Efficiency and Safety of Dental Implantation in the Area of Hyperdense Jaw Lesions: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
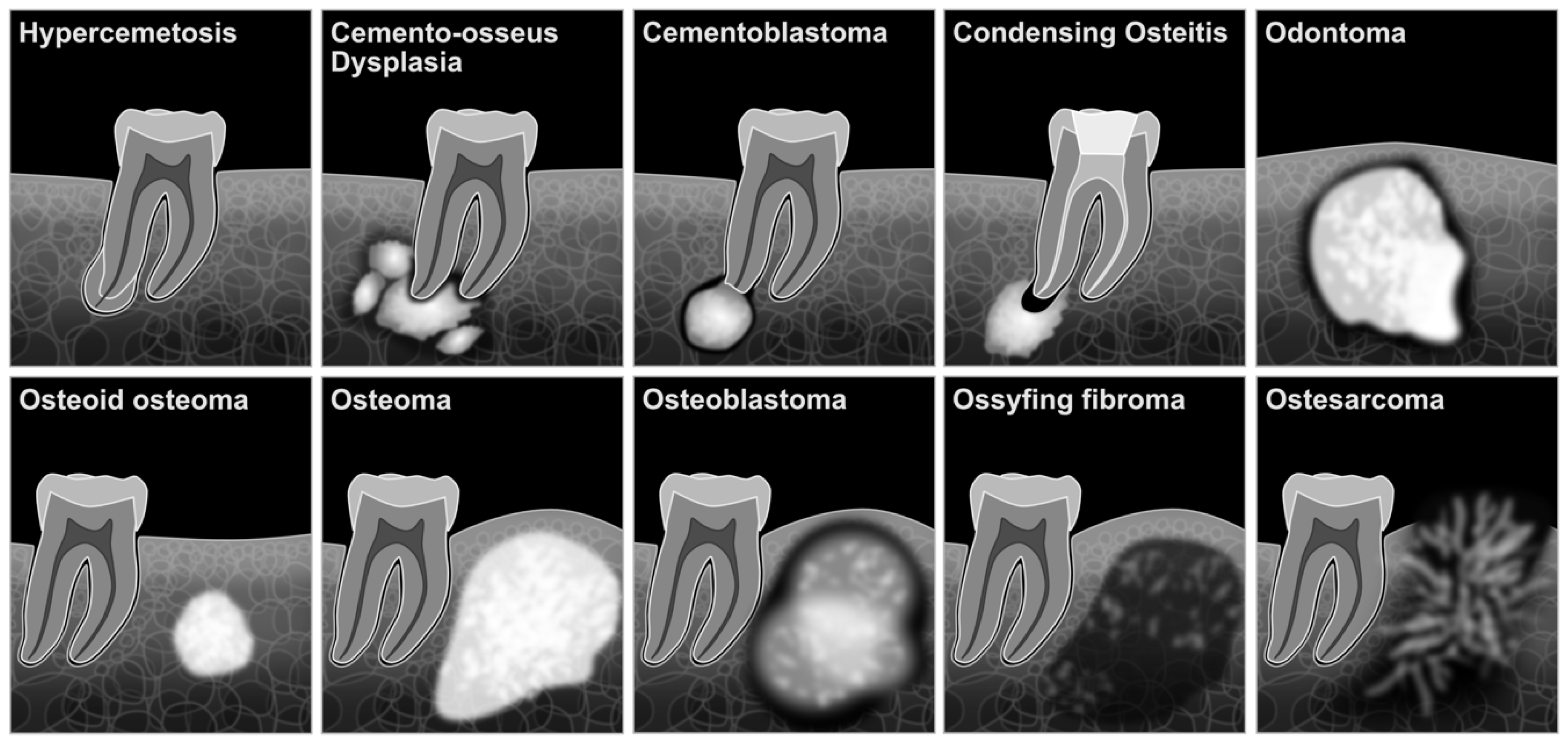
3. Results
3.1. Osteoid Osteoma
3.1.1. Histopathologic Features
3.1.2. Radiographic Features and Differential Diagnosis
3.1.3. Clinical Cases
3.2. Odontoma
3.2.1. Histopathologic Features
3.2.2. Radiographic Features and Differential Diagnosis
3.2.3. Clinical Cases
3.3. Osteoblastoma
3.3.1. Histopathologic Features
3.3.2. Radiographic Features and Differential Diagnosis
3.4. Cementoblastoma
3.4.1. Histopathologic Features
3.4.2. Radiographic Features and Differential Diagnosis
3.4.3. Clinical Cases
3.5. Cemento-Osseous Dysplasia (COD)
3.5.1. Histopathologic Features
3.5.2. Radiographical Investigation and Differential Diagnosis
3.5.3. Clinical Cases
3.6. Exostosis
Histopathologic, Radiological Features, and Differential Diagnosis
3.7. Idiopathic Osteosclerosis and Condensing Osteitis
3.7.1. Histopathologic Features
3.7.2. Radiographic Features and Differential Diagnosis
3.7.3. Clinical Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Lesion | Decades of Life/Age | Etiology | Gender Predilection | Anatomical Sites | Radiographic Characteristics | Histopathological Characteristics |
|---|---|---|---|---|---|---|
| Osteoid Osteoma | 2nd–3rd | Benign neoplastic lesion | No. | Angle of mandible | Less than 1.5 cm centrally radiopacity surrounds a well-circumscribed round to ovoid radiolucency or nidus with reactive surrounding sclerosis. | Centrally located lamellar trabeculae of cancellous bone with ample fibrofatty bone marrow, which are surrounded by osteoblasts and scattered osteoclasts. Or the dense, compact bone with sparse marrow tissue, well-circumscribed highly vascularized nidus contains mixture of trabeculae of variably mineralized woven bone which surround central radiopacity. |
| Cementoblastoma | 2nd–3rd | Benign neoplastic lesion | No. | Mostly in the area of the first molar of the mandible | Symptomatic round radiopaque mass with radiolucent rim. It is fused to the root of the tooth/teeth. May cause root resorption. | Dense mass of mineralized cementum-like material with numerous basophilic reversal lines. |
| Fibrous dysplasia | 1st–2nd | Non-inflammatory | No. | Maxilla | The lesion ranges from a radiolucent to an entirely radiopaque lesion with a ground glass appearance. | Irregular trabeculae of immature bone with a slight to moderate cellular fibrous connective tissue stoma. |
| Ossifying fibroma | 3rd–4th | Benign neoplasmic lesion | Female | Mandible in the area of molars and premolars | Asymptomatic, well-defined unilocular lesion with radiolucency or mixed radiolucency and radiopacity. | Cellular fibrous tissue with a mixture of cementicles, osteoid, and woven bone. |
| Osteosarcoma | 2nd | Malignant bone tumor | Male | Maxilla and molar regions of the mandible | Symptomatic, mixed radiolucent radiopaque lesion, widened periodontal ligament and loss of periodontal space, destruction of cortical plate. | Infiltrative margins, cartilage formation, and presence of malignant cells without osteoid production. |
