CAMBRA Protocol Efficacy: A Systematic Review and Critical Appraisal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
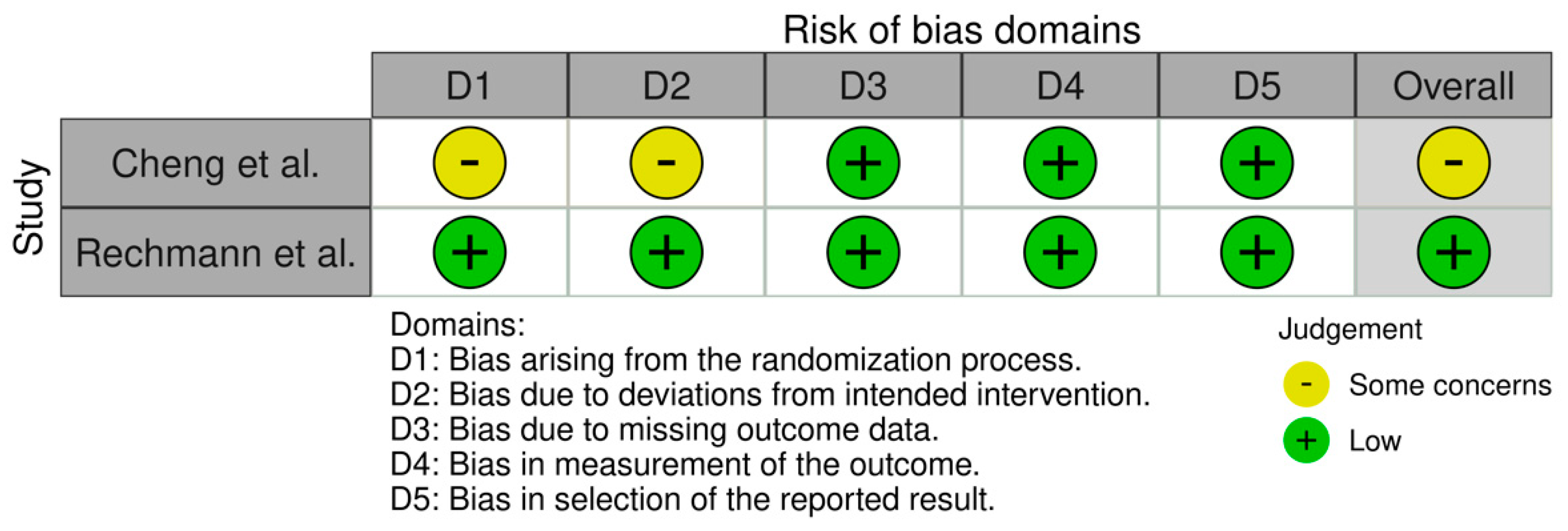
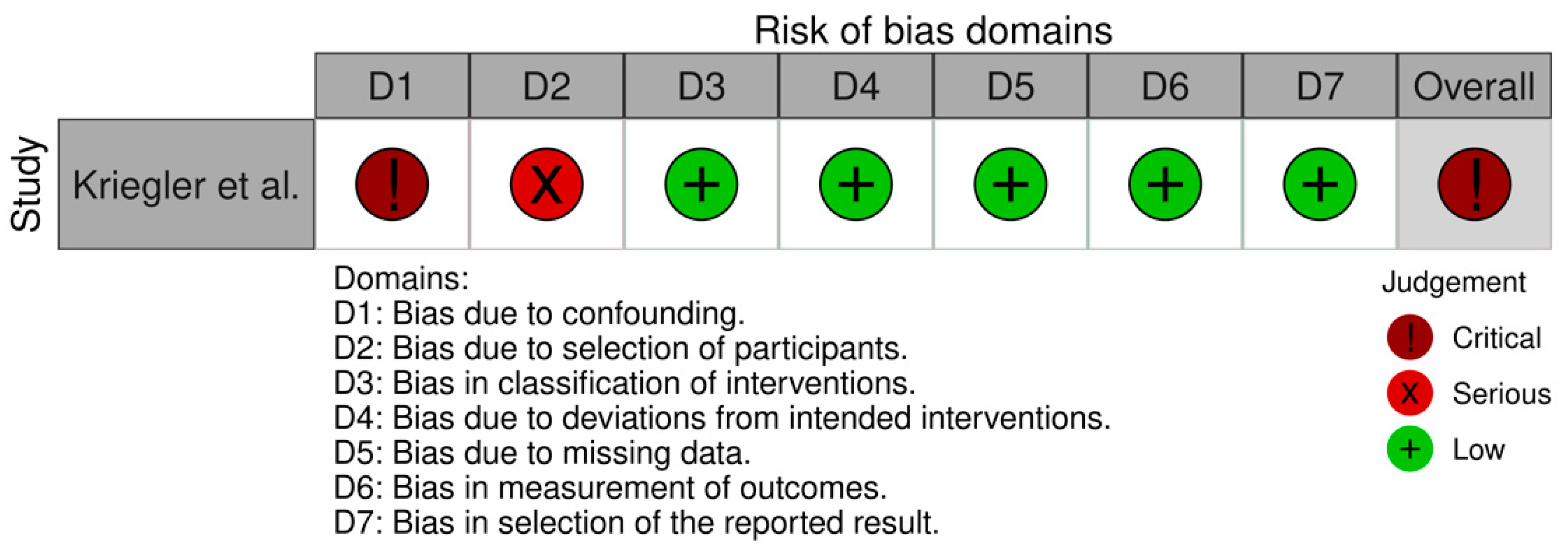
2.5. Quality Assessment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kutsch, V.K. Dental caries: An update medical model of risk assessment. J. Prosthet. Dent. 2013, 111, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dunamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 6, 51–59. [Google Scholar] [CrossRef]
- Manji, F.; Dahlen, G.; Fejerskov, O. Caries and Periodontitis: Contesting the Conventional Wisdom on Their Aetiology. Caries Res. 2018, 52, 548–564. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, S. Caries-Preventive Effects of Fluoride Products when Used in Conjunction with Fluoride Dentifrice. Caries Res. 2001, 35, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chaffee, B.W.; Cheng, N.F.; Gansky, S.A.; Featherstone, J.D. Understanding treatment effect mechanisms of the CAMBRA randomized trial in reducing caries increment. J. Dent. Res. 2015, 94, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Featherstone, J.D.; White, J.M.; Hoover, C.I.; Rapozo-Hilo, M.; Weintraub, J.A.; Wilson, R.S.; Zhan, L.; Gansky, S.A. A randomized clinical trial of anticaries therapies targeted according to risk assessment (caries management by risk assessment). Caries Res. 2012, 46, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Featherstone, J.D. The caries balance: The basis for caries management by risk assessment. Oral Health Prev. Dent. 2004, 2, 259–264. [Google Scholar]
- Walsh, L.J.; Brostek, A.M. Minimum intervention dentistry principles and objectives. Aust. Dent. J. 2013, 58, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, D.A.; Featherstone, J.D. Caries management by risk assessment. Community Dent. Oral Epidemiol. 2013, 41, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.B.R.; Jon, R. Caries moving restoration toward prevention—Part 1. J. Calif. Dent. Assoc. 2003, 31, 123–185. [Google Scholar]
- Featherstone, J.D.B.R.; Jon, R.; Anderson, M.H.; Donly, K.J.; Adair, S.M.; Hicks, J.; Garcia-Godoy, F.; Donly, K.; Flaitz, C.; Stewart, R.E.; et al. Caries moving restoration toward prevention—Part 2. J. Calif. Dent. Assoc. 2003, 31, 203–261. [Google Scholar]
