27 | 118,720 | |||
| Total | 3,711,659 | 29 | ||||
| AFH | Between groups | 111,739 | 2 | 55,870 | 0.632 | 0.539 |
| Within groups | 2,387,451 | 27 | 88,424 | |||
| Total | 2,499,190 | 29 | ||||
| SPAS | Between groups | 6855 | 2 | 3427 | 0.547 | 0.585 |
| Within groups | 169,140 | 27 | 6264 | |||
| Total | 175,995 | 29 | ||||
| PAS | Between groups | 11,703 | 2 | 5852 | 1.225 | 0.310 |
| Within groups | 129,006 | 27 | 4778 | |||
| Total | 140,710 | 29 | ||||
| MP-H | Between groups | 5243 | 2 | 2622 | 0.079 | 0.925 |
| Within groups | 900,285 | 27 | 33,344 | |||
| Total | 905,528 | 29 | ||||
| Pns-P | Between groups | 40,620 | 2 | 20,310 | 0.540 | 0.589 |
| Within groups | 1,015,350 | 27 | 37,606 | |||
| Total | 1,055,970 | 29 | ||||
| H-C3 | Between groups | 15,113 | 2 | 7557 | 0.198 | 0.821 |
| Within groups | 1,029,136 | 27 | 38,116 | |||
| Total | 1,044,250 | 29 | ||||
| Dependent Variable | (I) AHI_NOM | (J) AHI_NOM | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| PFH | 1 | 2 | −4.6806 | 5.2944 | 0.384 | −15.544 | 6.183 |
| 3 | 5.0135 | 4.8962 | 0.315 | −5.033 | 15.060 | ||
| 2 | 1 | 4.6806 | 5.2944 | 0.384 | −6.183 | 15.544 | |
| 3 | 9.6940 | 4.7248 | 0.050 | 0.000 | 19.388 | ||
| AHI | AHI Supine | ||
|---|---|---|---|
| AHI | Pearson correlation | 1 | 0.489 |
| Sig. (2-tailed) | 0.127 | ||
| N | 15 | 11 | |
| AHI supine | Pearson correlation | 0.489 | 1 |
| Sig. (2-tailed) | 0.127 | ||
| N | 11 | 11 | |
| SNA angle | Pearson correlation | −0.292 | −0.163 |
| Sig. (2-tailed) | 0.047 | 0.633 | |
| N | 15 | 11 | |
| SNB angle | Pearson correlation | −0.078 | 0.063 |
| Sig. (2-tailed) | 0.781 | 0.854 | |
| N | 15 | 11 | |
| ANB angle | Pearson correlation | −0.148 | −0.207 |
| Sig. (2-tailed) | 0.599 | 0.542 | |
| N | 15 | 11 | |
| FMA angle | Pearson correlation | 0.299 | 0.277 |