| Osteoblastoma | 2nd–3rd | Benign neoplasm lesion | No. | Posterior area of the mandible | Asymptomatic, well-or–ill-defined round to oval calcified area with or without radiolucency or fully radiolucent. | Irregular bony trabeculae, outstanding vascular network, and immature bone within the stroma. Bony trabeculae show various degrees of calcification, several layers of plump, hyperchromatic osteoblasts. |
| Hypercementosis | 2nd–3rd | Non-neoplasm excessive cementum deposition on the roots of teeth due to systemic and local factors | No. | Posterior region of the mandible | Asymptomatic, excessive dense mass around the root with irregular, surrounded by intact radiolucent periodontal ligament space and lamina dura. | Cellular or hypocellular excessive cementum. |
| Exostosis | 5th | Benign protuberances of bone | No. | The lingual aspect of the mandible near the canine and premolar teeth (torus mandibularis) or uni- or bilaterally in the palatal midline (torus palatinus and buccal exostoses) | Hyperplasic bone, consisting of mature cortical and trabecular bone. | Bony outgrowths located in the inner aspect of the alveolar bone of the jaw above the origin of the mylohyoid muscle (mandibular tori), buccal cortex of the maxilla (buccal exostosis), the midline on the hard palate (torus palatinus). |
| Odontoma | 1st–2nd | Odontogenic tumor-like malformation | No. | Incisor and canine areas of the maxilla and mandible | Amorphous radiopaque mass surrounded by slight radiolucency. Compound odontoma: radiopaque tooth structure in the tooth-bearing area, between roots, or over the crown of the impacted tooth. | Normal tooth component structures like enamel, dentine, cementum and even pulp, connective tissue capsules with islands of odontogenic epithelium. |
| Condensing osteitis (Focal sclerosing osteomyelitis) | 3rd–7th | Low-grade inflammatory stimulus from an inflamed dental pulp | No. | In the molar and premolar area of the mandible and associated with infected teeth | No radiolucent border, poorly defined nonexpanding sclerotic image, thickening of the periodontal ligament space, diffuse radiopaque lesions, and may be combined with adjacent radiolucent.inflammatory lesions. | Replacement of bone marrow and cancellous bone with dense compact bone fibrosis replacing fatty marrow. |
References
- Kumar, J.; Vanagundi, R.; Manchanda, A.; Mohanty, S.; Meher, R. Radiolucent Jaw Lesions: Imaging Approach. Indian J. Radiol. Imaging 2021, 31, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, T.; Bowles, W.R.; Rohrer, M. Frequency and Distribution of Radiolucent Jaw Lesions: A Retrospective Analysis of 9723 Cases. J. Endod. 2012, 38, 729–732. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D. Lesions of the Jaws Presenting as Radiolucencies on Cone-Beam CT. Clin. Radiol. 2016, 71, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Matsumoto, N.; Matsumoto, K.; Ohnishi, M.; Honda, K.; Komiyama, K. Asymptomatic Radiopaque Lesions of the Jaws: A Radiographic Study Using Cone-Beam Computed Tomography. J. Oral Sci. 2011, 53, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Esfahanizadeh, N.; Yousefi, H. Successful Implant Placement in a Case of Florid Cemento-Osseous Dysplasia: A Case Report and Literature Review. J. Oral Implant. 2018, 44, 275–279. [Google Scholar] [CrossRef]