- Young, D.A.F.; John, D.B.; Roth, J.R. Caries risk assessment—Part 1. J. Calif. Dent. Assoc. 2007, 35, 679–753. [Google Scholar]
- Young, D.A.F.; John, D.B.; Roth, J.R. Caries risk assessment—Part 2. J. Calif. Dent. Assoc. 2007, 35, 777–822. [Google Scholar]
- Featherstone, J.D.B.; Alston, P.; Chaffee, B.W.; Rechmann, P. Caries Management by Risk Assessment (CAMBRA): An Update for Use in Clinical Practice for Patients Aged 6 Through Adult. J. Calif. Dent. Assoc. 2019, 47, 25–34. [Google Scholar]
- Featherstone, J.D.B.; Crystal, Y.O.; Alston, P.; Chaffee, B.W.; Doméjean, S.; Rechmann, P.; Zhan, L.; Ramos-Gomez, F. Evidence-Based Caries Management for All Ages-Practical Guidelines. Front. Oral Health 2021, 27, 657518. [Google Scholar] [CrossRef] [PubMed]
- Jenson, L.; Budenz, A.W.; Featherstone, J.D.; Ramos-Gomez, F.J.; Spolsky, V.W.; Young, D.A. Clinical protocols for caries management by risk assessment. J. Calif. Dent. Assoc. 2007, 35, 714–723. [Google Scholar]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuytm, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Hernán, M.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Rechmann, P.; Chaffee, B.W.; Rechmann, B.M.T.; Featherstone, J.D.B. Changes in Caries Risk in a Practice-Based Randomized Controlled Trial. Adv. Dent. Res. 2018, 29, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriegler, K.; Blue, C.M. Caries Management by Risk Assessment vs. Traditional Preventive Strategies: Effect on Oral Health Behaviors and Caries Diagnoses: A Retrospective Case-Control Observational Design. Clin. Case Rep. 2021, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Chung, K.C. Observational Studies: Cohort and Case-Control Studies. Plast Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulraheem, S.; Bondemark, L. Hawthorne effect reporting in orthodontic randomized controlled trials: Truth or myth? Blessing or curse? Eur. J. Orhod. 2018, 28, 475–479. [Google Scholar] [CrossRef]
- Pacheco, K.G.; Enciso, P.A.; Martinez Lizan, I.; Pareja, G. Motivation: A Key Element to Adopt Healthy Habits and Improve Oral Health. Psychol. Behav. Sci. Int. J. 2019, 13, 555870. [Google Scholar]