| Sig. (2-tailed) | 0.047 | 0.409 | |
| N | 15 | 11 | |
| AO-BO | Pearson correlation | 0.159 | −0.034 |
| Sig. (2-tailed) | 0.571 | 0.922 | |
| N | 15 | 11 | |
| Occlusal plane angle | Pearson correlation | −0.070 | −0.275 |
| Sig. (2-tailed) | 0.803 | 0.414 | |
| N | 15 | 11 | |
| AFH | Pearson correlation | −0.109 | 0.271 |
| Sig. (2-tailed) | 0.700 | 0.421 | |
| N | 15 | 11 | |
| PFH | Pearson correlation | −0.355 | −0.181 |
| Sig. (2-tailed) | 0.050 | 0.595 | |
| N | 15 | 11 | |
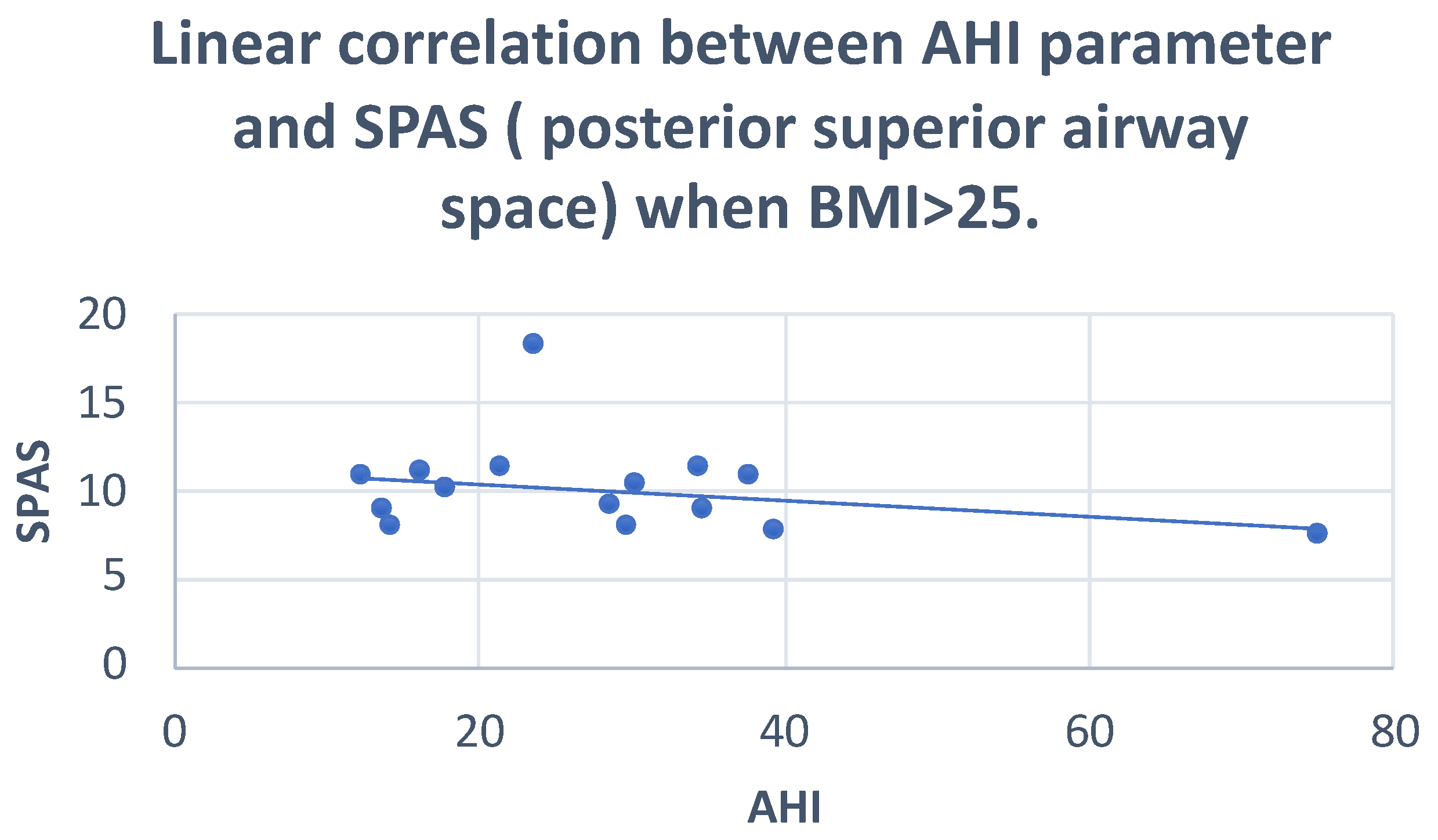
| SPAS | Pearson correlation | −0.020 | 0.294 |
| Sig. (2-tailed) | 0.943 | 0.381 | |
| N | 15 | 11 | |
| PAS | Pearson correlation | 0.137 | 0.173 |
| Sig. (2-tailed) | 0.627 | 0.610 | |
| N | 15 | 11 | |
| MP-H | Pearson correlation | 0.201 | 0.501 |
| Sig. (2-tailed) | 0.050 | 0.117 | |
| N | 15 | 11 | |
| Pns-P | Pearson correlation | 0.144 | −0.172 |
| Sig. (2-tailed) | 0.609 | 0.612 | |
| N | 15 | 11 | |
| H-C3 | Pearson correlation | 0.304 | 0.200 |
| Sig. (2-tailed) | 0.270 | 0.556 | |
| N | 15 | 11 | |
| AHI | AHI Supine | ||
|---|---|---|---|
| AHI | Pearson correlation | 1 | 0.489 |
| Sig. (2-tailed) | 0.127 | ||
| N | 15 | 11 | |
| AHI supine | Pearson correlation | 0.489 | 1 |
| Sig. (2-tailed) | 0.127 | ||
| N | 11 | 11 | |
| SNA angle | Pearson correlation | −0.292 | −0.163 |
| Sig. (2-tailed) | 0.291 | 0.633 | |
| N | 15 | 11 | |
| SNB angle | Pearson correlation | −0.078 | 0.063 |
| Sig. (2-tailed) | 0.781 | 0.854 | |
| N | 15 | 11 | |
| ANB angle | Pearson correlation | −0.148 | −0.207 |
| Sig. (2-tailed) | 0.599 | 0.542 | |
| N | 15 | 11 | |
| FMA angle | Pearson correlation | 0.247 | 0.277 |
| Sig. (2-tailed) | 0.046 | 0.409 | |
| N | 15 | 11 | |
| AO-BO | Pearson correlation | −0.300 | −0.034 |
| Sig. (2-tailed) | 0.046 | 0.922 | |
| N | 15 | 11 | |