- Carini, F.; Longoni, S.; Simone, M.; Monai, D.; Saggese, V.; Porcaro, G. Central Osteoma of the Maxilla: Implant Rehabilitation with Immediate Loading in Fresh Extraction Socket. Ann. Stomatol. 2014, 5, 10–14. [Google Scholar]
- Djanic, P.; Brajdic, D.; Biocic, J.; Tomislav, B.; Salaric, I.; Peric, B.; Macan, D. Dental Implants in a Patient with a Large Idiopathic Osteosclerosis of Maxilla. Clin. Oral Impl. Res. 2017, 28, 520. [Google Scholar] [CrossRef][Green Version]
- Sadda, R.S.; Phelan, J. Dental Management of Florid Cemento-Osseous Dysplosia. N. Y. State Dent. J. 2014, 80, 24–26. [Google Scholar]
- Larsson Wexell, C.; Bergenblock, S.; Kovács, A. A Case Report on Gardner Syndrome With Dental Implant Treatment and a Long-Term Follow-Up. J. Oral Maxillofac. Surg. 2019, 77, 1617–1627. [Google Scholar] [CrossRef]
- Davarpanah, M.; Szmukler-Moncler, S. Unconventional Implant Treatment: I. Implant Placement in Contact with Ankylosed Root Fragments. A Series of Five Case Reports. Clin. Oral Implant. Res. 2009, 20, 851–856. [Google Scholar] [CrossRef]
- Davarpahah, M.; Szmukler-Moncler, S. Unconventional Implant Placement. 2: Placement of Implants through Impacted Teeth. Three Case Reports. Int. J. Periodontics Restor. Dent. 2009, 29, 405–413. [Google Scholar]
- Davarpanah, M.; Szmukler-Moncler, S.; Rajzbaum, P.; Davarpanah, K.; Capelle-Ouadah, N.; Demurashvili, G. Unconventional Implant Placement. V: Implant Placement through Impacted Teeth; Results from 10 Cases with an 8- to 1-Year Follow-Up. Int. Orthod. 2015, 13, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Mithridade, D.; Serge, S.-M.; Keyvan, D.; Nedjoua, C.-O.; Georgy, D.; Philippe, R. Unconventional Implant Placement IV. Implant Placement through Impacted Teeth to Avoid Invasive Surgery. Long-Term Results of 3 Cases. Open Dent. J. 2015, 9, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Giardino, L.; Crespi, R.; Romagnoli, R. Cementum Formation around a Titanium Implant: A Case Report. Int. J. Oral Maxillofac Implant. 2002, 17, 729–732. [Google Scholar]
- Langer, L.; Langer, B.; Salem, D. Unintentional Root Fragment Retention in Proximity to Dental Implants: A Series of Six Human Case Reports. Int. J. Periodontics Restor. Dent. 2015, 35, 305–313. [Google Scholar] [CrossRef]
- Alqahtani, F. Implant Treatment for a Patient With Large Condensing Osteitis: Case Report. J. Oral Implantol. 2020, 46, 249–252. [Google Scholar] [CrossRef]