| Parameter | Description |
|---|---|
| Population (P) | Adults and children |
| Intervention (I) | Dental caries treatment + CAMBRA protocol |
| Control (C) | Dental caries treatment |
| Outcome (O) | Dental caries incidence and/or oral bacterial load |
| Database | Search Keys |
|---|---|
| PubMed | (CAMBRA) OR (“Caries Management by Risk Assessment”) |
| Cochrane Library | #1 CAMBRA #2 “Caries Management by Risk Assessment” #3 #1 OR #2 |
| Web of Science | CAMBRA (All Fields) OR “Caries Management by Risk Assessment” (All Fields) |
| Scopus | (TITLE-ABS-KEY (CAMBRA) OR TITLE-ABS-KEY (“Caries Management by Risk Assessment”) |
| Embase | (CAMBRA) OR (“Caries Management by Risk Assessment”) |
| Criteria | Description |
|---|---|
| Inclusion Criteria | Clinical studies evaluating the incidence of dental caries lesions and/or reduction of cariogenic bacterial load (Streptococcus mutans and/or Lactobacilli spp.) |
| Intervention group that follows the CAMBRA protocol | |
| Existence of a control group | |
| Exclusion Criteria | Reviews, animal and cellular studies, letters, clinical cases, comments, and abstracts |
| Author/Year | Type of Study | Groups (n) | Products (Based on Individual Risk Assessment) | Age (Mean) | Follow-Up (Months) | Dental Caries | Bacterial Load (CFU/mL, Log10) |
|---|---|---|---|---|---|---|---|
| Cheng et al., 2015 [7] | Randomized clinical trial | G1: Control (52) G2: CAMBRA (57) | G2 Chlorhexidine mouthwash (0.12%); Sodium fluoride toothpaste (1.100 ppm); Sodium fluoride mouthwash (0.05%); Sodium fluoride gel (1.1%) | 40 | 24 | DMFS increment = 0 G1 = 7.7% G2 = 12.3% (p = 0.32) DMFS increment G1 = 4.6 ± 4.1 G2 = 3.5 ± 3.5 (p = 0.02) | Streptococcus mutans Baseline: G1 = 4.51 ± 1.29; G2 = 4.27 ± 1.42 (p = 0.37) 12 months: G1 = 4.58 ± 1.49; G2 = 3.26 ± 1.95 (p < 0.01) Lactobacillus spp. Baseline: G1 = 3.71 ± 1.98; G2 = 3.60 ± 1.95 (p = 0.76) 12 months: G1 = 3.28 ± 2.00; G2 = 2.92 ± 2.10 (p = 0.25) |