| Occlusal plane angle | Pearson correlation | −0.070 | −0.275 |
| Sig. (2-tailed) | 0.803 | 0.414 | |
| N | 15 | 11 | |
| AFH | Pearson correlation | 0.321 | 0.271 |
| Sig. (2-tailed) | 0.050 | 0.421 | |
| N | 15 | 11 | |
| PFH | Pearson correlation | −0.375 | −0.181 |
| Sig. (2-tailed) | 0.168 | 0.595 | |
| N | 15 | 11 | |
| SPAS | Pearson correlation | −0.280 | 0.294 |
| Sig. (2-tailed) | 0.050 | 0.381 | |
| N | 15 | 11 | |
| PAS | Pearson correlation | −0.244 | 0.173 |
| Sig. (2-tailed) | 0.044 | 0.610 | |
| N | 15 | 11 | |
| MP-H | Pearson correlation | 0.183 | 0.501 |
| Sig. (2-tailed) | 0.514 | 0.117 | |
| N | 15 | 11 | |
| Pns-P | Pearson correlation | 0.144 | −0.172 |
| Sig. (2-tailed) | 0.609 | 0.612 | |
| N | 15 | 11 | |
| H-C3 | Pearson correlation | 0.304 | 0.200 |
| Sig. (2-tailed) | 0.270 | 0.556 | |
| N | 15 | 11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuzzi, F.; Santagostini, A.; Pollis, M.; Meola, F.; Segù, M. The Interaction of Craniofacial Morphology and Body Mass Index in Obstructive Sleep Apnea. Dent. J. 2022, 10, 136. https://doi.org/10.3390/dj10070136
Bertuzzi F, Santagostini A, Pollis M, Meola F, Segù M. The Interaction of Craniofacial Morphology and Body Mass Index in Obstructive Sleep Apnea. Dentistry Journal. 2022; 10(7):136. https://doi.org/10.3390/dj10070136
Chicago/Turabian StyleBertuzzi, Federica, Antonio Santagostini, Matteo Pollis, Fabio Meola, and Marzia Segù. 2022. "The Interaction of Craniofacial Morphology and Body Mass Index in Obstructive Sleep Apnea" Dentistry Journal 10, no. 7: 136. https://doi.org/10.3390/dj10070136
APA StyleBertuzzi, F., Santagostini, A., Pollis, M., Meola, F., & Segù, M. (2022). The Interaction of Craniofacial Morphology and Body Mass Index in Obstructive Sleep Apnea. Dentistry Journal, 10(7), 136. https://doi.org/10.3390/dj10070136