- Gerlach, R.C.; Dixon, D.R.; Goksel, T.; Castle, J.T.; Henry, W.A. Case Presentation of Florid Cemento-Osseous Dysplasia with Concomitant Cemento-Ossifying Fibroma Discovered during Implant Explantation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e44–e52. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Ju, Y.-R.; Lin, T.-M.; Chen, P.-L.; Chan, C.-P.; Pan, W.-L. Dental Implant Placement in the Edentulous. Area with Idiopathic Osteosclerosis—Two Case. Reports and Literature Review. J. Taiwan Soc. Oral Maxillofac. Surg. 2017, 28, 175–186. [Google Scholar]
- Ellingsen, T.; Nalley, A.; Oda, D.; Dodson, T.B.; Lee, P.P. Osteoblastoma and Osteoid Osteoma of the Mandible: Review of the Literature and Report of Two Cases. Case Rep. Dent. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Karandikar, S.; Thakur, G.; Tijare, M.; Shreenivas, K.; Agrawal, K. Osteoid Osteoma of Mandible. Case Rep. 2011, 2011, bcr1020114886. [Google Scholar] [CrossRef]
- Bhatt, G.; Gupta, S.; Ghosh, S.; Mohanty, S.; Kumar, P. Central Osteoma of Maxilla Associated with an Impacted Tooth: Report of a Rare Case with Literature Review. Head Neck Pathol. 2019, 13, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Regezi, J.A.; Sciubba, J.J.; Jordan, R.C.K.; Regezi, J.A. Oral Pathology: Clinical Pathologic Correlations; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017; ISBN 978-0-323-29769-1. [Google Scholar]
- Al-Shehry, A.; Hussein, M.R. Cutaneous myiasis of face. In Proceedings of the 15th National Conference of the IAOMFP, Chennai, India, 22–24 December 2006; Volume 11, p. 5. [Google Scholar]
- Devathambi, T.R.; Ambrose, W.; Niazi, K.T.M.; Raja, S. Osteoid Osteoma in Anterior Border of Ramus of Mandible: A Rare Entity. J. Indian Acad. Oral Med. Radiol. 2016, 28, 449. [Google Scholar] [CrossRef]
- Singh, A.; Solomon, M.C. Osteoid Osteoma of the Mandible: A Case Report with Review of the Literature. J. Dent. Sci. 2017, 12, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Walia, C.; Devi, P.; Vb, T.; Jayadev, S. Osteoid Osteoma of the Mandible: A Rare Entity. JIAOMR 2010, 22, 162–164. [Google Scholar] [CrossRef]
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Chi, A.C. Oral and Maxillofacial Pathology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; ISBN 978-0-323-34142-4. [Google Scholar]
- Chaudhary, M.; Kulkarni, M. Osteoid Osteoma of Mandible. J. Oral Maxillofac. Pathol. 2007, 11, 52. [Google Scholar] [CrossRef]
- An, S.-Y.; Shin, H.-I.; Choi, K.-S.; Park, J.-W.; Kim, Y.-G.; Benavides, E.; Kim, J.-W.; An, C.-H. Unusual Osteoid Osteoma of the Mandible: Report of Case and Review of the Literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e134–e140. [Google Scholar] [CrossRef]
- Ida, M.; Kurabayashi, T.; Takahashi, Y.; Takagi, M.; Sasaki, T. Osteoid Osteoma in the Mandible. Dentomaxillofacial Radiol. 2002, 31, 385–387. [Google Scholar] [CrossRef]
- Liu, C.J.; Chang, K.W.; Chang, K.M.; Cheng, C.Y. A Variant of Osteoid Osteoma of the Mandible: Report of a Case. J. Oral Maxillofac. Surg. 2002, 60, 219–221. [Google Scholar] [CrossRef]