| Rechmann et al., 2018 [23] | Randomized clinical trial | G1: Control (239) G2: CAMBRA (221) | G1 Nonfluoridated mouthwash; Fluoridated toothpaste (1.100 ppm); Placebo varnish; Sorbitol candies G2 Chlorhexidine mouthwash (0.12%); Fluoridated toothpaste (1.100 ppm); Fluoridated toothpaste (5.000 ppm); Fluoride mouthwash (0.05%); Fluoridated varnish; Xylitol candies | 36 | 24 | Cavities on radiograph into dentin Baseline: G1 = 17.1%; G2 = 17.5% (p = 0.33) 6 months: G1 = 7.0%; G2 = 6.3% (p = 0.86) 12 months: G1 = 4.3%; G2 = 10.3% (p = 0.33) 18 months: G1 = 7.5%; G2 = 3.7% (p = 0.49) 24 months: G1 = 15.4%; G2 = 0% Proximal enamel lesions on radiograph Baseline: G1 = 24.8%; G2 = 27.7% (p = 0.45) 6 months: G1 = 21.1%; G2 = 16.5% (p = 0.42) 12 months: G1 = 17.0%; G2 = 17.6% (p = 0.79) 18 months: G1 = 22.5%; G2 = 18.5% (p = 0.35) 24 months: G1 = 17.9%; G2 = 16.3% (p = 0.90) Active white spot lesions Baseline: G1 = 18.1%; G2 = 19.7% (p = 0.48) 6 months: G1 = 12.3%; G2 = 10.1% (p = 0.71); 12 months: G1 = 19.1%; G2 = 11.8% (p = 0.26) 18 months: G1 = 0.10%; G2 = 7.4% (p = 0.93) 24 months: G1 = 12.8%; G2 = 11.6% (p = 0.75) | _ |
| Kriegler et al., 2021 [24] | Case-control | G1: Control (100) G2: CAMBRA (107) | G2 Chlorhexidine mouthwash (0.12%); Fluoridated toothpaste (5.000 ppm); Sodium fluoride mouthwash (0.05%) | 69 | 12 | Dental caries incidence Baseline: G1 = 49.0%; G2 = 41.1% (p = 0.27) 12 months: G1 = 41.1%; G2 = 18.7% (p = 0.10) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, A.; Amaro, I.; Iunes, T.; Paula, A.; Marto, C.M.; Saraiva, J.; Ferreira, M.M.; Carrilho, E. CAMBRA Protocol Efficacy: A Systematic Review and Critical Appraisal. Dent. J. 2022, 10, 97. https://doi.org/10.3390/dj10060097
Coelho A, Amaro I, Iunes T, Paula A, Marto CM, Saraiva J, Ferreira MM, Carrilho E. CAMBRA Protocol Efficacy: A Systematic Review and Critical Appraisal. Dentistry Journal. 2022; 10(6):97. https://doi.org/10.3390/dj10060097
Chicago/Turabian StyleCoelho, Ana, Inês Amaro, Tainá Iunes, Anabela Paula, Carlos Miguel Marto, José Saraiva, Manuel Marques Ferreira, and Eunice Carrilho. 2022. "CAMBRA Protocol Efficacy: A Systematic Review and Critical Appraisal" Dentistry Journal 10, no. 6: 97. https://doi.org/10.3390/dj10060097
APA StyleCoelho, A., Amaro, I., Iunes, T., Paula, A., Marto, C. M., Saraiva, J., Ferreira, M. M., & Carrilho, E. (2022). CAMBRA Protocol Efficacy: A Systematic Review and Critical Appraisal. Dentistry Journal, 10(6), 97. https://doi.org/10.3390/dj10060097