- Chaurasia, A.; Balan, A. Osteoid Osteoma of Jaws: An Overview. J. Indian Acad Oral Med. Radiol 2008, 20, 6. [Google Scholar] [CrossRef]
- Resnick, D.; Kransdorf, M.J. Bone and Joint Imaging; Elsevier Saunders: Philadelphia, PA, USA, 2005; ISBN 978-1-4377-2092-1. [Google Scholar]
- Park, M.S.; Eo, M.Y.; Myoung, H.; Kim, S.M.; Lee, J.H. Early Diagnosis of Jaw Osteomyelitis by Easy Digitalized Panoramic Analysis. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 6. [Google Scholar] [CrossRef]
- Huvos, A.G. Bone Tumors: Diagnosis, Treatment, and Prognosis, 2nd ed.; W.B. Saunders Co: Philadelphia, PA, USA, 1991; ISBN 978-0-7216-2050-3. [Google Scholar]
- Masłowska, K.; Jakimiak, A.; Popowski, W. Comparison of Two Clinical Cases of Osteoma and Odontoma Complex Located in the Posterior Region of the Maxilla. J. Pre Clin. Clin. Res. 2019, 13, 42–49. [Google Scholar] [CrossRef]
- Silva, B.S.F.; Bueno, M.R.; Yamamoto-Silva, F.P.; Gomez, R.S.; Peters, O.A.; Estrela, C. Differential Diagnosis and Clinical Management of Periapical Radiopaque/Hyperdense Jaw Lesions. Braz. Oral Res. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Barba, L.T.; Campos, D.M.; Rascón, M.M.N.; Barrera, V.A.R.; Rascón, A.N. Descriptive Aspects of Odontoma: Literature Review. Rev. Odontológica Mex. 2016, 20, e265–e269. [Google Scholar] [CrossRef]
- Hidalgo-Sánchez, O.; Leco-Berrocal, M.I.; Martínez-González, J.M. Metaanalysis of the Epidemiology and Clinical Manifestations of Odontomas. Med. Oral Patol. Oral Cir. Bucal 2008, 13, E730–E734. [Google Scholar]
- Thompson, L. World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours. Ear Nose Throat J. 2006, 85, 74. [Google Scholar] [CrossRef]
- Amado Cuesta, S.; Gargallo Albiol, J.; Berini Aytés, L.; Gay Escoda, C. Review of 61 Cases of Odontoma. Presentation of an Erupted Complex Odontoma. Med. Oral 2003, 8, 366–373. [Google Scholar]
- Philipsen, H.P.; Reichart, P.A.; Praetorius, F. Mixed Odontogenic Tumours and Odontomas. Considerations on Interrelationship. Review of the Literature and Presentation of 134 New Cases of Odontomas. Oral Oncol. 1997, 33, 86–99. [Google Scholar] [CrossRef]
- Dunfee, B.; Sakai, O.; Pistey, R.; Gohel, A. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. Radiographics 2006, 26, 1751–1768. [Google Scholar] [CrossRef]
- Gedik, R.; Müftüoğlu, S. Compound Odontoma: Differential Diagnosis and Review of the Literature. West. Indian Med. J. 2014, 63, 793–795. [Google Scholar] [CrossRef]
- de Souza Batista, F.R.; de Souza Batista, V.E.; Vechiato-Filho, A.J.; Tieghi Neto, V.; Figueira, J.A.; Verri, F.R. Immediate Dental Implant Placement After Removal of Complex Odontoma. J. Craniofacial Surg. 2017, 28, e737–e738. [Google Scholar] [CrossRef]
- Ladani, P.; Shetye, A.; Shah, M. Dental Rehabilitation of Patient with Complex Odontoma: A Case Report and Review of Literature. J. Dent. Implant. 2017, 7, 28. [Google Scholar] [CrossRef]
- Öztürk, K.; DeliLbaşi, E.; Kahraman, S.; Kodaz, T. Huge Complex Odontoma and Treatment. Turk. Klin. J. Dent. Sci. 2019, 25, 113–116. [Google Scholar] [CrossRef][Green Version]
- Pacifici, A.; Carbone, D.; Marini, R.; Pacifici, L. Surgical Management of Compound Odontoma Associated with Unerupted Tooth. Case Rep. Dent. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gyulai-Gaal, S. Odontoma Removal and Oral Rehabilitation via Insertions of Albumin and Gentamycin Coated Bone Allograft and Dental Implants—A Case Report. BJSTR 2021, 33, 26116–26120. [Google Scholar] [CrossRef]
- Cavalcante, R.C.; Petinati, M.F.P.; de Oliveira, E.R.; Bergamaschi, I.P.; Rebelatto, N.L.B.; Klüppel, L.; Scariot, R.; da Costa, D.J. Benign Cementoblastoma Associated with an Impacted Third Molar inside Maxillary Sinus. Case Rep. Surg. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Hosalkar, R.; Sidana, S.; Swain, N.; Patel, S. Benign Cementoblastoma Involving Left Deciduous First Molar: A Case Report and Review of Literature. J. Oral Maxillofac. Pathol. 2019, 23, 422. [Google Scholar] [CrossRef]
- Brannon, R.B.; Fowler, C.B.; Carpenter, W.M.; Corio, R.L. Cementoblastoma: An Innocuous Neoplasm? A Clinicopathologic Study of 44 Cases and Review of the Literature with Special Emphasis on Recurrence. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 93, 311–320. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Gomez, R.S. Recurrence Probability for Keratocystic Odontogenic Tumors: An Analysis of 6427 Cases. J. Cranio-Maxillofac. Surg. 2017, 45, 244–251. [Google Scholar] [CrossRef]
- Huber, A.R.; Folk, G.S. Cementoblastoma. Head Neck Pathol. 2009, 3, 133. [Google Scholar] [CrossRef]
- Moraes, E.J.D.; Benevides Moraes, N.; Farias Miranda, D. Evolución de 1 Año de Un Paciente Rehabilitado Con Implantes Oseointegrados de Carga Inmediata Después de La Resección Quirúrgica de Un Cementoblastoma: Reporte Clínico. Rev. Esp. Cirug. Oral Y Maxilofac. 2009, 31, 386–391. [Google Scholar] [CrossRef]
- Edgar, C.; Alejandra, S.; Mariana, V.; Santos, D. Contemporary Management of Cementoblastoma: Surgical Technique and Oral Rehabilitation. Case Rep. 2021, 4. [Google Scholar]
- Eskandarloo, A.; Yousefi, F. CBCT Findings of Periapical Cemento-Osseous Dysplasia: A Case Report. Imaging Sci. Dent. 2013, 43, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Eversole, R.; Su, L.; ElMofty, S. Benign Fibro-Osseous Lesions of the Craniofacial Complex A Review. Head Neck Pathol. 2008, 2, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Shadid, R.; Kujan, O. Success of Dental Implant Osseointegration in a Florid Cemento-Osseous Dysplasia: A Case Report with 8-Year Follow-Up. Clin. Pract. 2020, 10, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Park, W.-B.; Han, J.-Y.; Jang, J.; Kang, K.; Kang, P. Long-Term Implant Survivability of an Implant Having Direct Contact with Cementum-Like Tissue in a Preexisting Mandibular Intraosseous Lesion with a 16-Year Longitudinal Follow-Up. Int. J. Periodontics Restor. Dent. 2019, 39, 895–902. [Google Scholar] [CrossRef]
- Mlouka, M.; Tlili, M.; Khanfir, F.; Hamrouni, A.; Khalfi, M.S.; Ben Amor, F. Implant Placement in a Focal Cemento-osseous Dysplasia: A Modified Protocol with a Successful Outcome. Clin. Case Rep. 2022, 10, e05307. [Google Scholar] [CrossRef]
- Oliveira, M.T.F.; Cardoso, S.V.; Silva, C.J.; Zanetta-Barbosa, D.; Loyola, A.M. Failure of Dental Implants in Cemento-Osseous Dysplasia: A Critical Analysis of a Case. Rev. Odontol. UNESP 2014, 43, 223–227. [Google Scholar] [CrossRef][Green Version]
- Shin, H.S.; Kim, B.C.; Lim, H.J.; Jo, S.Y.; Lee, J. Chronic Osteomyelitis Induced by the Placement of Dental Implants on Cemento-Osseous Dysplasia. Br. J. Oral Maxillofac. Surg. 2019, 57, 268–270. [Google Scholar] [CrossRef]
- Gupta, A.; Nayak, S.; Das, B.; Das, S. Florid Cemento-Osseous Dysplasia. J. Oral Maxillofac. Pathol. 2013, 17, 150. [Google Scholar] [CrossRef]
- Jainkittivong, A.; Langlais, R.P. Buccal and Palatal Exostoses: Prevalence and Concurrence with Tori. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 48–53. [Google Scholar] [CrossRef]
- Gorsky, M.; Raviv, M.; Kfir, E.; Moskona, D. Prevalence of Torus Palatinus in a Population of Young and Adult Israelis. Arch. Oral Biol. 1996, 41, 623–625. [Google Scholar] [CrossRef]
- Santhanakrishnan, M.; Rangarao, S. Mandibular Tori: A Source of Autogenous Bone Graft. J. Indian Soc. Periodontol. 2014, 18, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Puttaswamaiah, R.; Gowda, V.; Galgali, S. Exostosis: A Donor Site for Autograft. Indian J. Dent. Res. 2011, 22, 860. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Murakami, S.; Kishino, M.; Sakuda, M. Gigantic Dense Bone Island of the Jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1996, 82, 108–115. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Cai, C.; Liu, Z.; Zhang, L.; Wang, C.; Xu, J. Longitudinal Investigation of Idiopathic Osteosclerosis Lesions of the Jaws in a Group of Chinese Orthodontically-Treated Patients Using Digital Panoramic Radiography. J. Dent. Sci. 2022, 17, 113–121. [Google Scholar] [CrossRef]
- Williams, T.P.; Brooks, S.L. A Longitudinal Study of Idiopathic Osteosclerosis and Condensing Osteitis. Dentomaxillofac. Radiol. 1998, 27, 275–278. [Google Scholar] [CrossRef]
- Verzak, Z.; Celap, B.; Modrić, V.E.; Sorić, P.; Karlović, Z. The Prevalence of Idiopathic Osteosclerosis and Condensing Osteitis in Zagreb Population. Acta Clin. Croat. 2012, 51, 573–577. [Google Scholar]
- Chen, G.; Sung, P.-T. Gingival and Localized Alveolar Bone Necrosis Related to the Use of Arsenic Trioxide Paste--Two Case Reports. J. Med. Assoc. 2014, 113, 187–190. [Google Scholar] [CrossRef][Green Version]
- Lever, J.H. Paget’s Disease of Bone in Lancashire and Arsenic Pesticide in Cotton Mill Wastewater: A Speculative Hypothesis. Bone 2002, 31, 434–436. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mandalunis, P.M. A Review of Metal Exposure and Its Effects on Bone Health. J. Toxicol. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Green, T.L.; Walton, R.E.; Clark, J.M.; Maixner, D. Histologic Examination of Condensing Osteitis in Cadaver Specimens. J. Endod. 2013, 39, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Ruhani, M.; Zarandi, A. Frequency and Pattern of Idiopathic Osteosclerosis and Condensing Osteitis Lesions in Panoramic Radiography of Iranian Patients. Dent. Res. J. 2016, 13, 322. [Google Scholar] [CrossRef]
- Tsvetanov, T. Mandibular idiopathic osteosclerosis or condensing osteitis. A case report. Int. J. Med. Dent. 2020, 24, 604–606. [Google Scholar]
- Curé, J.K.; Vattoth, S.; Shah, R. Radiopaque Jaw Lesions: An Approach to the Differential Diagnosis. RadioGraphics 2012, 32, 1909–1925. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Montes, C.; Jiménez-Farfán, M.D.; Hernández-Guerrero, J.C. Idiopathic Osteosclerosis in the Maxillomandibular Area. Radiol. Med. 2019, 124, 27–33. [Google Scholar] [CrossRef]
- Santoro, A.; Pannone, G.; Ramaglia, L.; Bufo, P.; Lo Muzio, L.; Saviano, R. Central Odontogenic Fibroma of the Mandible: A Case Report with Diagnostic Considerations. Ann. Med. Surg. 2016, 5, 14–18. [Google Scholar] [CrossRef]
- Mao, W.; Lei, J.; Lim, L.Z.; Gao, Y.; Tyndall, D.A.; Fu, K. Comparison of Radiographical Characteristics and Diagnostic Accuracy of Intraosseous Jaw Lesions on Panoramic Radiographs and CBCT. Dentomaxillofacial Radiol. 2021, 50, 20200165. [Google Scholar] [CrossRef]
- Almazrooa, S.; Binmadi, N.O.; Khalifa, H.M.; Jadu, F.M.; Jan, A.M.; Meisha, D.E. The Agreement Rate between Radiographic Interpretation and Histopathologic Diagnosis of Jaw Lesions. Radiol. Res. Pract. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Peker, E.; Karaca, I.R.; Gltekin, E.; Fakir, M. A 5 Year Retrospective Study of Biopsied Jaw Lesions with the Assessment of Concordance between Clinical and Histopathological Diagnoses. J. Oral Maxillofac. Pathol. 2016, 20, 78. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghsimi, K.; Vasilyev, A.V.; Kuznetsova, V.S.; Galtsova, A.V.; Badalyan, V.A.; Babichenko, I.I. Efficiency and Safety of Dental Implantation in the Area of Hyperdense Jaw Lesions: A Narrative Review. Dent. J. 2022, 10, 107. https://doi.org/10.3390/dj10060107
Taghsimi K, Vasilyev AV, Kuznetsova VS, Galtsova AV, Badalyan VA, Babichenko II. Efficiency and Safety of Dental Implantation in the Area of Hyperdense Jaw Lesions: A Narrative Review. Dentistry Journal. 2022; 10(6):107. https://doi.org/10.3390/dj10060107
Chicago/Turabian StyleTaghsimi, Kimya, Andrey Vyacheslavovich Vasilyev, Valeriya Sergeevna Kuznetsova, Angelina Vladimirovna Galtsova, Varditer Agabekovna Badalyan, and Igor Ivanovich Babichenko. 2022. "Efficiency and Safety of Dental Implantation in the Area of Hyperdense Jaw Lesions: A Narrative Review" Dentistry Journal 10, no. 6: 107. https://doi.org/10.3390/dj10060107
APA StyleTaghsimi, K., Vasilyev, A. V., Kuznetsova, V. S., Galtsova, A. V., Badalyan, V. A., & Babichenko, I. I. (2022). Efficiency and Safety of Dental Implantation in the Area of Hyperdense Jaw Lesions: A Narrative Review. Dentistry Journal, 10(6), 107. https://doi.org/10.3390/dj10060107






